Review Article - Imaging in Medicine (2012) Volume 4, Issue 5
Image-guided radiotherapy for esophageal cancer
Maria A Hawkins*1 & Katharine Aitken11Department of Radiotherapy, The Royal Marsden NHS Foundation Trust, Sutton, Surrey, UK
- Corresponding Author:
- Maria A Hawkins
Department of Radiotherapy
The Royal Marsden NHS Foundation Trust
Sutton, Surrey, UK
Tel: +44 208 661 3374
Fax: +44 208 661 3470
E-mail: maria.hawkins@rmh.nhs.uk
Abstract
Esophageal cancer continues to be a diagnostic and therapeutic challenge in the multidisciplinary oncology setting. External beam radiotherapy has an established role in the radical management (i.e., preoperative or definitive) of esophageal malignancies. Successful delivery of accurate radiotherapy techniques requires a detailed understanding of esophageal anatomy, multimodality imaging (e.g., CT and endoscopic ultrasound and PET) to visualize the tumor and involved lymph nodes, an individualized assessment of organ motion, and precise patient immobilization prior to treatment. The practicing clinician is advised to utilize the current technological advances in imaging, prior to and during radiotherapy, to ensure precise radiotherapy delivery. This article will provide a summary of current imaging techniques incorporated in modern radiotherapy planning.
Keywordsf
4DCT ▪ cone beam CT ▪ CT ▪ esophageal cancer ▪ PET ▪ radical radiotherapy
Esophageal cancer is the eighth most common malignancy worldwide, with more than 480,000 new patients diagnosed annually. The incidence of esophageal adenocarcinoma is increasing, whereas the incidence of squamous cell carcinoma has not changed. According to the Surveillance, Epidemiology and End Results (SEER) statistics, the 5-year survival rate for esophageal carcinoma based on stage at diagnosis (2001–2007) is 17% overall: 37% for local disease; 18% for regional disease and 3% for distant disease [101].
Radical external beam radiotherapy plays an important role in the management of esophageal malignancies in the preoperative setting for operable patients [1,2], and as a definitive treatment for patients that are not resectable due to medical or technical considerations.
This article will focus on the current status of CT-guided radiotherapy planning in esophageal malignancies; discussing the challenges in defining disease extent and spread with current multimodality imaging, methods of target definition for radiotherapy planning, characterization of tumor motion and current best practice for treatment set-up. Future perspectives and challenges in the implementation of new imaging for radiotherapy delivery will also be considered.
Diagnostic considerations for cancer of the esophagus
Esophageal cancer is classified according to the 2010 American Joint Committee on Cancer (AJCC) tumor, lymph node and metastasis system. For the first time, this includes nonanatomic cancer characteristics, such as tumor location, tumor cell type and histological grade, which can be then used to stratify patients into prognostic groups and select treatment concepts [3].
Once the diagnosis of an esophageal cancer is established, staging usually begins with a contrast- enhanced CT scan of the chest and abdomen; in order to both evaluate the region of the primary tumor and adjacent lymph nodes and to explore if there is any distant metastatic disease. The extent of the primary tumor and lymph node status are the most important prognostic factors in esophageal cancer. Accurate detection of lymph node involvement is crucial in order to direct the correct therapeutic strategy [4,5].
Whilst CT has the advantage of being a readily available diagnostic test, it is of limited value for locoregional tumor staging, especially in early stage cancers. It is unable to consistently determine the depth of primary tumor invasion. In one study, local tumor staging was correctly predicted by CT in only 42% of patients [6]. CT is also limited in its ability to accurately assess nodal disease. It is unable to reliably detect disease in lymph nodes that are not enlarged by size criteria and sensitivity for celiac axis nodal disease is poor. A systematic review of 20 studies (1095 patients) found a pooled sensitivity (95% CI) of 0.51 (0.47–0.55) and a pooled specificity (95% CI) of 0.80 (0.76–0.84) for identification of nodal metastases [7]. It is a useful screening investigation for metastatic disease but has limited sensitivity for detecting small volume metastases, particularly within the peritoneum [8]. In addition, further investigations may be required to clarify equivocal CT findings, such as adrenal lesions, which may be benign or malignant in etiology.
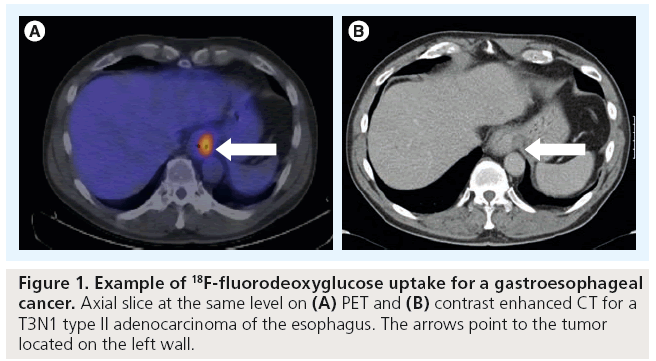
PET imaging is now well established to have a key role in the management of patients with esophageal cancer (Figure 1). PET, most commonly in combination with [18F]-fluorodeoxyglucose (FDG) as a metabolic marker, exploits the increased utilization of glucose uptake by tumor cells to provide functional information on tumor location and activity. The fusion of PET images with high resolution CT (PET-CT) enables this metabolic information to be combined with more accurate anatomical localization than PET imaging alone allows. PET-CT has a particular role in the improved detection of distant nodal and metastatic disease [9]. A systematic review undertaken by Facey et al. of 12 primary studies showed that PET had a sensitivity of 67% and a specificity of 97% in detecting esophageal tumors [10]. An incremental benefit of adding PET to endoscopic ultrasound (EUS) and CT was reported giving a sensitivity of 74% when compared with 53% for PET alone. However it has limited ability to detect local peritumoral lymphadenopathy where FDG uptake may be obscured by uptake from the primary tumor itself. It may also fail to detect disease in very small nodes (<1 cm) that are below the threshold for PET detection.
EUS is now used as an addition to CT as the locoregional tumor staging modality of choice. EUS has significantly higher sensitivity, but lower specificity, than CT and PET for the detection of regional lymph node metastases. EUS has the advantage of enabling evaluation of nodal architecture and fine-needle aspirate (FNA) sampling of nodes, which may be equivocal on imaging appearances. However, EUS may also have a potential risk of perforation [11] as the lumen has to be dilated to 14–16 mm to allow the completion of staging. Whilst PET is better than CT alone, EUS remains the most superior technique for assessing local lymph node disease, detecting significantly more patients with periesophageal and celiac lymphadenopathy than either PET or CT alone [12].
In view of all of the above, the combination of contrast-enhanced CT, EUS and PET-CT would appear to be optimal in order to stage the disease as accurately as possible.
Definitive treatment approaches
There is no international consensus on the optimal management of operable esophageal cancer. Surgery alone should be used in patients with very early stage disease. Currently, only 25% of patients treated with surgery have microscopically positive resection margins and the 5‑year survival rate rarely exceeds 40%. Phase III randomized clinical trials support the use of neoadjuvant chemotherapy in adenocarcinoma histology and a possible benefit in increased overall survival [13–15]. The role of neoadjuvant chemoradiotherapy in the treatment of patients with esophageal or esophagogastric-junction cancer is currently evolving, with promising outcomes in recent investigations [1].
Chemoradiation is the key treatment strategy, as a definitive therapy for locally advanced inoperable or unresectable disease [16,17]. Locoregional disease persistence or subsequent local disease recurrence are still high, despite combined modality treatment. Attempts to intensify treatment, either in the chemotherapy or radiotherapy components, have been hampered in the past by unacceptable toxicity [18–20]. However, radiation dose is an independent predictor of a pathological complete response and the addition of chemotherapy has further impact on this outcome [1,21]. Improvements in local treatment delivery are therefore needed to facilitate dose escalation, as justified by radiobiological considerations [21,22], and to minimize toxicity. The latter is especially relevant with the integration of newer chemotherapy drugs or targeted agents in the treatment pathway.
Tumor localization for radiotherapy planning
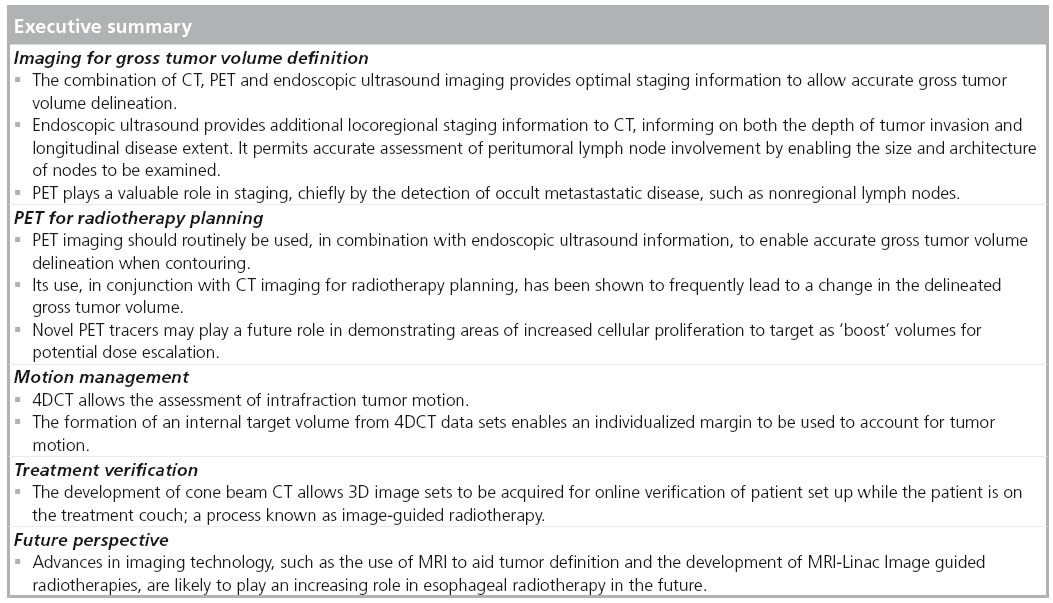
The current standard scheme for concurrent chemoradiation is radiotherapy at a dose of 50–50.4 Gy in 25–28 daily fractions, with cisplatin- and 5-fluorouracil-based concurrent chemotherapy. The radiation is delivered using a 3D conformal technique based on 3D CT planning. The International Commission on Radiation Units and Measurements (ICRU) introduced the concepts of gross tumor volume (GTV), clinical target volume (CTV) and planning target volume (PTV) [23,24]. The GTV and CTV are defined based on general oncological principles and these should be independent of any therapeutic approach.
■ GTV definition
The GTV is defined as the demonstrable gross extent of the tumor (GTV primary) and involved lymph nodes (GTV nodal). These concepts were further refined by ICRU 83 to state the imaging modality used for definition (e.g., GTV-PET) [25]. For defining the target volume in esophageal cancer, clinical information from all staging modalities is used (EUS and diagnostic CT scans). The integration of PET for GTV delineation in radiotherapy planning has increased the accuracy of target definition.
■ CTV definition
The CTV includes the areas at risk of microscopic spread of disease. The CTV is a volume of tissue that contains a demonstrable GTV and/or subclinical malignant disease with a certain probability of occurrence considered relevant for therapy. There is no general consensus on what probability is considered relevant for therapy but typically a probability of occult disease higher than 5–10% is assumed to require treatment. In general, it is recommended to use a cranio-caudal margin of 4 cm due to submucosal spread, and a 1 cm margin radially. These were derived using surgical data [26,27]; but most recently Gao et al. measured a mean (maximum) in vivo microscopic spread of 10.3 mm (29 mm) proximally and 18.3 mm (57 mm) distally in adenocarcinomas of the esophageal junction that underwent surgical resection [28]. This study provides the best data for CTV margins to use in radiotherapy planning; however, the EORTC-ROG recommend 3 cm proximally and 5 cm distally, measured along the mucosa [29]. In terms of locoregional lymph nodes at risk of microscopic disease to be included in the CTV, data from surgical series can be applied according to the location of the tumor, and guidelines regarding the nodal stations to be included are available [29]. However, it appears that patients with extensive nodal spread beyond the vicinity of the primary tumor are highly likely to have occult metastatic disease.
■ PTV definition
The PTV is a geometrical concept introduced for treatment planning and evaluation. It surrounds the CTV with a margin, such that the planned absorbed dose is delivered to the CTV. To obtain the PTV from the CTV, a margin is added to account for internal movement, such as respiratory motion, and external motion, such as set-up uncertainties. Respiratory motion cannot be characterized using respiratory-correlated CT. Many authors have proposed approaches to calculate the margins necessary for set-up uncertainties on the basis of systematic and random uncertainties derived form the position of patients during radiotherapy.
Tumor localization with PET for radiotherapy planning
FDG PET-CT has a proven role in the staging of esophageal cancer when combined with endoscopy, CT and EUS. Whilst EUS is superior to PET-CT for staging of the primary tumor and local lymph nodes, the additional value of PET-CT is in the improved detection of distant nodal and unsuspected metastatic disease, which can be present in up to 30% of patients at diagnosis [9]. In addition, PET-CT may have a role in the assessment of response to therapy [30,31]. Using metabolic response to treatment as a predictive marker, subsequent treatments can potentially be stratified depending on response. Following treatment, PET-CT can be used in patient follow-up to aid the detection of recurrent disease.
PET-CT has been shown to be of benefit in radiotherapy planning in many tumor types, such as non-small-cell lung cancer and lymphoma. Accurate delineation of the GTV must underpin the successful treatment of any malignancy by radiotherapy. PET-CT may help prevent geographical misses by enabling more accurate anatomical localization of the tumor than is possible by conventional imaging techniques alone. This is of particular importance in the era of highly conformal radiation techniques, such as intensity-modulated radiotherapy. Similarly, more accurate tumor delineation may improve the therapeutic window by allowing increased sparing of normal tissues and hence, potentially reducing toxicity. Finally, by combining PET with metabolic markers of proliferation, such as 18F-fluoro-deoxy-fluorothymidine (18F-FLT), it may be possible to not only make early assessments of response to therapy, but also to identify target subvolumes of the tumor with high proliferative activity or relative resistance. These areas could potentially serve as targets for future dose escalation studies [32].
■ Sensitivity & specificity of PET for tumor & lymph node localization
The ability of FDG PET-CT to accurately detect the primary tumor and involved locoregional lymph nodes has been widely studied. Increased uptake of FDG at the primary site has been reported to vary from 68 to 100% [33]. Whilst the sensitivity of PET-CT for detecting larger tumors is in the region of 95–100%, it has a reduced sensitivity for the detection of small tumors, as well as poorly cellular mucinous tumors [9]. The inability of PET-CT to reliably define the depth of invasion makes EUS a superior technique for accurately T-staging the tumor. In a study of 149 patients, Kato et al. demonstrated that PET was able to detect the primary tumor in only 80% of patients. This was largely due to its reduced ability to detect smaller tumors, with a sensitivity of only 43% for T1 tumors [34]. Whilst this study suggested a significant relationship between the intensity of FDG uptake (standard uptake value [SUV]) and depth of tumor invasion, this finding has not been corroborated by other authors.
The ability of PET-CT to accurately delineate tumor length has been validated by pathological studies. The direct comparison of tumor length, as evaluated by PET-CT, with length on pathological specimen is hampered by the difficulty in obtaining pathological specimens and the need to take into account factors such as shrinkage of surgical specimens. However, small studies have shown that FDG-PET CT-based estimates of tumor length correlate well with pathological tumor length, with an SUV cut-off of 2.5 most closely approximating tumor length [35]. Using an SUV cut-off of 2.5 has also been found to correlate well with tumor length as defined on EUS, the gold standard for T-stage imaging. Tumor length estimation from PET-CT was significantly shorter than length evaluated by CT alone and correlated better with endoscopy findings than CT [12].
Lymph node status is known to be one of the most significant prognostic factors in esophageal cancer. Therefore, the ability to define nodal status, number and location is of key importance in successful treatment. CT is limited in its value to accurately stage nodal disease. It is unable to reliably detect disease in lymph nodes that are not enlarged by size criteria or to differentiate between malignant and inflammatory causes of lymphadenopathy. Functional imaging, such as FDG-PET, may therefore be advantageous in the staging of lymph nodes in esophageal cancer. Reports of the sensitivity and specificity of FDG-PET in detecting lymph node disease vary considerably in the literature. A recent meta-analysis reported a pooled sensitivity of 0.57 (95% CI: 0.43–0.70) and specificity of 0.85 (95% CI: 0.76–0.95) compared with 0.50 (95% CI: 0.41–0.60) and 0.80 (95% CI: 0.77–0.89), respectively for CT [36]. The sensitivity and specificity of PET in detecting lymph node involvement can be improved by using integrated FDG-PET-CT techniques. In a recent study of 48 patients with squamous cell carcinoma of the esophagus, Yuan et al. recently confirmed the superiority of integrated FDG-PET-CT techniques over FDG PET, reporting an improved sensitivity of 94% and specificity of 92% in detecting nodal disease [37].
■ Effect of PET on target volume modification
Several studies have aimed to evaluate the effect of using PET on target volumes to aid radiotherapy planning. In the majority of these studies, visual interpretation of FDG-PET by the radiotherapist was used to guide target delineation. In a minority of studies, automatic contouring techniques were used. Here, a predefined level of SUV uptake, for example, the source:background ratio, is used to delineate the target volumes.
Using FDG-PET to aid radiotherapy volume delineation may lead to either an increase or decrease in the subsequent GTV. In a study of 34 patients, Moreau-Zabotto et al. evaluated the effect of coregistering FDG-PET images with the planning CT images on the target volumes. Target volumes were initially delineated on planning CT and then redefined with the PET images overlaid on the planning CT. The GTV was decreased by image fusion in 12 patients (35%) and increased in seven patients (21%). In four patients, the reduction in GTV was ≥25% due to a reduction in the length of the esophageal tumor. In 53% of patients, modifications in the GTV led to alterations in the subsequent PTV [38].
Discordance in GTVs when comparing CT alone with the combination of CT and PET appears to be predominantly in the longitudinal extent of disease within the esophagus. Leong et al. found that the GTV based on CT information alone excluded PET avid disease in 69% of patients. In five of these patients, areas of FDG avid GTV lay outside the CT-derived PTV, highlighting the potential risk of a geographical miss in patients planned with CT data alone [39].
Some authors have argued that, given the sensitivity of PET in disease detection, particularly for nodal disease, PET should not be used to reduce the GTVs due to the risk of a false-negative result [40]. They reported discordance in the assessment of nodal status between FDG-PET and EUS/CT in 47% of patients. In this study, six out of 30 patients were found to have abnormal nodes on FDG-PET with a normal EUS/CT. In three of these patients (10%), this would have led to a subsequent increase in the PTV.
Alterations in volume delineation may have consequent effects on the dose delivered to normal tissue. Whilst Leong et al. did not find any clinically significant difference in the dose delivered to the lung, cord and liver between CT- and CT/FDG-PET-based plans, others have reported that alterations in the PTV due to the influence of PET on target delineation resulted in significant changes in the radiotherapy dose received by normal tissues in nearly all cases, and hence, to the risk of normal tissue complication probability for organs such as the heart and lungs [38,41].
■ Technical considerations in using PET for radiotherapy planning
The use of PET for radiotherapy planning introduces several technical considerations. To enable accurate fusion of PET images with the planning CT, the patient must be positioned and immobilized in the radiotherapy treatment position.
One of the major concerns with the routine use of PET for radiotherapy planning is the lack of standardization in interpretation of the images. Visual interpretation of the PET signal is highly operator dependent. Changes in the windowing of the images will affect the volume of visible tumor; consequently, there can be significant variations between observers in interpretation of the images. Whilst semi-automatic contouring techniques using absolute SUV values avoid this, SUV can also be affected by a number of variables, such as scan acquisition and patient preparation. Further work is therefore required to ensure reproducibility and to standardize SUV values for use in target delineation.
■ Novel approaches to using PET for radiotherapy
18F-FLT is a marker for cellular proliferation that can be used in combination with PET imaging. In a study of patients receiving radical radiotherapy for esophageal cancer, serial FLT scans showed reduced uptake with increasing dose and a complete absence of uptake after 40 Gy. In this study, two patients showed subsequent increases in FLT uptake following unplanned treatment gaps, suggesting repopulation as a response to radiotherapy. End of treatment PET scans of patients with a confirmed pathological complete response on endoscopy showed no FLT uptake but a high degree of FDG uptake [42]. FLT-PET may therefore have a role in evaluating the early proliferative response to radiotherapy in an effort to identify subpopulations of cells with increased resistance to treatment. These areas could then, for example, be used as potential targets in future dose escalation studies as an example of PET-CT adaptive radiotherapy.
In summary, PET is currently an important technology for tumor localization in radiotherapy planning. Its use in conjunction with CT imaging for radiotherapy planning has been shown to frequently lead to a change in the delineated GTV. This may be a reduction in the target volume, for example, the cranio-caudal distance of the primary tumor being reduced, or alternatively, an increase in GTV, such as if previously unknown nodal disease is revealed. Further work is required to better understand the subsequent impact of this with regards to validating its effect on improving clinical outcome and reducing long-term treatment toxicity.
Motion definition with 4DCT
Intrafraction motion is defined as the organ motion that occurs while the patient is irradiated. Respiratory and cardiac motions are the main contributors to intrafraction motion, predominantly affecting the organs situated in the thorax and upper abdomen. Information about the magnitude and nature of the target motion is essential for the determination of the internal margin size. In order to include the intrafractional respiratory motion, a large volumetric expansion of the CTV is generally applied [43].
Initial reports regarding esophageal motion used CT scans acquired at the two extremes of the respiratory cycle (inhale and exhale) [44]. The displacement between inhale and exhale was measured at three levels (i.e., superior and inferior aspects of the tumor, and the isocenter) in all directions. They concluded that a 1 cm margin should account for esophageal motion.
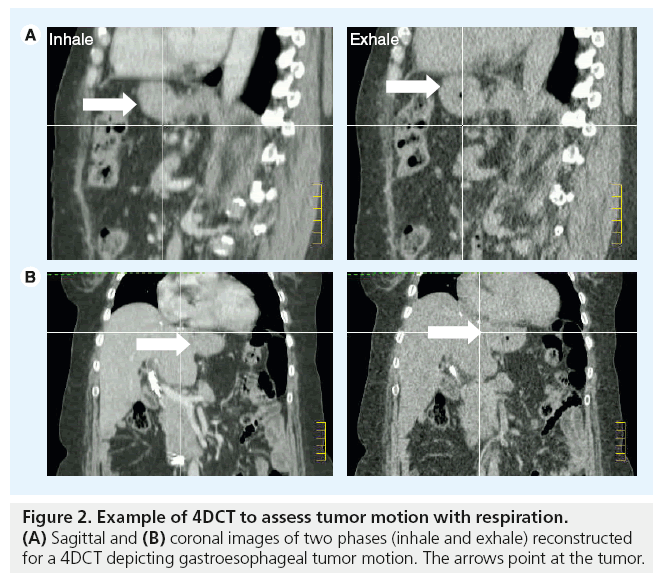
The respiratory motion of anatomical structures can now be characterized using respiratorycorrelated CT imaging (4DCT). This technology permits the capture and reconstruction of CT data sets in separate phases of the respiratory cycles. These can give information on the amplitude of motion and position of organs in different phases of respiration. In conventional 4DCT, the patient is scanned helically or axially over several breathing cycles, during which time the breathing cycle is tracked in real time and divided into bins of respiratory displacement or phases. After scanning, the images are sorted into these bins, from which several CT data sets (usually ten) are reconstructed at different time points over a single breathing cycle. This provides anatomical and motion information regarding tumor position and surrounding normal tissues. For radiotherapy treatment planning, internal target volumes are derived from these images to accommodate target motion and provide assurance in tumor coverage (Figure 2).
The mobility of the normal esophagus during respiration was characterized in 29 patients with nonesophageal malignancies [43]. The investigators concluded that the lower section of the esophagus was the most mobile. Margins to incorporate all movement in the left–right (LR) and anterior– posterior (AP) directions were 5 mm each proximally, 7 and 6 mm, respectively in the midesophagus, and 9 and 8 mm, respectively in the distal esophagus.
Investigators from the MD Anderson Cancer Center (TX, USA) have characterized motion of the tumors situated in the gastro-esophageal junction [45]. They found that the tumors exhibited deformation and asymmetrical motion. The mean ± standard deviation peak-to-peak tumor centroid motion was 0.39 ± 0.27 cm (range: 0.04–1.09 cm) in the LR direction, 0.38 ± 0.23 cm (range: 0.10–0.94 cm) in the AP direction and 0.87 ± 0.47 cm (range: 0.43–2.63 cm) in the superior–inferior (SI) direction. The investigators recommended the use of the following margins: 1.0 cm left (toward the stomach), 0.8 cm right, 1.1 cm anterior, 0.6 cm posterior, 1.0 cm superior (toward the distal esophagus) and 1.6 cm inferior (toward the stomach).
Yaremko et al. suggested that a radial margin of 0.8 cm and an axial margin of 1.8 cm would provide tumor motion coverage for 95% of the cases in the 31 consecutive patients with esophageal cancers that had 4DCT during radiotherapy planning [46]. Patel et al. retrospectively analyzed 30 patients with thoracic esophagus cancers [47]. They confirmed that the cranio–caudal direction of motion was always greater than (or equal to) AP or LR motion. They suggest that an approximation of the mean 3D motion of the primary esophageal tumors reported was (0.802 × 0.282 × 0.222)1/2 = 0.88 cm. They quantified celiac region lymph nodes peak-to-peak displacements mean (range, standard deviation) SI, AP and LR to be 0.92 (0.25–2.25, 0.56) cm, 0.46 (0.25–1, 0.27) cm and 0.19 (0–0.75, 0.26) cm, respectively. In the absence of 4DCT, the margins suggested for >95% internal target volumes coverage were 1.5 cm in the SI dimension, 0.75 cm in the AP dimension and 0.75 cm in the LR dimension. However, to achieve 100% coverage, a further 10 mm are needed SI and 5 mm AP.
The conventional method of acquiring 4DCT is very effective at assessing respiratory motion for treatment simulation. The drawbacks of limited sampling are evident when irregular breathing motion is present as the images are acquired only over one or two respiratory cycles. Yamashita et al. have investigated a time resolved volumetric 4DCT using a 320 multislice CT scanner with a coverage of 160 mm per rotation [48]. They acquired images over 20 s in 12 patients with esophageal cancers after two metal clips were placed at the superior and inferior aspects of the cancer. They characterized the 3D movement of the esophageal wall using the clips and related it to a respiratory trace derived from the changes in lung volumes. Concurring with previous studies, a larger motion in the cranio–caudal direction in the middle and lower esophagus was described. The motion was strongly correlated with the respiratory curve (R2 > 0.4).
Tumors of the gastroesophageal junction can exhibit considerable respiratory-induced motion, therefore, methods to quantify esophageal motion should be contemplated for radiotherapy planning [45,46]. If 4DCT is not available, sufficient margins derived from reported studies relevant to tumor location should be applied to ensure the tumor receives an adequate dose for the duration of radiotherapy.
In the future, methods to limit respiratory motion could allow for margin reduction and therefore possibly decrease treatment-related toxicity.
Set-up errors during radiotherapy delivery
Image-guided radiotherapy (IGRT) describes the process of imaging the patient in the treatment position on the linear accelerator before radiation delivery, with the aim of minimizing set-up errors. The magnitude of set-up errors depends on several factors, including immobilization for treatment and tumor visualization on treatment.
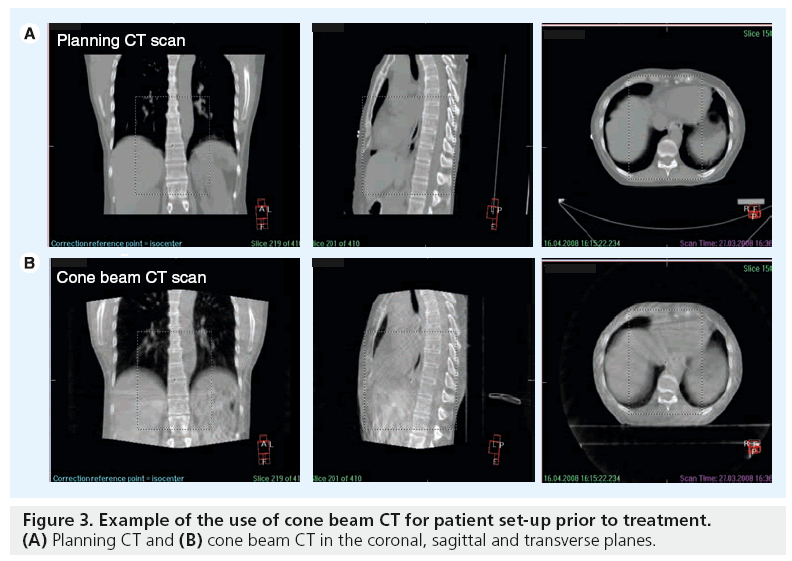
The ability to visualize soft tissue targets in the treatment room has been a challenge and a limitation of conformal radiation therapy. Traditionally, imaging during radiotherapy consists of 2D megavoltage serial portal images evaluated offline to determine the systematic errors in position of bony anatomy, lacking 3D anatomical information of the target volume and normal tissues. With the introduction of volumetric imaging on the linear accelerator, such as cone beam CT, soft tissue information is available for investigation. Cone beam CT represents a recent and significant advance for image verification as it has the ability to acquire 3D images in the treatment unit, while the patient is on the linear accelerator bed. kV cone beam CT can acquire volumetric images with very low dose radiation (~3 cGy) [49,50] and in a timeframe of 2 min or less, opening the possibility for online or offline volumetric image guidance. The IGRT software permits image acquisition, image reconstruction then matching with the reference images and providing data for individual set-up corrections (Figure 3).
Visualizing esophageal tumors in cone beam CT imaging continues to pose a challenge due to the image quality. As the images have to be acquired over 2 min, the intrafraction motion, due to respiration, causes a blurring of the image, especially at the diaphragm interface. The frequency of image guidance can influence the margins required. Even with alternate days of image guidance, Han et al. reported that 10% of fractions had a reduction in the 95% dose coverage [51].
Once the magnitude of the errors is known there are established formulas to derive the margins necessary to create the PTV (Table 1).
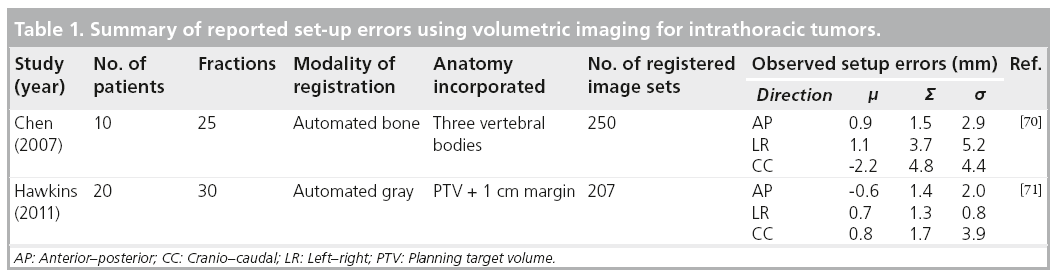
The most common formulas are described by Stroom et al., which ensures that at least 95% of the prescribed dose to 99% of the CTV is equal to approximately 2 Σ + 0.7 s, where Σ is the standard deviation of the distribution of systemic deviations and s is the standard deviation of the distribution of random deviations [52]. van Herk et al. describe the PTV margin as 2.5 S + 0.7 s. These margins were calculated based on the requirement that 90% of the patients received a minimum dose to the CTV of 95% the prescribed dose [53].
The volumetric information obtained with IGRT could be used further to define the volume to match (potentially using PTV) [54], or to define patient-specific PTVs using information acquired during the treatment [55]. IGRT-derived protocols require knowledge of normal tissue anatomy, tumor and lymph node location, and awareness of the possibility of volume changes during the radiotherapy.
By incorporating improvements in target definition (using CT, EUS and PET), accounting for intrafraction motion with 4DCT and interfraction motion with IGRT, modern radiotherapy approaches in esophageal cancer aim to improve local control and minimize normal tissue toxicity.
Future perspective
Lymph node status is the single most important prognostic factor in esophageal cancer. The introduction of new techniques to detect lymph node metastases and micrometastases can be expected in the near future. Sentinel lymph node biopsy is currently under investigation and accurate localization is essential when a minimal surgical approach is selected [7].
As a noninvasive imaging technique, CT is the most common approach to assess the response to neoadjuvant chemotherapy and radiation therapy [56–58]. However, more recently, several imaging procedures, such as endoscopy and EUS, have been used to assess response to neoadjuvant chemotherapy and radiation therapy by comparison of tumor volume between pre- and postchemoradiation imaging [59]. These methods are limited by their inability to traverse a malignant stricture occurring in 20–30% esophageal carcinoma patients, and by their operator dependency [60,61]. Early reports of measurements of changes in tumor uptake during radiotherapy suggests that PET could be used to stratify prognosis [62]. The incorporation of PET to define response may permit early adaptation of therapeutic concepts; however, the optimal timing of imaging and sensitivity in detection of residual disease needs to be further investigated before integrating into standard treatment paradigms.
For GTV-definition, MRI is emerging as a promising modality. MRI provides images with excellent anatomical detail and soft tissue contrast giving information regarding the tumor thickness and its relationship with surrounding organs [63,64]. The additional value of MRI as a predictive marker for resectability is being evaluated as a substudy of the current randomized Phase II/III trial of perioperative chemotherapy with or without bevacizumab in operable adenocarcinoma of the stomach and gastroesophageal junction (ISRCTN46020948). The aim is to investigate the accuracy of high resolution T2-weighted MRI to predict the likelihood of a positive circumferential margin at surgery. In addition, functional MRI could be used to assess response to treatment [65]. The concept of combined linear accelerator MRI for IGRT is under development and esophageal cancer radiotherapy delivery could benefit, potentially allowing tumor visualization and assessment of motion [66].
Assessing respiratory motion using 4DCT on the linear accelerator, also known as correlated cone beam CT [67], would permit characterization of motion range immediately prior to radiotherapy delivery. This IGRT technique would confirm that adequate margins have been applied.
Finally, CT plays a role in the dosimetric planning of intraluminal brachytherapy, which may also be utilized in the treatment of esophageal cancer. Currently, interest is centered on the combination of intraluminal brachytherapy as a ‘boost dose’ following radical external beam radiotherapy, potentially enabling dose escalation to the tumor, while minimizing the dose delivered to adjacent organs at risk [68].
Conclusion
There have been sequential improvements in tumor localization, as well as radiation planning and delivery, over the past two decades [69]. 3D conformal radiotherapy is now standard, with EUS and FDG-PET being incorporated to aid definition of the tumor and involved nodal areas. The use of FDG-PET to detect occult nodal disease and correlation with detailed histopathological specimens analysis have provided further guidance on CTV margin definition [28].
Curative therapy for esophageal cancer remains a challenge. Further efforts to improve the outcomes and toxicity of radiotherapy in the neoadjuvant and definitive treatment setting continue to be made. Randomized trials testing the benefits of any new technology are required; the demonstration of even small incremental improvements in survival or quality of life are of key importance in this disease, where the treatment outcome currently remains poor.
Financial & competing interests disclosure
The authors acknowledge the National Health Service funding to the NIHR Biomedical Research Centre. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• • of considerable interest
- Van Hagen P, Hulshof MC, Van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366(22), 2074–2084 (2012). nn New data strengthening the role of preoperative chemoradiation in the management of esophageal cancers.
- Sjoquist KM, Burmeister BH, Smithers BM et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 12(7), 681–692 (2011).
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL. AJCC Cancer Staging Manual. (7th Edition). Springer, NY, USA (2010).
- Zingg U, Montani M, Busch M, Metzger U, Went P, Oertli D. Prognostic influence of immunohistochemically detected lymph node micrometastasis and histological subtype in pN0 oesophageal cancer. Eur. J. Surg. Oncol. 35(6), 593–599 (2009).
- Mcguill Mj, Byrne P, Ravi N, Reynolds J. The prognostic impact of occult lymph node metastasis in cancer of the esophagus or esophago-gastric junction: systematic review and meta-analysis. Dis. Esophagus 21(3), 236–240 (2008).
- Lowe VJ, Booya F, Fletcher JG et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol. Imaging Biol. 7(6), 422–430 (2005).
- Sgourakis G, Gockel I, Lyros O, Hansen T, Mildenberger P, Lang H. Detection of lymph node metastases in esophageal cancer. Expert Rev. Anticancer. Ther. 11(4), 601–612 (2011).
- Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 72(5), 1631–1636 (1993).
- Chowdhury FU, Bradley KM, Gleeson FV. The role of 18F-FDG PET/CT in the evaluation of oesophageal carcinoma. Clin. Radiol. 63(12), 1297–1309 (2008).
- Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol. Assess. 11(44), iii–iv, xi–267 (2007).
- Wallace MB, Hawes RH, Sahai AV, Van Velse A, Hoffman BJ. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest. Endosc. 51(3), 309–313 (2000).
- Konski A, Doss M, Milestone B. The integration of 18-fluoro-deoxy- positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 61(4), 1123–1128 (2005).
- Kelsen DP, Ginsberg R, Pajak TF et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N. Engl. J. Med. 339(27), 1979–1984 (1998).
- Kelsen DP, Winter KA, Gunderson LL et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J. Clin. Oncol. 25(24), 3719–3725 (2007).
- Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355(1), 11–20 (2006).
- Malthaner RA, Wong RK, Rumble RB, Zuraw L. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med. 2, 35 (2004).
- Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst. Rev. (1), CD002092 (2006).
- Al-Sarraf M, Martz K, Herskovic A et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J. Clin. Oncol. 15(1), 277–284 (1997).
- Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. JAMA 281(17), 1623–1627 (1999).
- Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94–05) Phase III trial of combinedmodality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J. Clin. Oncol. 20(5), 1167–1174 (2002).
- Geh JI, Bond SJ, Bentzen SM, Glynne-Jones R. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: evidence of a radiation and chemotherapy dose response. Radiother. Oncol. 78(3), 236–244 (2006).
- Bedford JL, Viviers L, Guzel Z, Childs PJ, Webb S, Tait DM. A quantitative treatment planning study evaluating the potential of dose escalation in conformal radiotherapy of the oesophagus. Radiother. Oncol. 57(2), 183–193 (2000).
- International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy ICRU report 50. Bethesda, MD, USA (1992).
- International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy (Suppl. to ICRU report 50) ICRU report 62. Bethesda, MD, USA (1999).
- Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). J. ICRU 10(1), 83 (2010).
- Mariette C, Castel B, Balon JM, Van Seuningen I, Triboulet JP. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur. J. Surg. Oncol. 29(7), 588–593 (2003).
- Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann. Surg. 203(2), 173–176 (1986).
- Gao XS, Qiao X, Wu F et al. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 67(2), 389–396 (2007).
- Matzinger O, Gerber E, Bernstein Z et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother. Oncol. 92(2), 164–175 (2009).
- Zum Buschenfelde CM, Herrmann K, Schuster T et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J. Nucl. Med. 52(8), 1189–1196.
- Lordick F, Ott K, Krause BJ et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON Phase II trial. Lancet Oncol. 8(9), 797–805 (2007).
- Bussink J, Kaanders JH, Van Der Graaf WT, Oyen WJ. PET-CT for radiotherapy treatment planning and response monitoring in solid tumors. Nat. Rev. Clin. Oncol. 8(4), 233–242 (2011).
- Muijs CT, Beukema JC, Pruim J et al. A systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancer. Radiother. Oncol. 97(2), 165–171 (2010).
- Kato H, Miyazaki T, Nakajima M et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 103(1), 148–156 (2005).
- Zhong XJY, Zhang B et al. Using 18-F fluorodeoxyglucose positron emission tomography to estimate the length of gross tumour in patients with squamous cell carcinoma of the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 73(1), 136–141 (2008).
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br. J. Cancer 98, 547–557 (2008).
- Yuan S, Yu Y, Chao KS et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J. Nucl. Med. 47, 1255–1259 (2006).
- Moureau-Zabotto L, Touboul E, Lerouge D et al. Impact of CT and 18F-deoxyglucose positron emission tomography image fusion for conformal radiotherapy in esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 63(2), 340–345 (2005).
- Leong T, Everitt C, Yuen K et al. A prospective study to evaluate the impact of FDG-PET on CT-based radiotherapy treatment planning for oesophageal cancer. Radiother. Oncol. 78(3), 254–261 (2006).
- Vrieze O, Haustermans K, De Wever W et al. Is there a role for FGD-PET in radiotherapy planning in esophageal carcinoma? Radiother. Oncol. 73(3), 269–275 (2004).
- Muijs CT, Schreurs LM, Busz DM et al. Consequences of additional use of PET information for target volume delineation and radiotherapy dose distribution for esophageal cancer. Radiother. Oncol. 93(3), 447–453 (2009).
- Yue J, Chen L, Cabrera AR et al. Measuring tumor cell proliferation with 18F-FLT PET during radiotherapy of esophageal squamous cell carcinoma: a pilot clinical study. J. Nucl. Med. 51(4), 528–534 (2010).
- Dieleman EM, Senan S, Vincent A, Lagerwaard Fj, Slotman BJ, Van Sornsen De Koste JR. Four-dimensional computed tomographic analysis of esophageal mobility during normal respiration. Int. J. Radiat. Oncol. Biol. Phys. 67(3), 775–780 (2007).
- Lorchel F, Dumas JL, Noel A, Wolf D, Bosset JF, Aletti P. Esophageal cancer: determination of internal target volume for conformal radiotherapy. Radiother. Oncol. 80(3), 327–332 (2006).
- Zhao KL, Liao Z, Bucci MK et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother. Oncol. 84(3), 283–289 (2007).
- Yaremko BP, Guerrero TM, Mcaleer MF et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 70(1), 145–153 (2008).
- Patel AA, Wolfgang JA, Niemierko A, Hong TS, Yock T, Choi NC. Implications of respiratory motion as measured by fourdimensional computed tomography for radiation treatment planning of esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 74(1), 290–296 (2009).
- Yamashita H, Kida S, Sakumi A et al. Four-dimensional measurement of the displacement of internal fiducial markers during 320-multislice computed tomography scanning of thoracic esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 79(2), 588–595 (2011).
- Islam MK, Purdie TG, Norrlinger BD et al. Patient dose from kilovoltage cone beam computed tomography imaging in radiation therapy. Med. Phys. 33(6), 1573–1582 (2006).
- Amer A, Marchant T, Sykes J, Czajka J, Moore C. Imaging doses from the Elekta Synergy X-ray cone beam CT system. Br. J. Radiol.80(954), 476–482 (2007).
- Han C, Schiffner DC, Schultheiss TE, Chen YJ, Liu A, Wong JY. Residual setup errors and dose variations with less-than-daily image guided patient setup in external beam radiotherapy for esophageal cancer. Radiother. Oncol. 102(2), 309–314 (2012).
- Stroom JC, De Boer HC, Huizenga H, Visser AG. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int. J. Radiat. Oncol. Biol. Phys. 43(4), 905–919 (1999).
- van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 47(4), 1121–1135 (2000).
- Hawkins MA, Aitken A, Hansen VN, Mcnair HA, Tait DM. Cone beam CT verification for oesophageal cancer - impact of volume selected for image registration. Acta Oncol. 50(8), 1183–1190 (2011).
- Hawkins MA, Brooks C, Hansen VN, Aitken A, Tait DM. Cone beam computed tomography-derived adaptive radiotherapy for radical treatment of esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 77(2), 378–383 (2010).
- Umeoka S, Koyama T, Togashi K et al. Esophageal cancer: evaluation with triple-phase dynamic CT--initial experience. Radiology 239(3), 777–783 (2006).
- Yoon YC, Lee KS, Shim YM, Kim BT, Kim K, Kim TS. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology 227(3), 764–770 (2003).
- Wu LF, Wang BZ, Feng JL et al. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J. Gastroenterol. 9(2), 219–224 (2003).
- Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J. Thorac. Cardiovasc. Surg. 129(6), 1232–1241 (2005).
- Mallery S, Van Dam J. Increased rate of complete EUS staging of patients with esophageal cancer using the nonoptical, wire-guided echoendoscope. Gastrointest. Endosc. 50(1), 53–57 (1999).
- Pfau PR, Ginsberg GG, Lew RJ, Faigel DO, Smith DB, Kochman ML. Esophageal dilation for endosonographic evaluation of malignant esophageal strictures is safe and effective. Am. J. Gastroenterol. 95(10), 2813–2815 (2000).
- Ma JB, Song YP, Yu JM, Zhou W, Cheng EC, Zhang XQ. Linear correlation between patient survival and decreased percentage of tumor [(18)F]fluorodeoxyglucose uptake for late-course accelerated hyperfractionated radiotherapy for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 82(4), 1535–1540 (2012).
- Riddell AM, Allum WH, Thompson JN, Wotherspoon AC, Richardson C, Brown G. The appearances of oesophageal carcinoma demonstrated on high-resolution, T2-weighted MRI, with histopathological correlation. Eur. Radiol. 17(2), 391–399 (2007).
- Riddell AM, Davies DC, Allum WH, Wotherspoon AC, Richardson C, Brown G. High-resolution MRI in evaluation of the surgical anatomy of the esophagus and posterior mediastinum. AJR Am. J. Roentgenol.188(1), W37–W43 (2007).
- Aoyagi T, Shuto K, Okazumi S, Shimada H, Kazama T, Matsubara H. Apparent diffusion coefficient values measured by diffusionweighted imaging predict chemoradiotherapeutic effect for advanced esophageal cancer. Dig. Surg. 28(4), 252–257 (2011).
- Lips I, Lever F, Reerink O et al. SU-E-J-57: MRI-Linac (MRL) guided treatment for esophageal cancer. Med. Phys. 39(6), 3665 (2012).
- Bissonnette JP, Franks KN, Purdie TG et al. Quantifying interfraction and intrafraction tumor motion in lung stereotactic body radiotherapy using respiration-correlated cone beam computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 75(3), 688–695 (2009).
- Muijs CT, Beukema JC, Mul VE, Plukker JT, Sijtsema NM, Langendijk JA. External beam radiotherapy combined with intraluminal brachytherapy in esophageal carcinoma. Radiother. Oncol. 102(2), 303–308 (2012).
- Hong TS, Crowley EM, Killoran J, Mamon HJ. Considerations in treatment planning for esophageal cancer. Semin. Radiat. Oncol. 17(1), 53–61 (2007).
- Chen YJ, Han C, Liu A et al. Setup variations in radiotherapy of esophageal cancer: evaluation by daily megavoltage computed tomographic localization. Int. J. Radiat. Oncol. Biol. Phys. 68(5), 1537–1545 (2007).
- Hawkins MA, Aitken A, Hansen VN, Mcnair HA, Tait DM. Set-up errors in radiotherapy for oesophageal cancers - Is electronic portal imaging or conebeam more accurate? Radiother. Oncol. 98(2), 249–254 (2011).
- Howlader N, Krapcho M, Neyman N et al. (Eds). SEER Cancer Statistics Review, 1975–2008, National Cancer Institute, MD, USA (2011). www.seer.cancer.gov/csr/1975_2008
• • Review of functional imaging for radiotherapy planning and future directions.
■ Website