Review Article - Imaging in Medicine (2011) Volume 3, Issue 1
Imaging in thromboembolic disease
Aine M Kelly*
B1 132K Taubman Center, 1500 East Medical Center Court, Ann Arbor, MI 48109-0302, USA
- *Corresponding Author:
- Aine M Kelly
B1 132K Taubman Center 1500 East Medical Center Court
Ann Arbor MI 48109-0302, USA
E-mail: ainekell@med.umich.edu
Abstract
Venous thromboembolic disease is common, but is difficult to diagnose clinically. Diagnostic imaging therefore plays a central and increasing role in its diagnosis. Many modalities are available and there are many published guidelines on how to work-up patients suspected of venous thromboembolic disease. This article summarizes the findings on the various diagnostic imaging techniques and the accuracies of the modalities available. Guidelines are provided for the work-up of patients, including special patient populations, such as intensive care patients, patients with contrast allergy or patients of childbearing age. Future perspectives on venous thromboembolism work-up and advanced diagnostic imaging are also discussed.
Keywords
computed tomography; deep venous thrombosis; imaging; pulmonary embolism; thromboembolic disease
Venous thromboembolic disease (pulmonary ainekell@med.umich.edu embolism [PE] and deep venous thrombosis [DVT]) is a worldwide problem for people who have known risk factors [1]. The annual incidence for venous thromboembolic disease (VTE) in the USA is estimated at 1 per 1000 registered patients [2]. Up to 300,000 deaths occur due to PE annually in the USA, and the diagnosis is often not made until autopsy [2,3]. In Europe, the estimated number of VTE events annually is 1 million, with up to 370,000 deaths estimated due to VTE annually [4]. It is estimated that in only 20% of cases, the diagnosis is confirmed by objective testing [5]. Virchow’s classic triad of risk includes vascular wall damage, venous stasis,
Acquired risk factors for DVT, and thus PE, include knee replacement, hip replacement and surgery, surgery for cancer, trauma and spinal cord injury [7]. Acute medical illness is the most common setting for VTE and advanced age is a major risk factor [1,7]. Reduced mobility confers an increased risk but the degree and duration required are unclear [1]. Other acquired risk factors include antiphospholipid antibody syndrome, chemotherapy, central venous catheters, immobilization devices (such as casts), hormone replacement therapy, obesity, oral contraceptives, pregnancy and polycythemia vera [1]. and hypercoagulability of blood [6].
Hereditary risk factors, more frequent in the general population and common in patients with VTE, include factor V Leiden thrombophilia, increased Factor XI, increased Factor VIII and hyperhomocystinemia [6]. Other hereditary risk factors, which are rare in the general population and in patients with VTE, include protein C deficiency, protein S deficiency and antithrombin III deficiency [6]. These entities should be considered in patients with recurrent VTE, in patients with unprovoked VTE episodes, in young patients or in patients with VTE in an unusual location [1].
Thrombi commonly form in the deep veins of the calf and propagate proximally, to above the popliteal veins, from which they are more likely to embolize [1]. Approximately 80% of patients presenting with PE will have DVT in their leg veins [8]. Conversely, PE is found in 50% of patients with proximal leg DVT [1]. Owing to the dual supply of the pulmonary circulation, from the bronchial and pulmonary arteries, pulmonary infarction is not usually present [1]. With a large acute PE, anatomical obstruction may lead to increased right ventricular afterload, right ventricular wall tension increases leading to dilatation, dysfunction and infarction [1]. Death with PE usually occurs due to right ventricular failure.
Clinical manifestations of DVT include leg warmth, pain and swelling and, on examination, leg tenderness may be elicited. PE may present as chest pain or dyspnea, and pulmonary infarction may present as pleuritic chest pain and hemoptysis. On examination, tachypnea and tachycardia (right-sided gallop) may be found, with elevated neck veins, a loud second heart sound (pulmonic valve) and a right ventricular heave may occur with pulmonary hypertension. Finally, a pleural rub may be heard in cases of pulmonary infarction. The clinical symptoms and signs of VTE overlap with many other entities and are therefore neither sensitive nor specific.
Owing to the difficulties that can arise in clinical assessment, VTE may go undiagnosed, which is associated with significant morbidity and mortality. Anticoagulation therapy for VTE also carries a significant morbidity and mortality risk. Therefore, it is important to accurately diagnose VTE, and diagnostic imaging has emerged to play a crucial role in the work-up of patients with suspected VTE. Many diagnostic imaging modalities are now widely available and in recent years, clinical and radiological societies have published useful guidelines for the appropriate work-up of patients with suspected VTE, which incorporate clinical prediction rules, D-dimer testing and diagnostic imaging. This article summarizes the roles of the various diagnostic imaging modalities employed in the work-up of suspected VTE.
Diagnostic imaging appearances & diagnostic test performance
Acute PE are most often multiple as they are thought to fragment when passing through the right heart chambers and main pulmonary arteries. Acute PE also occurs more commonly in the lower lobe pulmonary arteries because of increased perfusion here. When it comes to documenting the sites of acute PE, the pulmonary arteries are ordered by size with the main pulmonary arteries (main, right and left) representing the first order, lobar arteries are second order, segmental arteries are third order and the subsegmental arteries are fourth order [9].
Chest radiography
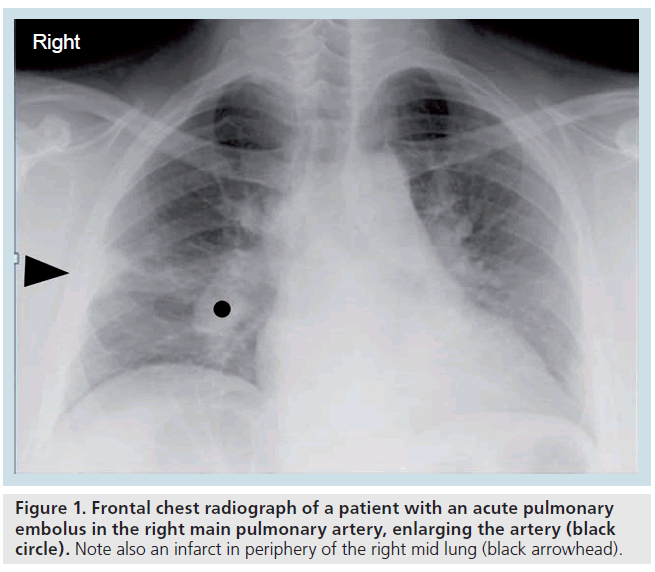
The chest radiographic (CXR) features of acute PE (such as the clinical symptoms and signs) are nonspecific and not sensitive [10]. There may be atelectasis or elevation of the hemidiaphragm secondary to hypoventilation, if the patient is suffering from pleuritic chest pain. There may be a small (hemorrhagic) pleural effusion if infarction has occurred. Classic radiographic signs include the enlarged occluded central vessel or ‘Fleischners’ sign’ and the pulmonary infarct or ‘Hamptons’ hump’ (Figure 1 & Supplementary Figure 1, see online www.futuremedicine.com/ doi/suppl/10.2217/iim.11.1) or the oligemic lung peripheral to a large embolus or ‘Westermarks’ sign’. However, these classic chest radiographic signs have been shown to be of no discriminatory value in the diagnosis or exclusion of PE [10].
Figure 1.Frontal chest radiograph of a patient with an acute pulmonary embolus in the right main pulmonary artery, enlarging the artery (black circle). Note also an infarct in periphery of the right mid lung (black arrowhead).
Since the CXR features of acute PE are nonspecific and are not sensitive, the main role of the CXR is to exclude other entities that may mimic PE, such as pneumothorax (pleuritic chest pain and shortness of breath), pneumonia (shortness of breath and pleuritic chest pain) and aortic dissection (chest pain and possibly hypotension) [10]. Chest radiography is a reasonable first-line test to perform, with the purpose excluding other disease entities.
Ventilation perfusion scintigraphy
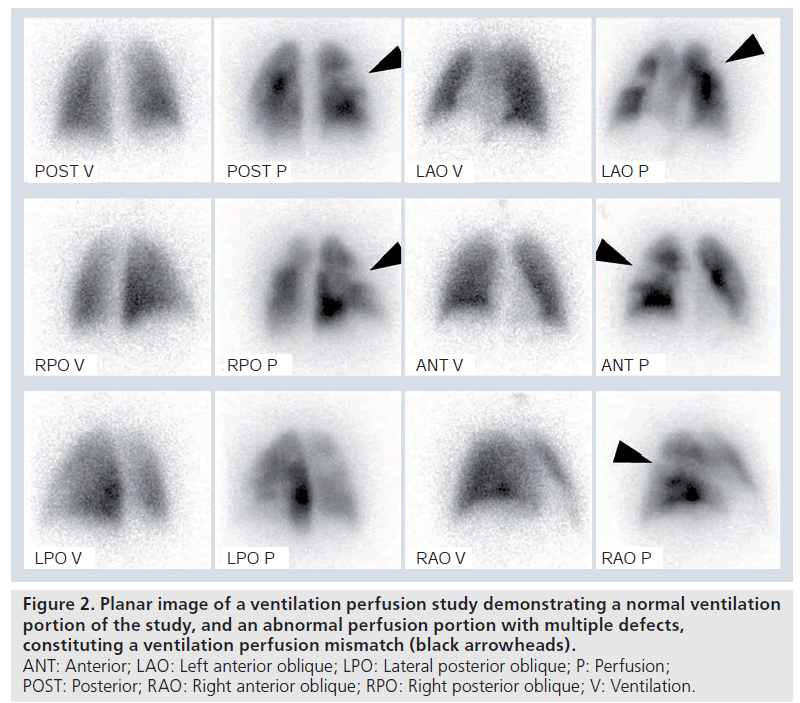
The scintigraphic (ventilation perfusion study or VQ) features of acute PE rely on the anatomy and blood supply of the bronchopulmonary segments, which are supplied by end arteries [11]. Each bronchopulmonary segment is conical in shape with the apex towards the hilum and the base projecting on to the pleural surface [11]. Occlusive pulmonary emboli therefore produce characteristic lobar, segmental, or subsegmental peripheral, wedge (triangular)-shaped defects with their base towards the pleura (Supplementary Figure 2) [11]. With acute PE, occlusion of arterial branches with preserved ventilation produces perfusion defects that are not matched by corresponding ventilation defects, known as ‘ventilation perfusion mismatches’ (Figure 2). The ventilation portion of the study acts as a guide to define the lung borders. The combination of ventilation and perfusion studies increases the specificity for PE diagnosis, and aids recognition of alternative processes such as chronic obstructive airways disease or pneumonia. However, in young patients, ventilation studies are often normal, and are sometimes omitted in females in the first trimester of pregnancy, to decrease ionizing radiation (Supplementary Figure 3) [11]. Later on, with recanalization or resolution of embolus, the ventilation perfusion mismatch becomes less distinct. It is generally accepted that a normal pulmonary perfusion is adequate to exclude PE. Ventilation perfusion mismatches can also be caused by congenital pulmonary vascular abnormalities, tuberculous lymphnode enlargement, lung cancer, vasculitis and venoocclusive disease [11].
Figure 2.Planar image of a ventilation perfusion study demonstrating a normal ventilation portion of the study, and an abnormal perfusion portion with multiple defects, constituting a ventilation perfusion mismatch (black arrowheads). ANT: Anterior; LAO: Left anterior oblique; LPO: Lateral posterior oblique; P: Perfusion; POST: Posterior; RAO: Right anterior oblique; RPO: Right posterior oblique; V: Ventilation.
The original Prospective Investigation Of Pulmonary Embolism Diagnosis (PIOPED 1) study used probabilistic interpretation based on simplistic criteria with diagnostic categories of high, intermediate, low or very low probability or nondiagnostic [12]. The imaging criteria have been revised to incorporate clinical information, pretest probability and the overall opinion or ‘gestalt’. Imaging criteria have also been revised since the technology used has evolved [13,14]. In order to be clinically useful, the result of the imaging test should be positive or negative with respect to PE (present or absent) [15]. A study is positive for PE if there is a ventilation perfusion mismatch of at least one segment or two subsegments that conforms to the pulmonary vascular anatomy [16]. A study is negative for PE if there is a normal perfusion pattern conforming to the anatomic boundaries of the lungs; the mismatches are reversed; ventilation and perfusion defects are matched, or mismatches do not have a lobar, segmental or subsegmental pattern [16].
Using composite reference standards (including clinical and laboratory information, multidetector CT [MDCT] and scintigraphy results and physician judgment), planar VQ demonstrates sensitivities in the range of 76 to 98% and specificities in the range of 85 to 93% [17–20]. Using composite reference standards (including four multidetector row CT), the sensitivity of single-photon emission CT VQ is 83–97% and the specificity is 91–98% [18,21].
Using composite reference standards, and the PIOPED criteria, perfusion scintigraphy combined with chest radiography has sensitivities of 85–89% and specificities of 92–93% for the detection of PE [20,22].
By both imaging and clinical outcome reference standards, negative predictive values of a normal or very-low-probability VQ range between 96 and 100%, while positive predictive values of high-probability VQ range between 83 and 92% [23–29]. Therefore, a VQ study with a normal result can be used to safely withhold anticoagulation in a patient with suspected PE, and a high probability scan can be used to justify treatment.
In a recent study, by Stein and colleagues, using a negative chest radiograph as a triage, a negative VQ study had a low false-negative rate of 1.2%, which was comparable to CT (1.1%), and it confers less radiation dose to the thorax and breast tissues [30].
However, a high percentage of patients have indeterminate probability or nondiagnostic studies (43% in PIOPED I and 20.6% in PIOPED II), which limits the usefulness of this modality, particularly in sick patients (68% of patients in PIOPED I and 11% of patients in PIOPED II were inpatients) [12,22]. Using the PIOPED criteria, interobserver agreements of 0.70 (substantial agreement) have been reported, with a k statistic of 0.65 (substantial agreement) for ‘gestalt’ impressions [31].
CT pulmonary angiography
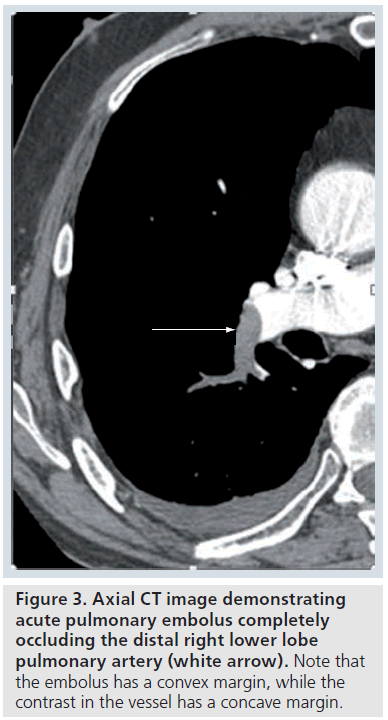
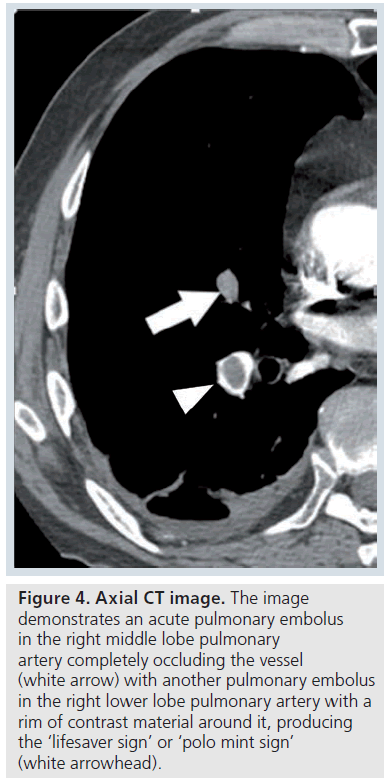
The contrast-enhanced CT features of acute PE include a central low attenuation filling defect. If the embolus completely fills or occludes the vessel, there will be no contrast enhancement visible at that level (Figure 3). At the level of the thrombus, the vessel is usually enlarged due to impaction of thrombus by pulsatile flow [9]. On an image parallel to the vessel, the thrombus has a convex margin (or the intravenous contrast cut-off has a concave margin) (Figure 3). Acute PE that is not completely occluding the artery and is centrally placed will be surrounded by contrast material producing the ‘lifesaver sign’ or ‘polo mint sign’ on an image acquired transversely or the ‘railway track sign’ on an image obtained parallel to the artery (Figure 4 &Supplementary Figure 4) [9]. Acute PE that is eccentrically located in the vessel will demonstrate acute angles with the vessel wall and some eccentric contrast enhancement (Supplementary Figure 5) [9]. Acute PE frequently occurs at branch points in vessels, due to more sluggish flow here (Supplementary Figure 6).
Figure 4.Axial CT image. The image demonstrates an acute pulmonary embolus in the right middle lobe pulmonary artery completely occluding the vessel (white arrow) with another pulmonary embolus in the right lower lobe pulmonary artery with a rim of contrast material around it, producing the ‘lifesaver sign’ or ‘polo mint sign’ (white arrowhead).
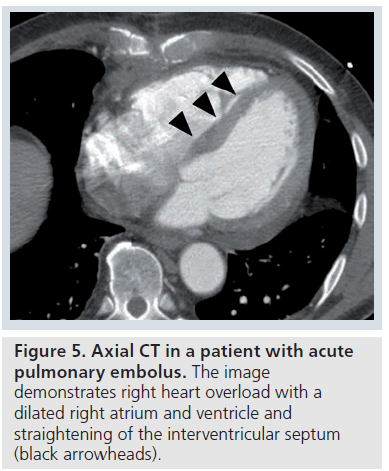
Ancillary findings (indirect or secondary signs) may be seen on CT in acute PE. These include pulmonary infarct (nonenhancing consolidation), hemorrhagic pleural effusion, atelectasis, pulmonary oligemia or hypoperfusion and heterogenous (mosaic) attenuation [32–35]. Pleural effusions and pulmonary infarcts are the most common abnormalities found, and were seen in a third of patients in studies [32,33]. However, in other series, atelectasis was the most common finding described [34,35]. Signs of right heart overload may also be seen, with dilatation of the right atrium and ventricle and straightening of the interventricular septum (Figure 5).
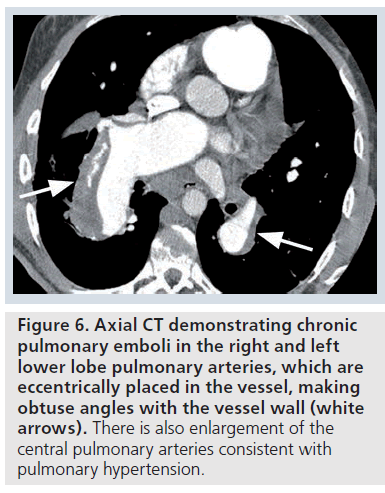
The CT features of chronic PE include a filling defect, which may completely occlude the vessel. In contrast to an acute PE, the vessel is usually smaller than adjacent arteries, and the thrombus has a straight or concave margin (with the intravenous contrast cut-off seen as a convex margin or ‘pouch’ defect) [9]. A chronic PE that is eccentrically placed within the vessel forms a crescent shape, and makes obtuse angles with the vessel wall (Figure 6). Chronic PE often becomes recanalized with contrast material visible centrally, with the thrombus seen peripherally (Supplementary Figure 7) [9]. With further recanalization, all that may remain of a chronic PE may be several bands or ‘webs’ within the contrast-enhanced vessel. The band is a ribbonlike structure attached to the vessel wall at both ends. Webs are complex bands with branches [9].
Figure 6.Axial CT demonstrating chronic pulmonary emboli in the right and left lower lobe pulmonary arteries, which are eccentrically placed in the vessel, making obtuse angles with the vessel wall (white arrows). There is also enlargement of the central pulmonary arteries consistent with pulmonary hypertension.
CT pulmonary angiography (CTPA) is increasingly being used for the diagnosis of PE. In studies using pulmonary angiography (PA) as the reference standard, four-detector row CTPA demonstrated sensitivities of 96–100% and specificities of 86–89% for detection of emboli to subsegmental level [19,36]. In PIOPED II, fourdetector row CTPA had a sensitivity of 83% and specificity of 96% compared with a composite reference standard [37]. With use of thinner ‘slice thickness’ or collimation, visualization of smaller subsegmental vessels was significantly improved [38,39]. The positive predictive values for four-detector row CTPA range from 92 to 96%, and the negative predictive values range from 94 to 100% [37,40]. Interobserver agreement for four-detector row CTPA (using thin collimation) was reported to be excellent in studies with values of 0.73–0.79 reported on a per-patient basis and values of 0.79–0.80 reported at segmental vessel level [38,39]. In the PIOPED II study, fourdetector row CTPA was nondiagnostic in 6.1% of cases and combined CTPA and indirect CT venography (CTV) nondiagnostic in 10.5% [35].
Multidetector CT, with increased number of detector rows, has higher image acquisition rates, reduces the rate of respiratory and motion artifacts, particularly in sections obtained during the end of the scan when patients may not be able to maintain breath holding, and improves overall spatial resolution. Visualization of smaller segmental and subsegmental pulmonary arteries is improved with 16 and 64 detector rows and beyond [41,42]. Increased detector rows allows for thinner collimation and multiplanar reformatting with isotropic voxels [41,42].
Indirect CT venography
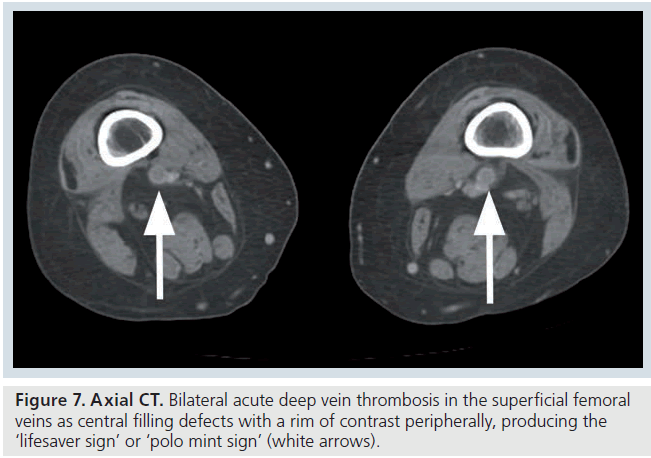
The CT features of acute DVT include a sharply marginated low attenuation filling defect, which may completely occlude the vessel, with no contrast enhancement visible [43]. If the thrombus is nonocclusive, some contrast enhancement will be visible surrounding the central thrombus (Figure 7) [43]. At the level of the thrombus, there is often venous expansion and occasionally vein wall enhancement and perivenous edema or stranding (Supplementary Figure 8) [43]. The vein can expand to twice the size of the adjacent artery in some cases and wall enhancement may be equal or greater to that of adjacent muscle [43].
Figure 7.Axial CT. Bilateral acute deep vein thrombosis in the superficial femoral veins as central filling defects with a rim of contrast peripherally, producing the ‘lifesaver sign’ or ‘polo mint sign’ (white arrows).
The CT features of chronic DVT may include recanalization with central contrast due to fragmentation of the thrombus [43]. In contrast to an acute DVT, the vessel is usually smaller than the adjacent vein, although occasionally the wall can be thickened due to fibroelastic tissue formation [43]. Wall thickening can also occur due to residual thrombus and the criteria for chronic DVT on indirect CTV are not yet fully established [43]. There is frequently extensive superficial collateral flow [43].
Indirect CTV can be performed at the same time as the CT pulmonary angiogram, without the need for additional intravenous contrast material. Indirect CTV is useful as a ‘one stop shop’ in sick patients, such as intensive care unit (ICU) patients. Indirect CTV has the same accuracy as compression ultrasound of the lower extremity [44–47]. With the addition of indirect CTV to CTPA in PIOPED II, sensitivity was increased from 83 to 90% and specificity was decreased from 96 to 95% [37]. This translated into additional diagnoses of VTE in only 2% of patients, which was similar to the rates found in other studies [37,48]. In the PIOPED II study, a structured pretest clinical assessment (Wells score) and CT angiography were equivalent to CT angiography and indirect CTV in predictive value [37,49].
Current data suggest that most patients do not benefit from the routine use of CTV after CT angiography [50]. There is increasing justification in the literature to eliminate CTV or to limit its use to patients at a high risk for DVT (patients with a past history of DVT or who have signs and symptoms of DVT) [50]. CT imaging of the lower extremity adds little to the overall diagnosis of VTE and 3-month follow-up outcome studies showed the same low rates of recurrence with or without lower extremity imaging [51]. High-risk patients receive moderate but potential benefit from lower extremity imaging [52,53].
Catheter PA
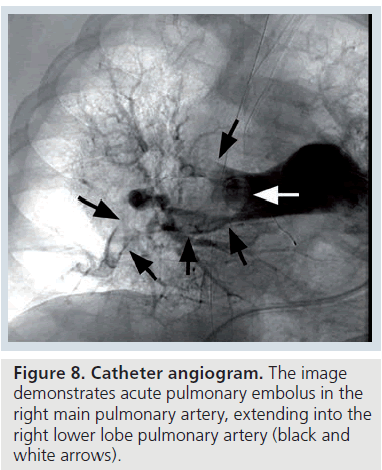
The catheter PA features of acute PE include a filling defect or an abrupt cut-off of contrast material in a vessel with visible thrombus (Figure 8) [54]. The catheter PA features of chronic PE include apparent stenoses in a vessel, arterial bands or webs or decreased caliber or absent vessels (Supplementary Figure 9) [55].
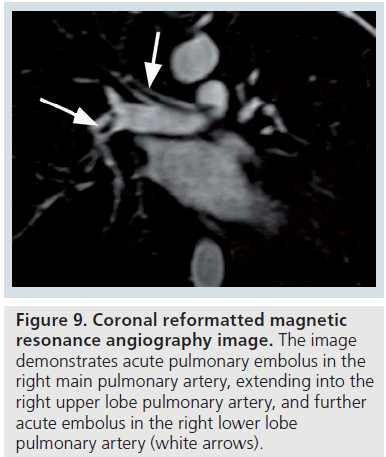
Figure 9.Coronal reformatted magnetic resonance angiography image. The image demonstrates acute pulmonary embolus in the right main pulmonary artery, extending into the right upper lobe pulmonary artery, and further acute embolus in the right lower lobe pulmonary artery (white arrows).
Catheter PA has traditionally been considered the reference or ‘gold standard’ diagnostic test in the evaluation of PE [54,56]. Consequently, the major articles that evaluated the accuracy of PA have used clinical outcome as the reference standard [54,56]. Several studies have demonstrated deterioration in angiographic interobserver agreement as the size of pulmonary arteries being visualized decreases, with poor interobserver agreement for subsegmental arteries [57–59]. The risk of recurrent PE following negative PA is low, with negative predictive values in the range of 99 to 100% reported in original studies and in a systematic review [60–63].
Magnetic resonance angiography
The magnetic resonance angiography (MRA) features of acute occlusive PE include an abrupt ‘cut-off ’ to the contrast material, with a meniscus that outlines the proximal end of the embolus [64]. With a partially occlusive thrombus, a small amount of contrast may be seen between the filling defect and the vessel wall, resulting in the ‘lifesaver sign’ or ‘polo mint sign’ on a transverse image, or the ‘railway track sign’ on a longitudinal image (Figure 9) [64]. The MRA features of chronic PE include a filling defect, which makes obtuse angles with the vessel wall [64].
Gadolinium-enhanced MRA has undergone evaluation for the detection of PE, and demonstrated sensitivities in the range of 87 to 100% and specificities in the range of 95 to 100% when compared with conventional PA [65,66]. In earlier studies, interobserver agreement for MRA was good, and in more recent studies was very good with a k value of 0.75 [65,66]. In PIOPED III, MRA and venous phase magnetic resonance venography (MRV) were compared with a composite reference standard, which included clinical assessment, D-dimer, venous ultrasound, VQ and MDCT [64,67]. Technically adequate MRA, which represents 75% of studies averaged across all centers, demonstrated sensitivity of 78% and specificity of 99% [67]. Technically adequate MRA and MRV combined, representing 48% of studies, showed sensitivity of 92% and specificity of 96% [67]. In PIOPED III, MRA was technically inadequate in 25% of cases, and combined MRA and MRV technically inadequate in 48% of cases [67].
Magnetic resonance venography
The MRV features of occlusive acute DVT will show a lack of contrast opacification in that segment of deep vein [64]. If the thrombus only partially occludes the vessel, contrast may be visible between the filling defect and the vessel wall (Supplementary Figure 10). The MRV features of chronic DVT include a filling defect, which makes obtuse angles with the vessel wall [64].
Gadolinium-enhanced MRV can be performed at the same time as the pulmonary artery MRA, without the need for a separate injection of contrast [64]. In early studies compared with contrast venography, MRV showed sensitivity, specificity, positive and negative predictive values of 100, 95–96, 90 and 100%, respectively [68,69]. When compared with duplex ultrasound, MRV had sensitivity, specificity, positive and negative predictive values of 100, 96, 94 and 100%, respectively [68]. Time-offlight MRV has been compared with contrast venography, with sensitivities and specificities of 100% to detect DVT above the knee and sensitivity and specificity of 95 and 99% to detect proximal extent of DVT [70]. Contrast venography is now almost obsolete and studies comparing both MRV and duplex ultrasound to venography have confirmed similar accuracies for both modalities in the detection of proximal DVT [70,71]. In PIOPED III, MRV combined with MRA demonstrated sensitivity of 92% and specificity of 96% (in the 48% of studies that were technically adequate) when compared with a composite reference standard [67].
Ultrasound of the pelvic & lower-limb veins
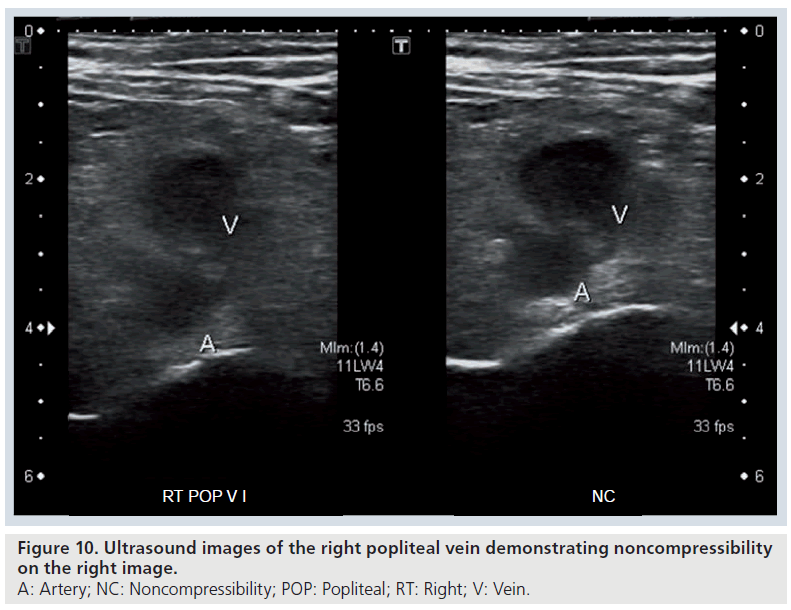
The ultrasound features of acute DVT include a deep vein (or veins) that are not completely compressible with the probe (the vein or veins should completely disappear) and visible thrombus (Figure 10 & Supplementary Figure 11) [72,73]. Doppler ultrasound will demonstrate loss of the normal phasic pattern of flow, which occurs with respiration or with manual compression and instead there will be continuous flow due to venous outflow obstruction [72]. In addition, thrombus will be seen as a filling defect on color Doppler (duplex) ultrasound (Supplementary Figures 12 & 13) [73]. Color Doppler ultrasound may show reversal of flow in a vein to indicate venous outflow obstruction upstream.
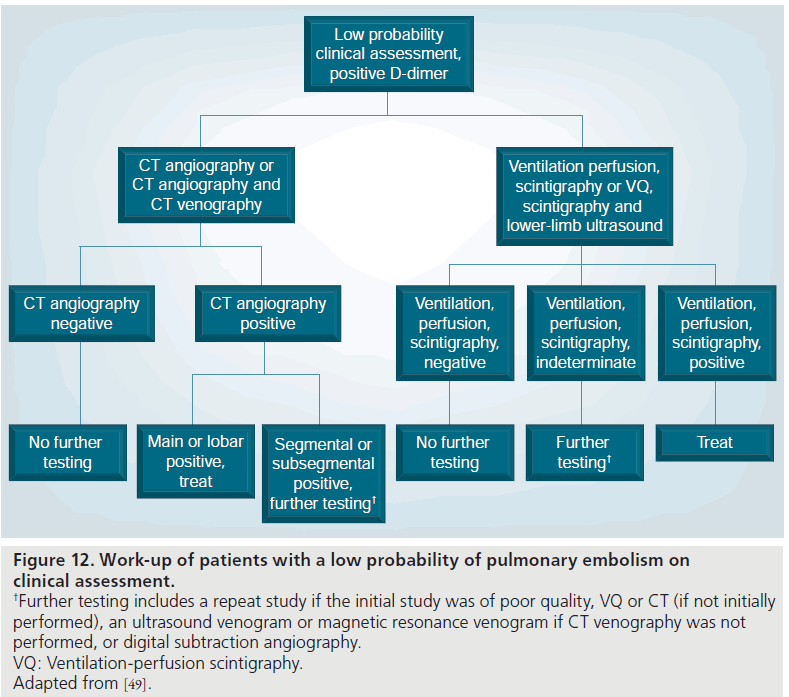
Figure 12.Work-up of patients with a low probability of pulmonary embolism on clinical assessment. †Further testing includes a repeat study if the initial study was of poor quality, VQ or CT (if not initially performed), an ultrasound venogram or magnetic resonance venogram if CT venography was not performed, or digital subtraction angiography. VQ: Ventilation-perfusion scintigraphy. Adapted from [49].
The ultrasound features of chronic DVT include a filling defect, and noncompressibility as for acute DVT. In addition, the vein walls may be thickened due to decreased compliance [73]. Recanalization and resolution of thrombus may occur with fibrin bands and webs visible in the deep veins [73]. Residual DVT may be seen as echogenic material adherent to the vessel wall, mimicking acute thrombus [73]. Segments ofs veins that are atretic in cases of chronic DVT may not be visible adjacent to their accompanying artery on ultrasound imaging [73].
Ultrasound of the lower-limb veins with compression sonography, duplex ultrasound and color flow are the mainstay for evaluation for DVT in many centers. When compared with the classic gold standard of contrast venography, compression ultrasound compared favorably with sensitivities and specificities of 91 and 99% [74]. Other studies using combinations of direct visualization of thrombus, compression ultrasound, Doppler ultrasound and flow have demonstrated sensitivities in the range of 92 to 95% and specificities in the range of 83 to 87% [75]. Contrast venography is now almost obsolete and has been replaced by ultrasound of the lower-limb veins as the ‘new’ gold standard. Following a negative ultrasound lower-limb examination for the first episode of DVT, anticoagulation can be safely withheld, as the recurrence rate is less than 1% [76–78].
Echocardiography (surface & transesophageal)
Surface and transesophageal echocardiography (TEE) can be used for the evaluation of PE. Abnormalities found on surface echocardiography in PE patients include right ventricular dilatation and hypokinesis, flattening and abnormal motion of the interventricular septum, tricuspid regurgitation, and lack of collapse of the inferior vena cava on expiration [79]. Other findings include patent foramen ovale (when right atrial pressure exceeds left atrial pressure), right ventricular hypertrophy (increased trabeculations and a wall thickness of approximately 6 mm), pulmonary arterial hypertension (detected by Doppler flow velocity in the right ventricular outflow tract), direct visualization of pulmonary arterial embolus and diastolic left ventricular (LV) impairment (little difference between LV area during diastole and systole) [80]. TEE can diagnose PE by direct visualization of thrombus in the pulmonary arteries [81]. The right pulmonary artery can be followed until it branches at the lobar level. Visualization of the mid-left main pulmonary artery is limited by the left main bronchus in between [82]. Embolus is demonstrated as an echogenic (bright) focus within the affected pulmonary artery.
Studies on the use of transthoracic echocardiography (TTE) have employed various criteria in the evaluation of known PE patients. These abnormalities include right ventricular dilatation and hypokinesis, flattening and abnormal motion of the interventricular septum, tricuspid regurgitation, lack of collapse of the inferior vena cava on expiration, patent foramen ovale, right ventricular hypertrophy, pulmonary arterial hypertension, direct visualization of pulmonary embolus and diastolic LV impairment [79,80]. TTE compared with CT demonstrated a sensitivity of 56% and a specificity of 90% [83].
Transesophageal echocardiography has the potential to diagnose PE by direct visualization of embolus [81]. Compared with CT, in the detection of central PE, TEE had a sensitivity and specificity of 84% and an overall sensitivity of 58% for PE [84]. TEE is most useful in the confirmation of central PE in patients with hemodynamic compromise [82]. When compared with helical CT, combinations of TTE and TEE yielded sensitivities and specificities of 59 and 77% for all PE (and 82 and 92% for central PE), respectively, when compared with CT [85].
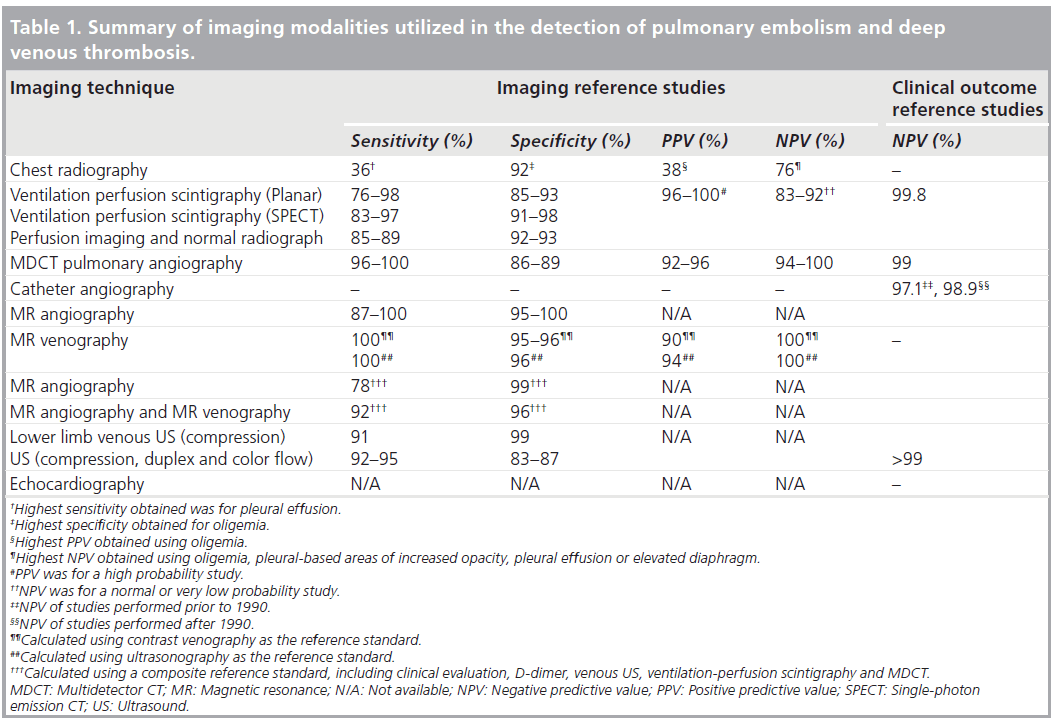
The various imaging modalities used in the work-up for patients with suspected PE and DVT and their diagnostic performances are summarized in Table 1.
Major society guidelines & recommendations for the diagnostic imaging work-up of patients with suspected acute PE
A variety of imaging tests are useful in the diagnosis of suspected PE in patients with intermediate and high pretest probability, as determined by clinical prediction rules such as the Wells score (Table 1) [86–89]. In addition, many of the clinical and radiological societies have recently published excellent guidelines that propose appropriate imaging pathways [49,90,91]. Most of these guidelines employ a combination of clinical evaluation, serum D-dimer measurement, lower-limb ultrasound, CTPA, VQ imaging, conventional PA and MRI in the diagnosis of PE [49,90,91].
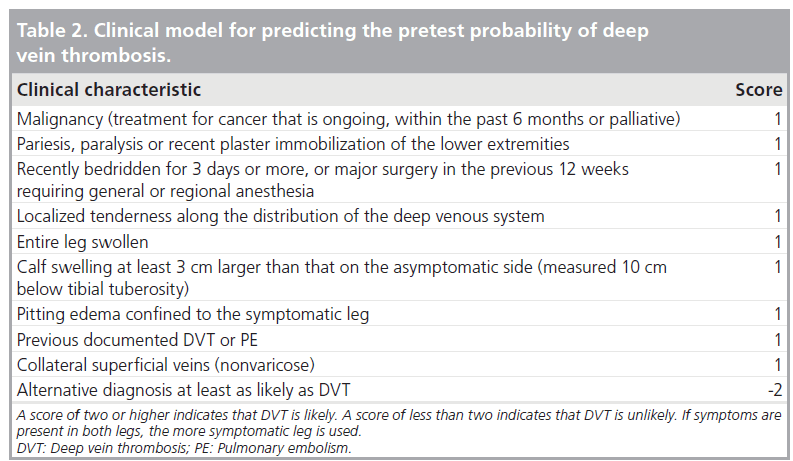
The first step to be taken is to determine the pretest probability for PE using a clinical prediction rule, such as the Wells Score [86–88]. The Wells score is outlined in Table 2. The Wells score consists of a series of questions regarding risk factors for PE and the points total is calculated for the various responses (Table 2) [88]. Criteria include past history of VTE, recent surgery or immobilization, history of cancer, hemoptysis, clinical signs of DVT, heart rate greater than 100 bpm and whether an alternative diagnosis is more likely [88]. Points scores are added up and from these scores probabilities (unlikely/likely) are estimated [88].
Although chest radiography does not have a primary role in the diagnosis or follow-up of PE, it is a reasonable first-line test to perform, with the purpose of quickly excluding other disease entities, such as pneumothorax or pneumonia, which overlap clinically with PE, but which have very different management approaches [10].
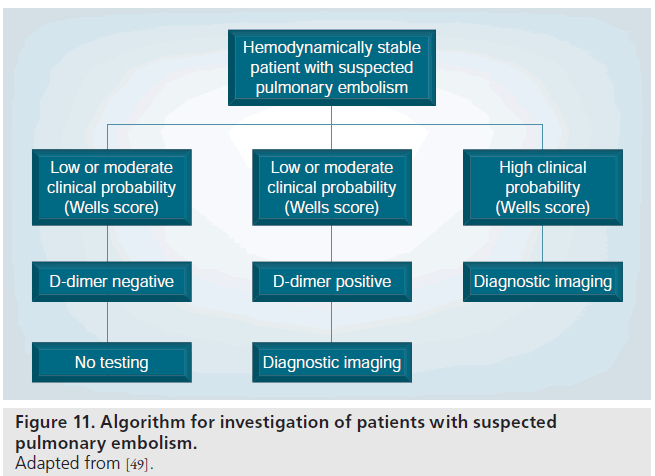
In hemodynamically stable patients with a low or intermediate pretest probability for PE, as determined by the Wells score, many authorities recommend performing a serum D-dimer assay as the next step (Figure 11) [49,90]. If a high-quality rapid ELISA D-dimer assay is of sufficiently low value or ‘negative’, it is safe to withhold further investigation for PE or treatment [49,92,93].
If the D-dimer assay is positive, or the pretest probability for PE is high, further testing is recommended (Figure 1) [5]. Most of the PIOPED II investigators, and the European Society of Cardiology (ESC) Taskforce recommended MDCT PA as the first-line imaging test in patients with a positive D-dimer or high pretest probability patients [49,90]. The Fleischner society recommended either MDCT or VQ scintigraphy in these patients [91]. The PIOPED II investigators had recommended performing indirect CTV at the same time as the CTPA [49]. Since then, more papers have been published demonstrating equivalent accuracies for CTV and lower-limb venous ultrasound [46]. Therefore, the choice of either test will depend on the individual patient’s circumstances with CTPA and CTV advocated as a ‘one stop shop’ in sick and intensive care patients [91]. For patients of a young age or where there are concerns surrounding ionizing radiation, lower-limb ultrasound is a suitable alternative to CTV.
In hemodynamically unstable patients, MDCT is recommended as the first-line test, with echocardiography as an alternative if MDCT is not available [90].
In low pretest probability patients following a negative MDCT, it is safe to withhold therapy due to the very high negative predictive value of MDCT of over 99% (Figures 2 & 11) [94].
In low pretest probability patients with a positive MDCT, the ESC Taskforce recommended treatment with anticoagulation (Figure 12) [90]. The PIOPED II investigators stratified the management based upon the size of the vessels involved with emboli [49]. For emboli in the main, right, left or lobar pulmonary arteries, treatment was recommended due to the high positive predictive value at 97% [49]. For emboli in the segmental or subsegmental vessels, further (imaging) evaluation was recommended due to the lower positive predictive value (segmental 68% and subsegmental 25%) of MDCT in these patients [49]. These patients represent a discordant group, that is, a low clinical probability with a positive test. Further imaging could be performed with repeat MDCT if there was a technical issue with the initial MDCT, or alternatively, conventional PA or VQ scintigraphy could be performed [49]. If the lower-limb veins had not been assessed, this could be carried out with ultrasound or MRV.
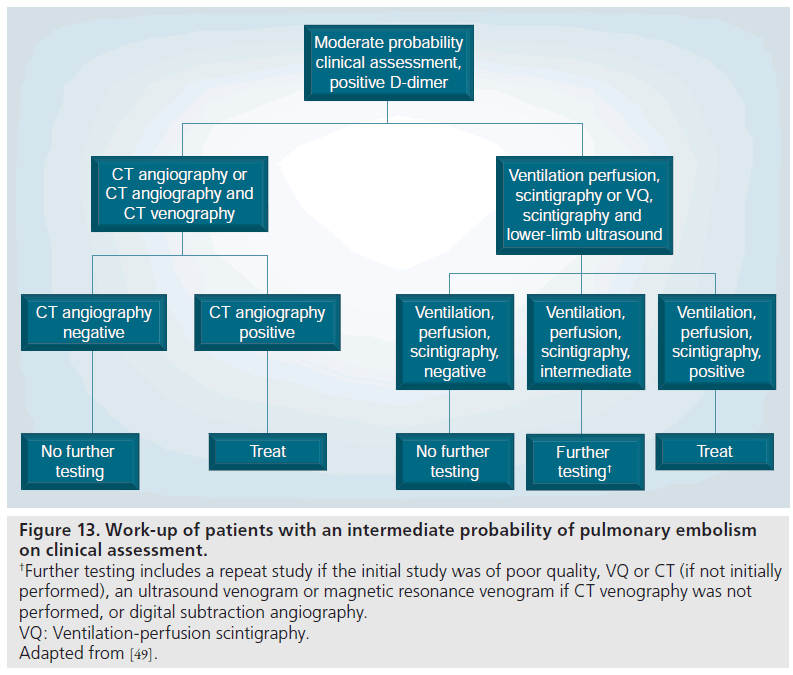
Figure 13.Work-up of patients with an intermediate probability of pulmonary embolism on clinical assessment. †Further testing includes a repeat study if the initial study was of poor quality, VQ or CT (if not initially performed), an ultrasound venogram or magnetic resonance venogram if CT venography was not performed, or digital subtraction angiography. VQ: Ventilation-perfusion scintigraphy. Adapted from [49].
In moderate pretest probability patients with a negative MDCT, it is safe to withhold therapy due to the relatively high negative predictive value of MDCT of 89% (Figure 13) [37]. The PIOPED investigators mentioned lowerlimb evaluation with ultrasound or MRV as alternative options, if this had not yet been performed [49].
In moderate pretest probability patients with a positive MDCT, the positive predictive value is high at 92% and both the PIOPED II investigators and the ESC Taskforce recommended treatment (Figure 13) [49,90]. Further evaluation could be performed with repeat MDCT if there was a technical issue with the initial MDCT, or alternatively, conventional PA or VQ scintigraphy could be performed [49]. In addition, if the lower-limb veins had not been assessed, this could be carried out with ultrasound or MRV.
In high pretest probability patients with a negative MDCT, the positive predictive value is lower at 60%, and both the PIOPED II investigators and ESC taskforce recommended further evaluation (Figures 4 & 13) [49,90]. These patients represent a discordant group, that is, a high clinical probability with a negative test. Again, a repeat MDCT was recommended if there were technical faults with the initial study, an alternative test such as catheter PA or VQ, or evaluation of the lower-limb veins with ultrasound or MRV if the lower limbs were not yet evaluated.
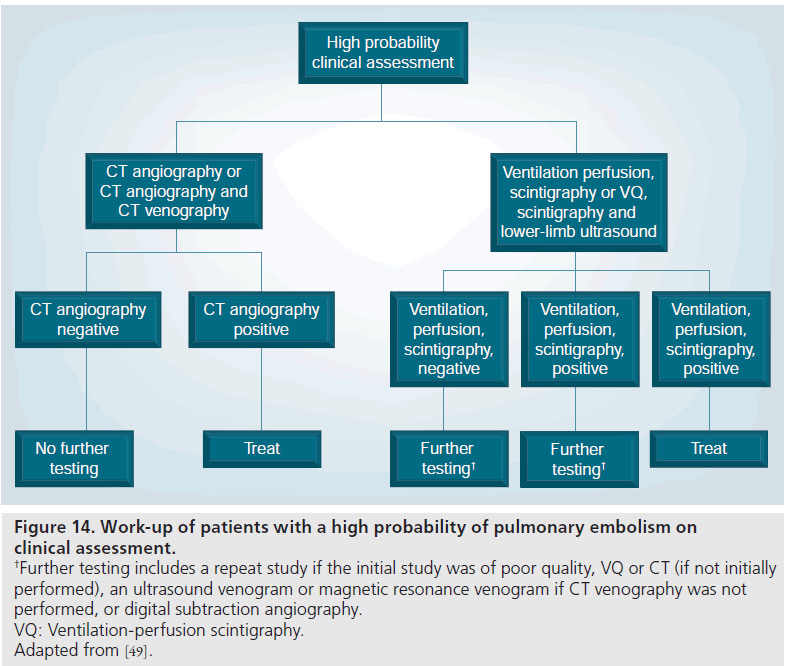
In high pretest probability patients with a positive MDCT, the PIOPED II investigators and the ESC Taskforce recommended treatment due to the very high positive predictive value of 96% (Figure 14) [49,90].
Figure 14.Work-up of patients with a high probability of pulmonary embolism on clinical assessment. †Further testing includes a repeat study if the initial study was of poor quality, VQ or CT (if not initially performed), an ultrasound venogram or magnetic resonance venogram if CT venography was not performed, or digital subtraction angiography. VQ: Ventilation-perfusion scintigraphy. Adapted from [49].
Special patient populations
Intensive care patients
In the patient with suspected PE who presents with shock or hypotension (representing a higher risk for PE), it is recommended to proceed straight to testing with MDCT [90]. A similar recommendation was applied to ICU patients, who could have other causes for a positive D-dimer, thus reducing its sensitivity. If MDCT is not available, echocardiography is an alternative possibility [90].
Contraindication to iodinated contrast
In patients with iodinated contrast allergy and suspected PE, the risks from undiagnosed PE must be weighed against the risks of contrast allergy. In patients who have a history of mild allergy to contrast, such as a few hives, but no respiratory symptoms, it is thought to be safe to proceed with a steroid preparation, but this could delay the imaging work-up of the patient [49]. With moderate or severe contrast allergy, an alternative test not utilizing iodinated contrast material is recommended [49]. Lowerlimb ultrasound and VQ scintigraphy can be performed, provided that the CXR is normal, as otherwise VQ accuracy will be decreased. MRA and MRV can be performed as alternatives, but contraindications, such as pacemakers, cochlear implants, and vascular clips and bands, need to be excluded.
Patients with renal impairment
With renal impairment and suspected PE, the risks from undiagnosed PE must be weighed against the risks of worsening renal failure from iodinated contrast material. Prehydration is recommended and is of proven benefit in the prevention of contrast-induced nephropathy [95]. NSAIDs and metformin should be discontinued. Lower-limb ultrasound and VQ scintigraphy can be safely performed in the presence of renal failure. MRA and MRV may be considered, but the risks of nephrogenic systemic fibrosis need to be weighed against the risks of undiagnosed PE [96]. In patients with glomerular filtration rates below 30 ml/min, gadolinium-containing contrast agents should be avoided and they should be used with caution in patients with glomerular filtration rates between 30 and 60 ml/min [96].
Patients of childbearing age
In patients of child bearing age with suspected PE, the risks of radiation-induced cancer must be considered and weighed against the risks of undiagnosed PE. MDCT delivers an absorbed dose of 10–50 mGy to the breast, while perfusion scintigraphy delivers a lower absorbed dose (0.28 mGy) to the breast [97–99]. In PIOPED I, VQ studies in patients with normal CXRs were diagnostic (high probability, very low probability, normal) in 52% of patients, and more recently VQ studies were shown to be diagnostic in 91% of patients with normal CXRs [12,100,101]. In patients of childbearing years, ultrasound is recommended as a first-line test, following a positive D-dimer [49]. Most PIOPED investigators (69%) recommended MDCT as the next test, and the remainder recommended VQ scintigraphy (31%) [49]. A CTPA could be combined with a lower-limb ultrasound as an alternative to CTV [49].
There are measures that can be taken to reduce radiation from CTV. In a study using discontinuous images of the pelvis and thighs (two thirds of images omitted) there was good sensitivity (89%) and substantial agreement (continuous vs discontinuous images) with an estimated dose reduction of 75% [102]. Alternatively, by omitting imaging of the pelvis, detection of VTE was not altered and dose was significantly reduced by an estimated 90% [103].
Pregnant patients
In pregnant patients with suspected PE, D-dimer testing is still indicated, although it is often positive during pregnancy. The reason to test is that a negative D-dimer has the same high negative predictive value as in the nonpregnant population [90]. Lower-limb ultrasound is recommended as the first-line imaging test [49,90]. Venous ultrasound demonstrated DVT in 13–15% of patients with suspected PE, and in 29% of patients with proven PE in one study, and could thus eliminate the need for further imaging in some cases [104]. The risks to the mother and fetus from radiation exposure should be considered and weighed against the risk of undiagnosed PE to the mother. Some sources estimate greater absorbed radiation doses to the fetus with VQ imaging compared with MDCT (0.12 mGy VQ versus 0.01 mGy for MDCT and 100–370 μGy VQ versus 3.3–20.2 μGy for 4 MDCT) [98,105]. Other studies suggest equivalent absorbed fetal doses from both modalities in all three trimesters (0.24–0.66 mGy) [106,107]. In pregnant patients with normal chest radiographs, a perfusion study can be performed initially and if this is negative, no further testing is needed [16]. Another step that can be taken to further decrease radiation is to reduce the dose of the perfusion study [98,107]. For the proliferating breast tissue in the pregnant female, MDCT is estimated to impart a much greater absorbed dose compared with a perfusion scan, that is 10 versus 0.28 mGy [98]. The role of MRA is not established since the safety of gadolinium during pregnancy, and especially the first trimester, have not been established [108].
Future perspective
Screening for hereditary or acquired thrombophilias to reduce the need for imaging?
Hereditary or acquired causes (or usually a combination of the two) are identified in over 80% of cases of thromboembolism, according to recent reports [109]. Investigators are now looking at identifying underlying hereditary and acquired risk factors in patients who present with a thrombotic episode and using this information to help decide the duration of therapy and the need for prophylactic anticoagulation in the future [109].
At present, there is limited consensus as to who should be tested for inherited thrombophilia, but some authorities agree that this should include patients aged 50 years and under presenting with an episode of thromboembolism, or thromboembolism in an unusual location (e.g., mesenteric, splenic, portal, hepatic or cerebral), or who have a first-degree relative (particularly ≤50 years) with an episode of thrombosis, or who have a thrombotic episode during pregnancy or while taking the oral contraceptive pill [109]. The inherited thrombophilias include factor V Leiden, elevated Factor VIII, Factor IX, Factor X, lipoprotein (a), or thrombin-activatable fibrinolysis inhibitor and hyperhomocystinemia [109]. It should be noted, however, that the finding of a thrombophilic disorder does not alter the clinical management, and so many have questioned the usefulness of this approach [110]. However, it may be helpful to identify possible asymptomatic family members. In addition, identifying a thrombophilic defect does not exclude other risk factors, given the multifactorial etiology of venous thrombosis [110].
Screening for acquired thrombophilias is recommended by some for patients presenting with their first episode of thromboembolism, who do not fulfill the criteria mentioned. The acquired thrombophilias include antiphospholipid antibody syndrome, myeloproliferative disorders, malignancy, heparin-induced thrombocytopenia and paroxysmal nocturnal hemoglobinuria [109].
Many of the inherited thrombophilias are transmitted in an autosomal dominant fashion, with variable expression so it is unclear who would need treatment. In up to 50% of firstdegree relatives who inherited thrombophilia, a predisposing acquired risk factor was also present and the mean age for thrombosis was lower than the general population. By identifying people with inherited thrombophilias, it may be appropriate to recommend prophylaxis in certain situations that increase their risk. By identifying and treating patients who have inherited or acquired risk factors after their first episode of VTE, this may decrease the numbers of patients who are referred for imaging. In the long term, this might reduce the numbers of inappropriately performed CTs for PE.
Using D-dimer testing appropriately
D-dimer is a fibrin degradation product that is generated in the final step of thrombus formation and is typically elevated in acute VTE. D-dimer is elevated in many clinical settings where thrombosis and fibrinolysis are increased, including malignancy, trauma, surgery, stroke, acute coronary syndrome, infection, pregnancy and advancing age [111]. D-dimer is a sensitive but nonspecific test, so if it is negative, it can be used to ‘rule out’ the diagnosis of VTE. D-dimer tests rely on the binding of monoclonal antibodies to the specific byproduct of circulating cross-linked fibrin, and to be useful in clinical practice they need to be rapid, convenient, inexpensive, accurate and reliable. Many D-dimer tests are available and it is important for users to be aware of the assay characteristics (sensitivity, specificity, positive and negative predictive values) as they relate to the local patient population (disease prevalence). D-dimers may be quantitative or qualitative, with the latter having a lower sensitivity and higher specificity compared with the former [111]. D-dimer levels are reduced in the setting of anticoagulation therapy and decrease with longer standing thrombus. D-dimer sensitivity is also affected by size and location of the thrombus. In pregnant patients with suspected PE, D-dimer testing is still indicated, although it is often positive during pregnancy. The reason to test is that a negative good-quality D-dimer has the same high negative predictive value as in the nonpregnant population [90].
Many authorities recommend D-dimer testing to be performed in hemodynamically stable patients with a low and intermediate pretest probability (as determined by a clinical prediction rule such as the Wells score) for PE prior to diagnostic imaging [86–88] (Figure 11) [49,90]. For hemodynamically unstable (shocked or hypotensive) patients (with a higher probability to have PE), sick patients, such as those in an ICU, or for other patients with a high pretest probability, it is recommended to proceed immediately to further testing without testing the D-dimer [90].
If a high-quality rapid ELISA D-dimer assay is of sufficiently low value or ‘negative’, it is safe to withhold further investigation for PE or treatment [49,92,93]. In a recent study of 2003 patients, for those who had a low (negative) D-dimer result, the negative predictive value to have a negative CTPA was 100% [112].
If the D-dimer assay is positive, or the pretest probability for PE is high, then further testing is recommended as the next step (Figure 1) [5].
Prognostic value of CTPA
Risk stratification is important since work-up, treatment and monitoring of PE will depend on the overall prognosis. With fatal PE, patients usually die as a consequence of right ventricular (RV) failure and circulatory collapse, which frequently occur within a few hours of admission [113]. Therefore, RV dysfunction should be diagnosed rapidly to identify patients who may benefit from thrombolytic therapy. Acute rightsided heart failure can be diagnosed on CTPA by assessing the dimensions of the right-sided heart chambers, superior vena cava or azygos vein. Measurements include RV to left ventricular short axis ratios and main pulmonary artery diameter to aorta diameter ratios, which are usually not greater than 1. Other features of RV dysfunction include a dilated inferior vena cava, reflux of contrast medium into the inferior vena cava, and leftward bowing or a sigmoid shape of the interventricular septum. With the use of electrocardiographic gating, it is possible to measure LV and RV ejection fractions [113].
Clot load scores can also be used to assess the magnitude or severity of PE, such as the Miller and Walsh scores [114,115]. The Miller Index for pulmonary angiograms used an objective score for the vessel level of arterial obstruction (with 1 for segmental vessels and larger arteries scored on all the segments that arise distally, with a possible maximum score of 16) and a subjective score for peripheral perfusion in the lungs (three zones each side, each scored 0 for normal perfusion, 1 if moderately reduced, 2 if severely reduced, and 3 if absent, with a possible maximum score of 18) [114]. With the advent of CT and MDCT, the Miller and Walsh scores have been adapted to CT [116]. In addition, newer scoring systems have been developed that include qualitative assessments of the degree of vascular obstruction (0 for no occlusion, 1 for partial occlusion and 2 completely occlusive) and correlated well with the Miller index and echographic assessment of right heart overload and dilatation [117]. Newer scoring systems that use quantitative assessments of the percentage of obstruction in vessels (with 1 for <25%; 2 for 25–49%; 3 for 50–74%; 4 for 75–99%; and 5 for 100%) also correlated well with echographic assessment of cor pulmonale and pulmonary hypertension [118].
Dual-energy CT
Dual-source (or energy) CT is a form of MDCT and was introduced in 2006. Dual-energy CT employs two tubes and two detectors, which are mounted orthogonally to each other [119,120]. Tube voltages of the same energy (dual-source scanning) or x-ray spectra with two different energies (dual-energy scanning) can be emitted at the same phase of contrast enhancement. The application of dual-energy CT in the diagnosis of PE relies mainly on its ability to separately identify iodinated contrast medium or tissue infiltration, which cannot be precisely detected with single-energy CT [121]. Iodine and tissues containing iodine, such as pulmonary arteries, produce higher attenuation at lower tube voltages and the spectral information obtained can be used to differentiate iodine-containing tissues from other tissues [120]. Selective visualization of tissues containing iodine, such as the pulmonary parenchyma, can be used to detect defects, indicating areas of pulmonary infarction [120]. Another advantage of dual-energy CT compared with other diagnostic modalities is that blood volume compared with blood flow can be visualized [121]. Dual-energy CT can provide highquality morphologic analysis and functional information on the pulmonary circulation from a single dataset, comparable to MRA or scintigraphy [121]. In cases where there is suboptimal pulmonary arterial concentration of contrast medium, the low-energy acquisition enables one to generate an image set with increased vascular enhancement [121]. Dual-source CT can also be used in combination with xenon CT to generate ventilation perfusion maps and detect ventilation perfusion mismatches [122]. A disadvantage of dual-energy CTPA is the higher radiation exposure compared with single source CT [121]. In a study of 13 patients, the sensitivity, specificity and negative predictive value of dual-energy CTPA compared with pulmonary perfusion scintigraphy were estimated as 75, 80 and 66%, respectively [113]. Dual-energy CT as an imaging method for PE is not widely used at present, but shows great promise.
Conclusion
Venous thromboembolic disease is a common and serious problem, the causes of which are not fully understood. In addition, the symptoms and signs of PE on clinical examination overlap those of other entities, which require different management. Diagnostic imaging therefore plays a central role in the work-up of the patient suspected of having PE or DVT. Chest radiography is not sensitive or specific but can identify the other entities that can overlap with PE clinically. There are many other imaging options available and many of the medical societies have published excellent guidelines to help practitioners choose the optimal imaging strategy for a particular patient. An understanding of the options available for special patient populations is also necessary in order to increase the speed and accuracy of diagnosis, reduce the side effects from imaging and minimize exposure to ionizing radiation.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Tapson VF: Acute pulmonary embolism. N. Engl. J. Med. 358, 1037–1052 (2008).
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd: Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch. Intern. Med. 158, 585–593 (1998).
- Heit JA, Silverstein MD, Mohr DN et al.: The epidemiology of venous thromboembolism in the community. Thromb. Haemost. 86, 452–463 (2001).
- Cohen AT, Agnelli G, Anderson FA et al.: Venous thromboembolism (VTE) in Europe. the number of VTE events and associated morbidity and mortality. Thromb. Haemost. 98, 756–764 (2007).
- Agnelli G, Becattini C: Acute pulmonary embolism. N. Engl. J. Med. 363, 266–274 (2010).
- Dalen JE: Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest 122, 1440–1456 (2002).
- Francis CW: Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N. Engl. J. Med. 356, 1438–1444 (2007).
- Sandler DA, Martin JF: Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J. R. Soc. Med. 82, 203–205 (1989).
- Hoang JK, Lee WK, Hennessy OF: Multidetector CT pulmonary angiography features of pulmonary embolus. J. Med. Imaging Radiat. Oncol. 52, 307–317 (2008).
- Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE: Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED study. Radiology 189, 133–136 (1993).
- Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B: EANM guidelines for ventilation/perfusion scintigraphy: part 1. Pulmonary imaging with ventilation/perfusion single photon emission tomography. Eur. J. Nucl. Med. Mol. Imaging 36, 1356–1370 (2009).
- The PIOPED Investigators: Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED). JAMA 263, 2753–2759 (1990).
- Freeman LM, Krynyckyi B, Zuckier LS: Enhanced lung scan diagnosis of pulmonary embolism with the use of ancillary scintigraphic findings and clinical correlation. Semin. Nucl. Med. 31, 143–157 (2001).
- Hagen PJ, Hartmann IJ, Hoekstra OS, Stokkel MP, Teule GJ, Prins MH: How to use a gestalt interpretation for ventilationperfusion lung scintigraphy. J. Nucl. Med. 43, 1317–1323 (2002).
- Schuemichen C: Pulmonary embolism: is multislice CT the method of choice? Against. Eur. J. Nucl. Med. Mol. Imaging 32, 107–112 (2005).
- Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B: EANM guidelines for ventilation/perfusion scintigraphy: Part 2. Algorithms and clinical considerations for diagnosis of pulmonary emboli with V/P(SPECT) and MDCT. Eur. J. Nucl. Med. Mol. Imaging 36, 1528–1538 (2009).
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L: Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 246, 941–946 (2008).
- Reinartz P, Wildberger JE, Schaefer W, Nowak B, Mahnken AH, Buell U: Tomographic imaging in the diagnosis of pulmonary embolism: a comparison between V/Q lung scintigraphy in SPECT technique and multislice spiral CT. J. Nucl. Med. 45, 1501–1508 (2004).
- Coche E, Verschuren F, Keyeux A et al.: Diagnosis of acute pulmonary embolism in outpatients: comparison of thin-collimation multi-detector row spiral CT and planar ventilation-perfusion scintigraphy. Radiology 229, 757–765 (2003).
- Wang F, Fang W, Lv B et al.: Comparison of lung scintigraphy with multi-slice spiral computed tomography in the diagnosis of pulmonary embolism. Clin. Nucl. Med. 34, 424–427 (2009).
- Miles S, Rogers KM, Thomas P et al.: A comparison of SPECT lung scintigraphy and CTPA for the diagnosis of pulmonary embolism. Chest 136(6), 1546–155 (2009).
- Sostman HD, Miniati M, Gottschalk A, Matta F, Stein PD, Pistolesi M: Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. J. Nucl. Med. 49, 1741–1748 (2008).
- van Beek EJ, Kuyer PM, Schenk BE, Brandjes DP, ten Cate JW, Buller HR: A normal perfusion lung scan in patients with clinically suspected pulmonary embolism. Frequency and clinical validity. Chest 108, 170–173 (1995).
- Henry JW, Stein PD, Gottschalk A, Raskob GE: Pulmonary embolism among patients with a nearly normal ventilation/ perfusion lung scan. Chest 110, 395–398 (1996).
- Leblanc M, Leveillee F, Turcotte E: Prospective evaluation of the negative predictive value of V/Q SPECT using 99mTc-Technegas. Nucl. Med. Commun. 28, 667–672 (2007).
- Gottschalk A, Stein PD, Sostman HD, Matta F, Beemath A: Very low probability interpretation of V/Q lung scans in combination with low probability objective clinical assessment reliably excludes pulmonary embolism: data from PIOPED II. J. Nucl. Med. 48, 1411–1415 (2007).
- Harris B, Bailey D, Miles S et al.: Objective analysis of tomographic ventilation-perfusion scintigraphy in pulmonary embolism. Am. J. Respir. Crit. Care Med. 175, 1173–1180 (2007).
- Macdonald WB, Patrikeos AP, Thompson RI, Adler BD, van der Schaaf AA: Diagnosis of pulmonary embolism: ventilation perfusion scintigraphy versus helical computed tomography pulmonary angiography. Australas. Radiol. 49, 32–38 (2005).
- Bajc M, Olsson B, Palmer J, Jonson B: Ventilation/perfusion SPECT for diagnostics of pulmonary embolism in clinical practice. J. Intern. Med. 264, 379–387 (2008).
- Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM: Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. AJR Am. J. Roentgenol. 194, 392–397 (2010).
- Hagen PJ, Hartmann IJ, Hoekstra OS, Stokkel MP, Postmus PE, Prins MH: Comparison of observer variability and accuracy of different criteria for lung scan interpretation. J. Nucl. Med. 44, 739–744 (2003).
- Coche EE, Muller NL, Kim KI, Wiggs BR, Mayo JR: Acute pulmonary embolism: ancillary findings at spiral CT. Radiology 207, 753–758 (1998).
- Johnson PT, Wechsler RJ, Salazar AM, Fisher AM, Nazarian LN, Steiner RM: Spiral CT of acute pulmonary thromboembolism: evaluation of pleuroparenchymal abnormalities. J. Comput. Assist. Tomogr. 23, 369–373 (1999).
- Karabulut N, Kiroglu Y: Relationship of parenchymal and pleural abnormalities with acute pulmonary embolism: CT findings in patients with and without embolism. Diagn. Interv. Radiol. 14, 189–196 (2008).
- Shah AA, Davis SD, Gamsu G, Intriere L: Parenchymal and pleural findings in patients with and patients without acute pulmonary embolism detected at spiral CT. Radiology 211, 147–153 (1999).
- Winer-Muram HT, Rydberg J, Johnson MS et al.: Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology 233, 806–815 (2004).
- Stein PD, Fowler SE, Goodman LR et al.: Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 354, 2317–2327 (2006).
- Schoepf UJ, Holzknecht N, Helmberger TK et al.: Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology 222, 483–490 (2002).
- Patel S, Kazerooni EA, Cascade PN: Pulmonary embolism: optimization of small pulmonary artery visualization at multidetector row CT. Radiology 227, 455–460 (2003).
- Guilabert JP, Manzur DN, Tarrasa MJ, Llorens ML, Braun P, Arques MP: Can multislice CT alone rule out reliably pulmonary embolism? A prospective study. Eur. J. Radiol. 62, 220–226 (2007).
- Heuschmid M, Mann C, Luz O et al.: Detection of pulmonary embolism using 16-slice multidetector-row computed tomography: evaluation of different image reconstruction parameters. J. Comput. Assist. Tomogr. 30, 77–82 (2006).
- Nishino M, Kubo T, Kataoka ML, Gautam S, Raptopoulos V, Hatabu H: Evaluation of pulmonary embolisms using coronal reformations on 64-row multidetector-row computed tomography: comparison with axial images. J. Comput. Assist. Tomogr. 30, 233–237 (2006).
- Ciccotosto C, Goodman LR, Washington L, Quiroz FA: Indirect CT venography following CT pulmonary angiography: spectrum of CT findings. J. Thorac. Imaging 17, 18–27 (2002).
- Garg K, Kemp JL, Wojcik D et al.: Thromboembolic disease: comparison of combined CT pulmonary angiography and venography with bilateral leg sonography in 70 patients. AJR Am. J. Roentgenol. 175, 997–1001 (2000).
- Lim KE, Hsu WC, Hsu YY, Chu PH, Ng CJ: Deep venous thrombosis: comparison of indirect multidetector CT venography and sonography of lower extremities in 26 patients. Clin. Imaging 28, 439–444 (2004).
- Goodman LR, Stein PD, Matta F et al.: CT venography and compression sonography are diagnostically equivalent: data from PIOPED II. AJR Am. J. Roentgenol. 189, 1071–1076 (2007).
- Thomas SM, Goodacre SW, Sampson FC, van Beek EJ: Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin. Radiol. 63, 299–304 (2008).
- Richman PB, Wood J, Kasper DM et al.: Contribution of indirect computed tomography venography to computed tomography angiography of the chest for the diagnosis of thromboembolic disease in two United States emergency departments. J. Thromb. Haemost. 1, 652–657 (2003).
- Stein PD, Woodard PK, Weg JG et al.: Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Radiology 242, 15–21 (2007).
- Goodman LR, Sostman HD, Stein PD, Woodard PK: CT venography: a necessary adjunct to CT pulmonary angiography or a waste of time, money, and radiation? Radiology 250, 327–330 (2009).
- Perrier A, Bounameaux H: Accuracy or outcome in suspected pulmonary embolism. N. Engl. J. Med. 354, 2383–2385 (2006).
- Hunsaker AR, Zou KH, Poh AC et al.: Routine pelvic and lower extremity CT venography in patients undergoing pulmonary CT angiography. AJR Am. J. Roentgenol. 190, 322–326 (2008).
- Rosen MP, Sheiman RG, Weintraub J, McArdle C: Compression sonography in patients with indeterminate or low-probability lung scans: lack of usefulness in the absence of both symptoms of deep-vein thrombosis and thromboembolic risk factors. AJR Am. J. Roentgenol. 166, 285–289 (1996).
- Dalen JE, Brooks HL, Johnson LW, Meister SG, Szucs MM Jr, Dexter L: Pulmonary angiography in acute pulmonary embolism: indications, techniques, and results in 367 patients. Am. Heart J. 81, 175–185 (1971).
- Mills SR, Jackson DC, Sullivan DC et al.: Angiographic evaluation of chronic pulmonary embolism. Radiology 136, 301–308 (1980).
- Williams JR, Wilcox C, Andrews GJ, Burns RR: Angiography in pulmonary embolism. JAMA 184, 473–476 (1963).
- Quinn MF, Lundell CJ, Klotz TA et al.: Reliability of selective pulmonary arteriography in the diagnosis of pulmonary embolism. AJR Am. J. Roentgenol. 149, 469–471 (1987).
- Diffin DC, Leyendecker JR, Johnson SP, Zucker RJ, Grebe PJ: Effect of anatomic distribution of pulmonary emboli on interobserver agreement in the interpretation of pulmonary angiography. AJR Am. J. Roentgenol. 171, 1085–1089 (1998).
- Stein PD, Henry JW, Gottschalk A: Reassessment of pulmonary angiography for the diagnosis of pulmonary embolism: relation of interpreter agreement to the order of the involved pulmonary arterial branch. Radiology 210, 689–691 (1999).
- Novelline RA, Baltarowich OH, Athanasoulis CA, Waltman AC, Greenfield AJ, McKusick KA: The clinical course of patients with suspected pulmonary embolism and a negative pulmonary arteriogram. Radiology 126, 561–567 (1978).
- Stein PD, Athanasoulis C, Alavi A et al.: Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 85, 462–468 (1992).
- Henry JW, Relyea B, Stein PD: Continuing risk of thromboemboli among patients with normal pulmonary angiograms. Chest 107, 1375–1378 (1995).
- van Beek EJ, Brouwerst EM, Song B, Stein PD, Oudkerk M: Clinical validity of a normal pulmonary angiogram in patients with suspected pulmonary embolism – a critical review. Clin. Radiol. 56, 838–842 (2001).
- Stein PD, Gottschalk A, Sostman HD et al.: Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III). Semin. Nucl. Med. 38, 462–470 (2008).
- Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR: Diagnosis of pulmonary embolism with magnetic resonance angiography. N. Engl. J. Med. 336, 1422–1427 (1997).
- Oudkerk M, van Beek EJ, Wielopolski P et al.: Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet 359, 1643–1647 (2002).
- Stein PD, Chenevert TL, Fowler SE et al.: Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann. Intern. Med. 152, 434–443, W142–433 (2010).
- Carpenter JP, Holland GA, Baum RA, Owen RS, Carpenter JT, Cope C: Magnetic resonance venography for the detection of deep venous thrombosis: comparison with contrast venography and duplex Doppler ultrasonography. J. Vasc. Surg. 18, 734–741 (1993).
- Evans AJ, Sostman HD, Knelson MH et al.: 1992 ARRS Executive Council Award. Detection of deep venous thrombosis: prospective comparison of MR imaging with contrast venography. AJR Am. J. Roentgenol. 161, 131–139 (1993).
- Laissy JP, Cinqualbre A, Loshkajian A et al.: Assessment of deep venous thrombosis in the lower limbs and pelvis: MR venography versus duplex Doppler sonography. AJR Am. J. Roentgenol. 167, 971–975 (1996).
- Evans AJ, Sostman HD, Witty LA et al.: Detection of deep venous thrombosis: prospective comparison of MR imaging and sonography. J. Magn. Reson. Imaging 6, 44–51 (1996).
- Tovey C, Wyatt S: Diagnosis, investigation, and management of deep vein thrombosis. BMJ 326, 1180–1184 (2003).
- Hamper UM, DeJong MR, Scoutt LM: Ultrasound evaluation of the lower extremity veins. Radiol. Clin. North Am. 45, 525–547, IX (2007).
- Lensing AW, Prandoni P, Brandjes D et al.: Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N. Engl. J. Med. 320, 342–345 (1989).
- Killewich LA, Bedford GR, Beach KW, Strandness DE Jr: Diagnosis of deep venous thrombosis. A prospective study comparing duplex scanning to contrast venography. Circulation 79, 810–814 (1989).
- Cogo A, Lensing AW, Koopman MM et al.: Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ 316, 17–20 (1998).
- Stevens SM, Elliott CG, Chan KJ, Egger MJ, Ahmed KM: Withholding anticoagulation after a negative result on duplex ultrasonography for suspected symptomatic deep venous thrombosis. Ann. Intern. Med. 140, 985–991 (2004).
- Subramaniam RM, Heath R, Chou T, Cox K, Davis G, Swarbrick M: Deep venous thrombosis: withholding anticoagulation therapy after negative complete lower limb US findings. Radiology 237, 348–352 (2005).
- Jardin F, Dubourg O, Bourdarias JP: Echocardiographic pattern of acute cor pulmonale. Chest 111, 209–217 (1997).
- Come PC: Echocardiographic evaluation of pulmonary embolism and its response to therapeutic interventions. Chest 101, 151S–162S (1992).
- Goldhaber SZ: Echocardiography in the management of pulmonary embolism. Ann. Intern. Med. 136, 691–700 (2002).
- Pruszczyk P, Torbicki A, Pacho R et al.: Noninvasive diagnosis of suspected severe pulmonary embolism: transesophageal echocardiography vs spiral CT. Chest 112, 722–728 (1997).
- Miniati M, Monti S, Pratali L et al.: Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am. J. Med. 110, 528–535 (2001).
- Vieillard-Baron A, Qanadli SD, Antakly Y et al.: Transesophageal echocardiography for the diagnosis of pulmonary embolism with acute cor pulmonale: a comparison with radiological procedures. Intensive Care Med. 24, 429–433 (1998).
- Steiner P, Lund GK, Debatin JF et al.: Acute pulmonary embolism: value of transthoracic and transesophageal echocardiography in comparison with helical CT. AJR Am. J. Roentgenol. 167, 931–936 (1996).
- Wells PS, Anderson DR, Rodger M et al.: Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann. Intern. Med. 135, 98–107 (2001).
- Wells PS, Ginsberg JS, Anderson DR et al.: Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann. Intern. Med. 129, 997–1005 (1998).
- Wells PS, Anderson DR, Rodger M et al.: Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb. Haemost. 83, 416–420 (2000).
- Wells PS, Anderson DR, Rodger M et al.: Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N. Engl. J. Med. 349, 1227–1235 (2003).
- Torbicki A, Perrier A, Konstantinides S et al.: Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 29, 2276–2315 (2008).
- Remy-Jardin M, Pistolesi M, Goodman LR et al.: Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 245, 315–329 (2007).
- Stein PD, Hull RD, Patel KC et al.: D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann. Intern. Med. 140, 589–602 (2004).
- Michiels JJ, Gadisseur A, van der Planken M et al.: Different accuracies of rapid enzymelinked immunosorbent, turbidimetric, and agglutination D-dimer assays for thrombosis exclusion: impact on diagnostic work-ups of outpatients with suspected deep vein thrombosis and pulmonary embolism. Semin. Thromb. Hemost. 32, 678–693 (2006).
- Quiroz R, Kucher N, Zou KH et al.: Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA 293, 2012–2017 (2005).
- Eisenberg RL, Bank WO, Hedgock MW: Renal failure after major angiography can be avoided with hydration. AJR Am. J. Roentgenol. 136, 859–861 (1981).
- Thomsen HS: How to avoid nephrogenic systemic fibrosis: current guidelines in Europe and the United States. Radiol. Clin. North Am. 47, 871–875, VII (2009).
- Parker MS, Hui FK, Camacho MA, Chung JK, Broga DW, Sethi NN: Female breast radiation exposure during CT pulmonary angiography. AJR Am. J. Roentgenol. 185, 1228–1233 (2005).
- Cook JV, Kyriou J: Radiation from CT and perfusion scanning in pregnancy. BMJ 331, 350 (2005).
- Managing patient dose in computed tomography. A report of the International Commission on Radiological Protection. Ann. ICRP 30, 7–45 (2000).
- Stein PD, Alavi A, Gottschalk A et al.: Usefulness of noninvasive diagnostic tools for diagnosis of acute pulmonary embolism in patients with a normal chest radiograph. Am. J. Cardiol. 67, 1117–1120 (1991).
- Forbes KP, Reid JH, Murchison JT: Do preliminary chest x-ray findings define the optimum role of pulmonary scintigraphy in suspected pulmonary embolism? Clin. Radiol. 56, 397–400 (2001).
- Goodman LR, Stein PD, Beemath A et al.: CT venography for deep venous thrombosis: continuous images versus reformatted discontinuous images using PIOPED II data. AJR Am. J. Roentgenol. 189, 409–412 (2007).
- Kalva SP, Jagannathan JP, Hahn PF, Wicky ST: Venous thromboembolism: indirect CT venography during CT pulmonary angiography – should the pelvis be imaged? Radiology 246, 605–611 (2008).
- Turkstra F, Kuijer PM, van Beek EJ, Brandjes DP, ten Cate JW, Buller HR: Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann. Intern. Med. 126, 775–781 (1997).
- Winer-Muram HT, Boone JM, Brown HL, Jennings SG, Mabie WC, Lombardo GT: Pulmonary embolism in pregnant patients: fetal radiation dose with helical CT. Radiology 224, 487–492 (2002).
- Ginsberg JS, Hirsh J, Rainbow AJ, Coates G: Risks to the fetus of radiologic procedures used in the diagnosis of maternal venous thromboembolic disease. Thromb. Haemost. 61, 189–196 (1989).
- Hurwitz LM, Yoshizumi T, Reiman RE et al.: Radiation dose to the fetus from body MDCT during early gestation. AJR Am. J. Roentgenol. 186, 871–876 (2006).
- Kanal E, Barkovich AJ, Bell C et al.: ACR guidance document for safe MR practices: 2007. AJR Am. J. Roentgenol. 188, 1447–1474 (2007).
- Whitlatch NL, Ortel TL: Thrombophilias: when should we test and how does it help? Semin. Respir. Crit. Care Med. 29, 25–39 (2008).
- Middeldorp S, van Hylckama Vlieg A: Does thrombophilia testing help in the clinical management of patients? Br. J. Haematol. 143, 321–335 (2008).
- Hargett CW, Tapson VF: Clinical probability and D-dimer testing: how should we use them in clinical practice? Semin. Respir. Crit. Care Med. 29, 15–24 (2008).
- Mamlouk MD, van Sonnenberg E, Gosalia R et al.: Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology 256, 625–632 (2010).
- Nikolaou K, Thieme S, Sommer W, Johnson T, Reiser MF: Diagnosing pulmonary embolism: new computed tomography applications. J. Thorac. Imaging 25, 151–160 (2010).
- Miller GA, Sutton GC, Kerr IH, Gibson RV, Honey M: Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br. Heart J. 33, 616 (1971).
- Walsh PN, Stengle JM, Sherry S: The urokinase-pulmonary embolism trial. Circulation 39, 153–156 (1969).
- Bankier AA, Janata K, Fleischmann D et al.: Severity assessment of acute pulmonary embolism with spiral CT: evaluation of two modified angiographic scores and comparison with clinical data. J. Thorac. Imaging 12, 150–158 (1997).
- Qanadli SD, El Hajjam M, Vieillard-Baron A et al.: New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am. J. Roentgenol. 176, 1415–1420 (2001).
- Mastora I, Remy-Jardin M, Masson P et al.: Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur. Radiol. 13, 29–35 (2003).
- Pontana F, Faivre JB, Remy-Jardin M et al.: Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad. Radiol. 15, 1494–1504 (2008).
- Thieme SF, Johnson TR, Lee C et al.: Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am. J. Roentgenol. 193, 144–149 (2009).
- Remy-Jardin M, Faivre JB, Pontana F et al.: Thoracic applications of dual energy. Radiol. Clin. North Am. 48, 193–205 (2010).
- Kang MJ, Park CM, Lee CH, Goo JM, Lee HJ: Dual-energy CT: clinical applications in various pulmonary diseases. Radiographics 30, 685–698 (2010).