Review Article - Imaging in Medicine (2012) Volume 4, Issue 4
Imaging techniques for presurgical evaluation of temporal lobe epilepsy
Andrea Bartoli1*, Serge Vulliemoz2, Sven Haller3, Karl Schaller1 and Margitta Seeck21Department of Neurosurgery Geneva University Hospital, 4, Rue Gabrielle Perret-Gentil, CH-1211 Geneva, Switzerland
2Department of Neurology, Faculty of Medicine, Geneva University Hospital, Geneva, Switzerland
3Department of Neuroradiology, Faculty of Medicine, Geneva University Hospital, Geneva, Switzerland
Abstract
The surgical treatment of pharmaco-resistant focal epilepsy has increased dramatically in recent years. This evolution is undoubtedly due to an improvement of electrophysiological and neuroradiological investigations to localize the epileptic focus. Temporal lobe epilepsy is the most frequent pharmacoresistent epileptic syndrome in adults and responds better to surgery than the so-called extratemporal epilepsy. Apart from the history and the clinical semiology, the investigations for temporal lobe epilepsy are based on core investigations, including electroencephalography (long-term video-EEG recordings) and MRI. Additional noninvasive imaging techniques to improve the localization of the epileptic focus include PET ictal and interictal SPECT, electric source imaging and magnetic source imaging, and simultaneous EEG and functional MRI. Advanced sequences and analysis of structural MRI data allow us to map subtle structural abnormalities as well as important white matter tracts while functional MRI of language/memory helps to identify eloquent cortical area and estimate the risk of postoperative deficits. Our aim is to review the current literature and summarize all available data on these validated imaging techniques for the assessment of focal temporal lobe epilepsy.
Keywords
ESI; fMRI; imaging; magnetic source imaging; MRI; PET-CT; SPECT; temporal lobe epilepsy
The neurosurgical activity of epilepsy surgery has increased dramatically in recent years. In Europe, 15 operations were performed on average between 1979 and 1984: this number grew to 42 operations between 1989 and 1994 on average per center [1]. This evolution is undoubtedly owing to an improvement of electrophysiological and neuroradiological investigations. Surgery for temporal lobe epilepsy (TLE) is indicated for drug-resistant epilepsy where the epileptogenic zone (EZ) can be localized and where the surgical removal is not related to inacceptable neurological or neuropsychological outcome.
Common pathologies that could underlie TLE are hippocampal sclerosis (HS), tumors, congenital malformations, vascular abnormalities and many other pathologies, summarized in Box 1.
TLE classically presents with complex partial seizures lasting 1–4 min, often preceded by auras with epigastric sensations, fear, anxiety, experiential phenomena, olfactory or gustatory hallucinations, or autonomic symptoms. Altered consciousness and oral or manual automatisms are typical and variably associated with contralateral dystonic postures or motor symptoms. Secondary generalization is rare but can occur, as well as postictal disturbances in mood, language and memory [2].
However, even if the MRI is normal, it is worthwhile pursuing surgery for epilepsy because if carefully selected, these patients also present good seizure control [3].
TLE is frequently drug-resistant and remains the most common cause of focal epilepsy among adults [4]. Consequently, around two-thirds of surgical procedures for intractable epilepsy are carried out on the temporal lobe. A systematic review and metanalysis from Téllez-Zenteno et al. of previous studies of TLE surgery showed that seizure-free outcome was achieved in 45% of patients suffering from a nonlesional TLE and 69% of patients with lesional TLE [5]. One recent study has only included TLE patients with normal structural MRI and has reported a rate of seizure freedom in 55% of patients after resective surgery of the temporal lobe [6]. The same authors showed that the long-term rate of seizure freedom is more likely to be achieved in patients with tumoral epilepsy (76%) and is lowest in studies with patients older than 50 years at the time of surgery, suggesting that early surgery is beneficial.
Presurgical evaluation of TLE includes clinical history and semiology, electroencephalographic studies (interictal EEG and continuous EEG), structural and functional neuroimaging techniques, neuropsychological assessment (for preoperative cognitive deficits and as a baseline for postoperative follow-up), invasive monitoring (epidural or subdural and/or intracerebral electrodes), and – if necessary – intracarotid amytal test (so-called Wada test) to verify good functioning of the nonresected hippocampus.
Here, we review the literature concerning all neuroimaging techniques currently used in TLE, with special emphasis on mesial temporal sclerosis associated with TLE (MTS-TLE).
Structural MRI
Standard MRI
The principal role of MRI is to define any structural abnormality that underlies TLE, and thus to correlate the structure with brain function. A recent study on surgical decision-making in TLE included, retrospectively, 186 patients and compared 18F-fluorodeoxyglucose (FDG)-PET, MRI and EEG: it was found that MRI seems to have the most influence on surgical candidacy and that FDG-PET predicts surgical outcome [7].
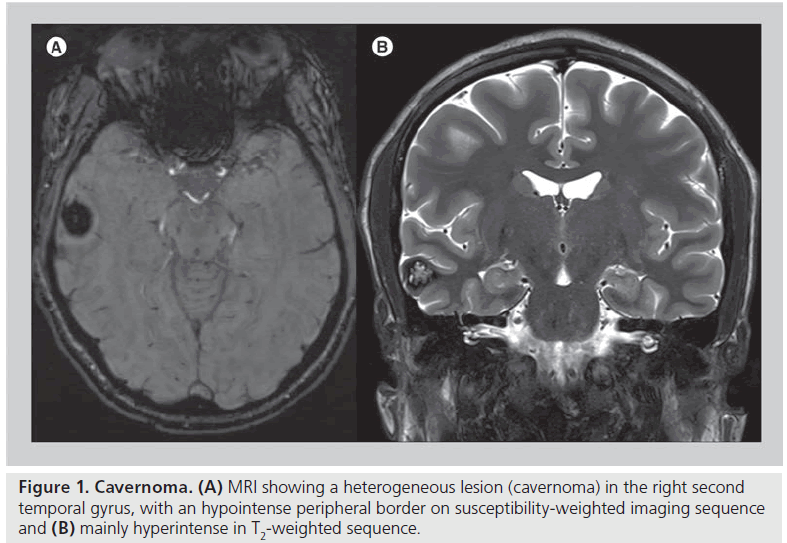
Since 1985 MRI has been shown to be more accurate than CT scanning regarding the etiology of epilepsy [8]. The most common MRI abnormal findings in TLE at 1T or 1.5T with T1- and T2-weighted sequences are vascular malformation (e.g., cavernomas see Figure 1), HS (Box 2 & Figure 2), malformation of cortical development, tumors and some acquired cortical damage (trauma, infarction or granulomas) [9–11]. On the other hand, CT can still be useful for skull fractures and intracranial calcification, which are both potentially causes of chronic TLE.
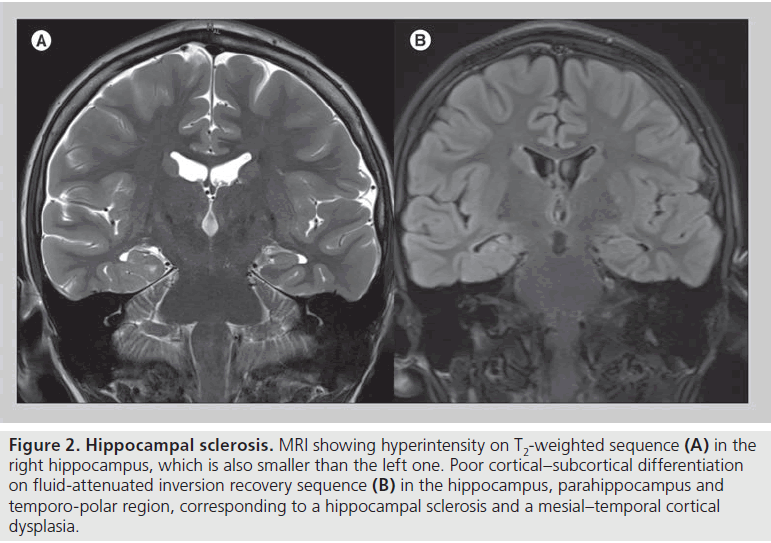
HS is the prevalent cause of TLE and is variably associated with focal cortical dysplasia (FCD) type IA in the temporo-polar region [12–14]. From a histopathological point of view, HS has been defined as loss of pyramidal neurons mainly in CA1 region with sprouting mossy fibers of dentate granule cells, often associated with CA3 and dentate neuronal loss. This lesion is frequently found with the so-called endfolium sclerosis, which is neuronal loss involving the CA4 region and dentate gyrus [15–17]. On the other hand, FCD is a dyslamination of cortical architecture but both pathologic and radiologic diagnostic criteria remain poor [18].
The validity of structural MRI has also been consolidated by some studies that compared electroclinical, radiological and histopathological findings in patients with TLE and nodular heterotopia, another cortical abnormality. In ten cases of nodular heterotopias (seven of which were temporal), both subependymal and subcortical signal intensity were isointense (nodules vs cortex), except for three cases where nodules were hyperintense in T2 and in fluid-attenuated inversion recovery (FLAIR) sequences. Cortex thickness was found to be normal over the nodules, gyrations altered and hemisphere size slightly reduced (mainly at temporal lobe). The histopathological study demonstrated the same microscopic organization within the nodules and that the overlying cortex was dysplastic (even if normal in thickness) [19]. Congruencies and discrepancies between ictal and interictal EEG and lesions on structural MRI in focal epilepsies have shown different values depending on the location of the lesion: patients with temporal lesions had the highest congruence between interictal epileptiform discharges, epileptic seizure patterns and lesions, more than patients with frontal, parieto-occipital regions but sur gical outcome did not differ between regions [20].
Series including both TLE and extratemporal epilepsy patients proved the usefulness of 3T MRI scanners for identifying focal cortical FCD and HS, especially with coronal and axial FLAIR sequences [21,22]. Surface coil placement did not improve the detection of previously undiagnosed focal lesions but improved the demarcation and provided more details about cortical lesions already diagnosed without coil placement [23].
Figure 2.Hippocampal sclerosis. MRI showing hyperintensity on T2-weighted sequence (A) in the right hippocampus, which is also smaller than the left one. Poor cortical–subcortical differentiation on fluid-attenuated inversion recovery sequence (B) in the hippocampus, parahippocampus and temporo-polar region, corresponding to a hippocampal sclerosis and a mesial–temporal cortical dysplasia.
With the exception of vascular malformations, tumors and other obvious lesions, subtle HS and cortical abnormalities (such as focal cortical dysplasia or cortical heterotopia) might be challenging to detect on 1 or 1.5T MRI because of the submillimetric size of these lesions, limited spatial resolution and contrast of MRI [11,23]. 3T MRI, as it is available in many centers today, provides a higher signal-to-noise ratio, leading to better identification of subtle lesions and/or better characterization of larger lesions in patients with epilepsy [21].
Additional advanced MRI techniques High-field structural MRI
Several studies and reviews showed that structural in vivo MRI at 1T or 1.5T can be unremarkable in patients with TLE [11,24]. Further improvement on detecting extremely small mesiotemporal structures by increasing spatial resolution and tissue contrast can be achieved by ultrahigh field strength MRI (7T and more) [25,26]. Normal hippocampal structures have been defined at 7T in young adults [27]. A combined T2-weighted 7T scanning and histopathological study on 13 surgical specimens from TLE patients has recently shown that highresolution MRI allows the study of intracortical organisa tion in normal and pathologic areas [28].
Henry et al. explored mesial temporal structures with T1- and T2-weighted sequences at 7T in eight patients suffering from TLE and 11 healthy subjects and found selectively greater Ammon’s horn atrophy in patients with TLE and HS. Furthermore, independently from hippocampal atrophy, some abnormalities of the dentate gyrus were visible, as well as paucity of digitations of the head of hippocampi and some malrotation of the hippocampus [23].
On the other hand, preliminary studies of frontal lobe malformations of cortical development using 7T MRI, multichannel coils, arterial spin labeling sequence, susceptibility-weighted imaging and diffusion tensor and spectrum imaging have been reported to show a better definition of the dysplasia than 3T MRI, and this can probably also apply in TLE [29].
Automated quantitative MRI-based analysis
Volumetric MRI analysis of the brain has become a common method to evaluate neurologic disorders including epilepsy and is a valuable tool, especially when structural abnormalities related to volume changes are less visible at visual inspection.
Manual hippocampal volumetry has been shown to detect unilateral or bilateral abnormality [30]. The degree of volume loss of enthorinal cortex measured by volumetry and the electrophysiological coupling of the enthorinal cortex and hippocampus measured by intracerebral recordings were strongly correlated, suggesting that volumetry can predict epileptogenesis of the enthorinal cortex [31].
On the other hand, manual volumetry is time consuming and prone to operator error. Therefore, attempts have been made to automate volume estimation of the brain, using MRI as a quantitative tool, and thus improving sensitivity and reducing subjectivity. It also has the advantage of not being restricted to only one region of interest but potentially to the whole brain.
Three automated methods are commonly used: voxel-based morphometry (VBM), deformation- based morphometry (DBM) and cortical thickness method [32–35].
VBM is a neuroimaging technique based on statistical parametric mapping of voxels derived from MRI scans and a brain template that takes into account brain anatomy differences among people. It allows the study of the probability that a given voxel is gray matter or white matter, and thus estimates the focal volumetric differences in the brain.
VBM is the most widely used automated quantitative analysis (more than 20 studies have been published concerning TLE) [33,36–41], it has been used extensively for other neurologic and neuropsychiatric disorders (e.g., schizophrenia and Alzheimer’s dementia) [36]. Bonilha et al. used VBM in 23 patients suffering from MTS-TLE and proved its utility for a reliable discrimination between atrophic and normal hippocampi [42]. However, interpretation of VBM analysis should be made cautiously because of some false-positive results [43,44] and requires correlation with other imaging data.
Compared with VBM, DBM allows the study of brain anatomical differences on a more macroscopic scale and is based on vector fields that describe global or gross differences in brain shape between the patient and a control group [45]. Only one study has been reported on DBM in TLE, and the same study compared VBM, DBM and the cortical thickness method in 29 TLE patients (14 with MTS and 15 with normal-appearing hippocampi, respectively) and 33 healthy subjects on a 4T MRI scan. Each method detects a different aspect of brain atrophy and should be used on the basis of the suspected pathology. In particular, VBM and DBM detect cortical and subcortical abnormalities in pathologies associated with macroscopic volume loss. Moreover, DBM seems to be an excellent method for subcortical abnormalities [33].
In contrast to VBM, cortical thickness is a more sensitive parameter, which has less group average standard deviation [46], which can be explained by the combination of a more precise spatial normalization (cortex-based alignment) and the more accurate estimation of cortical thickness.
Whole cortical thickness analysis also showed extra temporal neocortical thinning (especially bilaterally in sensorimotor cortex and to a lesser extent in the temporal, occipital and parietal lobes) in TLE patients, either with or without MTS (16 with MTS and 16 without MTS on structural MRI), compared with 44 healthy controls [47]. This finding corroborates what has been found in other reports about the coexistence of extrahippocampal lesion in MTSTLE patients [48] and supports the hypothesis that even in TLE without MTS, abnormalities in neocortical regions may be involved in the pathophysiology of the epilepsy. However, this method is not yet routinely used nor validated for clinical practice and limitations exist with regards to its value for identifying a small volume of tissue for resection: in fact, in children with frontal lobe epilepsy, cortical thinning was extensive within the frontal lobe and also in extrafrontal areas and this concept might also apply to TLE [49].
In a recent study, automatic quantitative FLAIR analysis promises to be a useful tool in quantifying hippocampal signal alterations, detecting HS and monitoring the evolution in time of the disease (following temporal lobe seizures, status epilepticus and limbic encephalitis) [50].
Diffusion studies
In an isotropic medium such as cerebrospinal fluid, water molecules are moving due to diffusion and they move at equal rates in all directions. Diffusion tensor imaging (DTI) is a sensitive method to measure the diffusion of water molecules in the intracellular and extracellular spaces: water molecules diffuse more rapidly along fiber bundles and more slowly perpendicular to the main axis of the fiber bundles, so that diffusion of water in the white matter is anisotropic. Decreased anisotropy of water diffusion can be due to reduced axonal density but also to the presence of crossing fibers in the region of interest. Studies comparing stereo-EEG and DTI showed spatial concordance between epileptiform activity and diffusivity abnormalities in up to 50% of patients with refractory focal epilepsy [51,52].
Furthermore, extratemporal abnormalities in TLE have been extensively studied and a considerable literature exist about co-existing white matter alterations detected by DTI [53]; however, its validity in localization of EZ has to be proven.
Owing to increased diffusivity of water in HS, apparent diffusion coefficient (which represents the net displacement of molecules in a tissue) has been found to be abnormal in patients with normal structural MRI hippocampi and ipsilateral onset of seizure [54,55].
Reduction of fractional anisotropy (FA) and higher diffusivity in the external capsule and corpus callosum in patients with TLE compared with healthy subjects was first reported by Arfanakanis et al. [56]. Several studies then showed diffusion abnormalities (both FA and apparent diffusion coefficient) in temporal and extratemporal structures of patients with TLE: the thalamus and hippocampus in children with TLE had decreased FA and increased apparent diffusion coefficient [57].
Reduced symmetry of FA was found in the fornix and cingulum of TLE patients compared with control subjects [58], also after surgery [59]. The FA of the uncinate fasciculus was found to be asymmetric in normal subjects (right greater than left) while this asymmetry was no longer observed in TLE patients with right HS, suggesting widespread alterations of the limbic system in these patients [60]. Concha et al. provided a good correlation between in vivo DTI abnormalities and postsurgical histopathology in the fimbria-fornix of TLE patients [61].
Some groups tried to overcome the subjectivity of assessment of diffusion indices of the white matter in TLE patients based on the ‘region of interest’ method by implementing some less arbitrary (quantitative) automated methods. These studies confirmed the existence of a significant reduction of FA in epileptogenic temporal lobes, corpus callosum and inferior frontal gyrus [62,63] and showed promising results for TLE patients with no MTS [64].
Diffusion abnormalities in TLE do not yet have clear explanations or implications: diverging studies exist on the question of whether white matter alterations are due to duration of epilepsy [65–68].
Some studies showed a correlation between white matter changes and neuropsychological memory tests; in other words, FA values of anterior temporal lobe and mesial temporal lobe were positively correlated with delayed memory and immediate memory, respectively [69,70]. Moreover, pre- and post-operative changes of white matter measured with DTI in patients undergoing anterior temporal lobe resection cor related with postoperative verbal fluency test [71].
Changes in white matter tracts, particularly an increase of FA values in the contra-lesional fornix after resective surgery, have also been demonstrated with tract-based spatial statistics on DTI sequences, a method that reduces the diffusion data to a skeleton of large white matter tract in order to allow more reliable intersubject and group comparisons [59].
Even though the clinical relevance of these findings remains unclear, white matter abnormalities seem to be extensive and bilateral in TLE patients with unilateral MTS and to a lesser extent in TLE patients without MTS [72].
Besides the still uncertain utility of DTI in the presurgical evaluation of TLE in terms of mapping subtle structural abnormalities, tractography algorithms can be used to estimate white matter tracts from the direction of preferred water diffusion measured by DTI. This technique has been shown to be useful for studying the anatomical variation in the course of the optic radiation in individual patients and to predict visual field loss after anterior temporal lobectomy (ATL): a superior homonymous quadrantanopia is a well-recognized complication following ATL and occurs because of disruption of the anterior part of the optic radiation (Meyer’s loop). Wallerian degeneration of fibers directly (parahippocampal cingulum, uncinate fasciculus, inferior longitudinal fasciculus and fornix) or indirectly (inferior fronto-occipital fasciculus and corpus callosum) affected by ATL is apparent 2 months after resection and DTI can accurately define the optic radiation preoperatively and in the future could be fused with intraoperative scans to reduce the risk of postoperative visual field defects [73–75]. Although promising, this application is not yet automated, and therefore requires lengthy analysis by experts in the field.
Relaxometry
Relaxometry is based on the creation of a map of relaxation time in a given sequence. T2-relaxometry is able to detect hippocampal and amygdala asymmetries in drug-resistant TLE [76,77]. Several approaches to quantitative estimation of T2 values exist and promising results stem from algebric T2 estimation for detection of hippocampal abnormalities [78,79] but no extensive use of this method has been published.
Spectroscopy
Magnetic resonance spectroscopy (MRS) can estimate different concentrations of chemicals within brain tissue. In a study by Guye et al., the metabolic index on spectroscopy (measured by N-acetyl aspartate:choline plus creatinine ratio) was spatially concordant with the site of epileptic abnormalities determined by intracranial EEG with depth electrodes as compared with normal intracranial EEG regions and compared with healthy control subjects. The metabolic changes were not dependent on the TLE subtype, nor on the structural alteration of the temporal lobe, but were linked to ictal and inter-ictal activity and extended to extramesial structures [80].
N-acetyl aspartate (a marker of neuronal integrity) has been found to be diminished not only in the sclerotic hippocampus, but also in extratemporal regions [81,82], in accordance to other MR studies in patients with MTS-TLE.
Creatinine and choline, markers of energy metabolism and cell membrane integrity, respectively, are usually unchanged [83]. Concentrations of N-acetylaspartate/creatine and choline-containing compounds have recently been found decreased in both hip pocampal and extrahippocampal structures in both MTS-TLE and no MTS-TLE, thus reducing the value of MRS for focus lateralization [84]. In healthy control subjects, metabolite concentration differences varied in different parts of the temporal lobe depending on the volume of hippocampal tissue within the region of interest (partial volume effect), which raises further questions about the reliability of MRS in TLE [85].
Considerations on advanced MRI techniques
Except for the standard structural MRI, more advanced MRI-based studies that have been shown here are not routinely used in the presurgical evaluation of TLE but serve more as anatomical studies of the alterations of the brain in epilepsy.
Wagner et al. have compared the standard visual analysis carried out by an experienced neuroradiologist to the morphometric MRI analysis for detecting focal cortical dysplasia type II (FCD II) and found that the combined use of both approaches provides additional diagnostic sensitivity for this subtle lesion; since the detection of FCDs can significantly improve the postsurgical outcome, his group apply the morphometric analysis in all patients with negative MRI after standard visual inspection [86].
On the other hand, studies on DTI, spectroscopy and relaxometry reported different results so they still lack true clinical validity.
Another consideration arises from the fact that many studies rely on average structure differences between TLE patients and control subjects and cannot be applied to individual patients. For example, whereas there is evidence that TLE patients have extratemporal structural abnormalities, it is unclear whether these abnormalities can guide surgical therapy.
Functional imaging
Functional MRI
Mapping brain functions has been revolutionized by functional MRI (fMRI), particularly with blood oxygen level-dependent (BOLD) contrast, which theoretically reflects the increased oxygenated blood delivery to a particular cerebral area involved during a specific task.
The main use of fMRI in surgical activity consists in the location of eloquent functions (vision, language, motor and sensory function) for surgical planning. Generally speaking images generated by fMRI can help surgical planning for intracerebral EEG recordings and in turn electrodes can better map a certain function on the cerebral surface [11].
With regards to TLE, fMRI has demonstrated a reorganization of language localization and this finding has also been demonstrated as language network reorganization with DTI and VBM studies, with involvement of the nondominant hemisphere [87,88]. This right reorganization has been related to a potential early (congenital) insult to the left hemisphere [89]. Moreover, after ATL patients with left-TLE seem to show greater bilateral fMRI activation in the inferior/middle frontal gyri and a stronger connectivity between these areas compared with patients with right- TLE. Interestingly, preoperative fMRI activation in the left middle frontal gyrus for verbal fluency in left TLE seems to be predictive of naming decline after ATL [90].
In a study by Binder et al., preoperative language fMRI has been shown to be more useful than the Wada test for identifying patients at high risk for verbal memory decline prior to left anterior temporal lobectomy [91].
The link between neocortical language and temporo-mesial memory region has been explored with fMRI in children with left-sided focal epilepsy and it has demonstrated that an atypical language lateralization is advantageous for verbal memory performance. In other words, verbal memory function in children with left-sided focal epilepsia provides a better idea of language lateralization than handedness and side of epilepsy [92].
Language fMRI activation may predict verbal memory outcome in left mesial TLE, the activation being greater in patients with severe verbal memory decline after surgery [93].
Limits exist for fMRI: variable paradigms used to explore a certain cerebral function, thresholds used to display data and spatial and temporal resolution limitation. Expertise both in neuropsychology and neuroradiology are therefore mandatory, especially in preoperative assessment of TLE and further validation with electrocorticography and postoperative follow-up is still needed.
Simultaneous EEG & fMRI
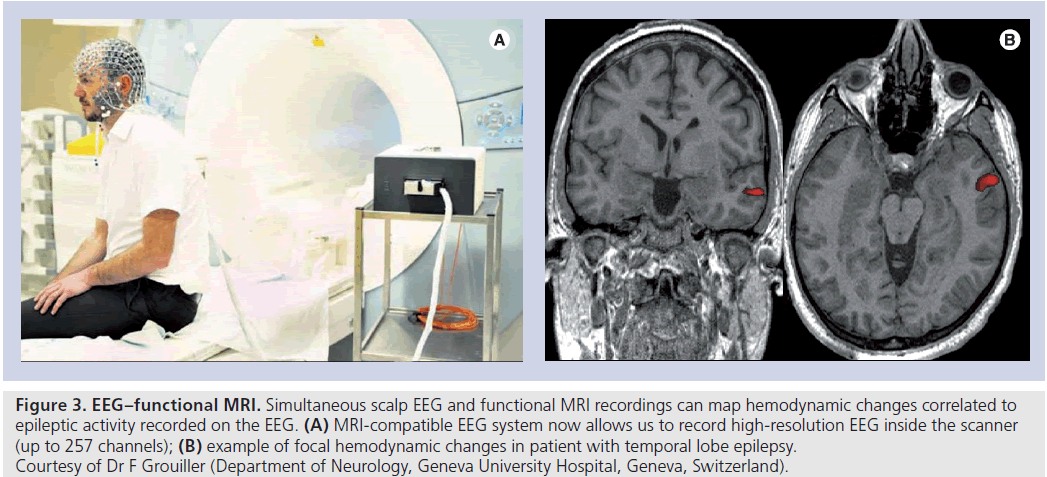
Simultaneous scalp EEG and fMRI maps hemo dynamic changes related to epileptic activity recorded on the EEG (Figure 3) [94]. The procedure is safe and the interaction between the EEG and fMRI equipment (artifacts) can be corrected to obtain data of high quality for analysis. The technique can provide a good spatial and temporal resolution of propagation pattern of epileptic activity [95–97] and could be useful in patients where clinical data, EEG and imaging are not concordant [98,99] and can provide further information on intracranial electrode placement [100,101]; sometimes EEG–fMRI studies of spike-correlated BOLD changes are inconclusive because of the absence of spikes during fMRI or the absence of significant spike-related BOLD changes.
Nevertheless, Grouiller et al. showed that it is possible to create an epilepsy-specific voltage map on a scalp EEG regardless of presence of discharges during simultaneous recording (correlating averaged interictal epileptiform discharges during long-term monitoring outside the MRI scanner with EEG recordings within the MRI scanner). This map was then correlated with BOLD hemodynamic changes in an fMRI (topography-related hemodynamic changes). The concordance between voltage changes and BOLD signals was better in patients with lateral temporal and extratemporal neocortical epilepsy compared with mesial/polar temporal epilepsy allowing good targeting for resection or implantation of intracranial electrodes [102].
Figure 3.EE G–functional MRI. Simultaneous scalp EEG and functional MRI recordings can map hemodynamic changes correlated to epileptic activity recorded on the EEG. (A) MRI-compatible EEG system now allows us to record high-resolution EEG inside the scanner (up to 257 channels); (B) example of focal hemodynamic changes in patient with temporal lobe epilepsy. Courtesy of Dr F Grouiller (Department of Neurology, Geneva University Hospital, Geneva, Switzerland).
In one patient suffering from a TLE, Vulliemoz et al. showed also a good correlation between interictal epileptiform discharges on intracranial EEG and hemodynamic changes on an fMRI [103]. This first study on simultaneous intracranial EEG and fMRI in humans is conceptually important as it confirmed that the presence of local hemodynamic changes correlated with very focal epileptic activity.
A group has compared interictal epileptic discharge- related hemodynamic BOLD changes, intracranial recordings and postsurgical outcome in patients with focal epilepsy and focal cortical dysplasia and found fMRI–EEG to provide additional information about seizure onset zone location but that in more widespread distributed interictal epileptic discharge-related hemodynamic changes the seizure onset zone location value and postsurgical outcome were poor [104].
Recently some researchers focused their attention on the brain connectivity in TLE explored by fMRI during both cognitive tasks and resting state. Hippocampal connectivity measured by interictal resting state fMRI has been found to increase linearly after 10 years of TLE duration, maybe because of the controlateral hippocampus exerting more influence on the EZ on the contralateral side [105].
Other authors have studied the resting state functional connectivity in 22 MTS-TLE patients and showed that basal functional connectivity is increased in the nonepileptic side, which could be used as a localizer for the EZ [106]. The same authors quantified functional connectivity in the resting state by fMRI (which should reflect spontaneous neuronal activity) and made a correlation with intracranial EEG in five patients with TLE during the interictal period. Intracranial recordings found functional connectivity in regions affected by epileptiform activity (compared with nonaffected zones), but BOLD signals showed the opposite pattern. To date, functional connectivity measurement by BOLD and iEEG does not give clinically useful information in TLE [107]. Results from functional connectivity studies during cognitive tasks are also inconclusive [11].
At the moment only a few small studies are available, understanding pathological brain networks requires replicable data and results must be assessed at the individual level to estimate any clinical benefit.
PET
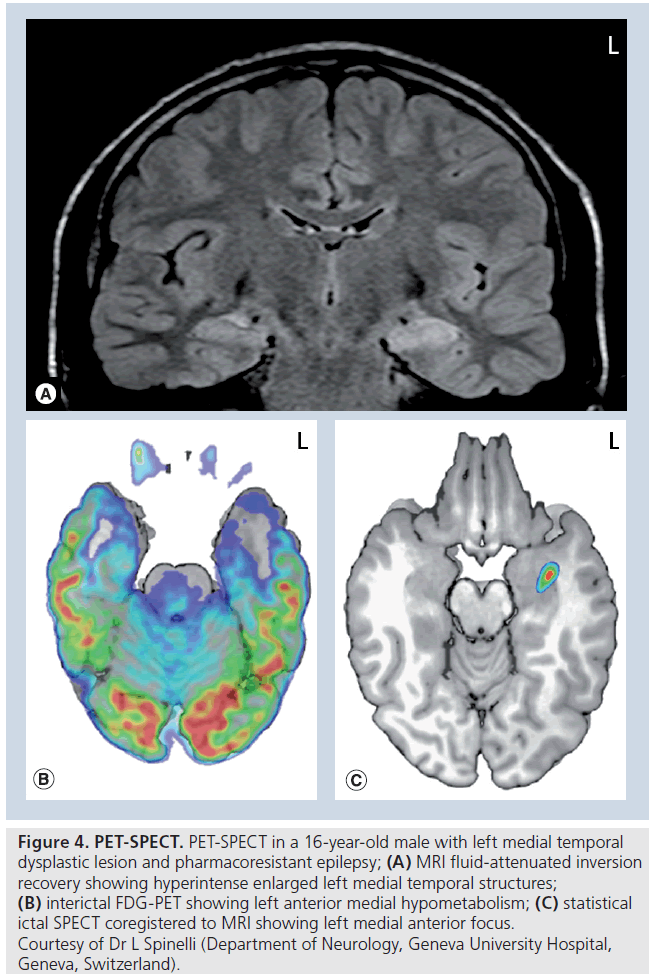
PET coupled to a CT or MRI allows assessment of cerebral metabolism or a certain chemical flux by using tracers labeled with a positron-emitting isotope. The most used tracer is FDG, which gives a good estimation of oxygen uptake and phosphorylation (usually but not always linearly related to brain oxygen utilization) FDG-PET is not readily available in many hospitals and it is mainly obtained during the interictal state; the hallmark of an interictal PET-CT for TLE (and other forms of focal epilepsy) is an area of hypometabolism that may involve more than the epileptogenic area (Figure 4B). It has been shown that the seizure onset better matches the margin of the hypometabolic area than the center [108]. PET-CT can be particularly useful for surgical planning of intracranial EEG in TLE patients with normal structural MRI and inconsistent video-EEG [109]. Furthermore, examining a posteriori the standard structural MRI in patients showing an area of hypometabolism on FDGPET, can also reveal some structural abnormalities previously not seen [110].
FDG-PET improves the detection of cortical abnormalities such as cortical dysplasia if coupled with MRI [111]. A hypometabolic area on FDG-PET correlates with a good-to-excellent outcome after resection surgery in TLE if it is ipsilateral to the lobe to resect [112]. Coregistration of FDG-PET and MRI is required to obtain a more precise spatial localization, and combined PET-MRI systems are promising advances in the field [113]. With regard to TLE, the extent of the resection of the hypometabolic area relates to the surgical outcome: the greater the extent of resection of hypometabolic area, the better the surgical outcome [114].
MRI-negative TLE patients also benefit from FDG-PET. Patients who were operated on on the basis of the PET without a visible lesion on a structural MRI had a similar outcome to patients with a lesion. There may be also a different pattern of hypometabolism between patients with MTS and patients without MTS on structural MRI: both MTS-positive and -negative patients had lateralized temporal hypometabolism compared with healthy control subjects, but in patients with MTS, hypometabolism was rather anteroinferomesial, while in patients without MTS the hypometabolism was seen more infe rolaterally, thus probably involving the neocortical temporal cortex more [115–117].
Other tracers have been developed, such as 11C-flumazenil (theoretically binding the central benzodiazepine receptor) and 11C-a-methyl-ltryptophan (AMT), in order to show cerebral abnormalities not visible on structural MRI, but their utility in clinical practice is not yet established [118,119].
A reduced level of GABA receptor binding has been demonstrated in the epileptic focus compared with the contralateral homotopic region and in the remaining neocortex [120]. Reduced binding of 11C-flumazenil has also been seen in frontal epilepsy patients [121]. In a study that compared 11C-flumazenil to FDG-PET in TLE patients, the first was found to have a more localized decreased density than the latter, being confined to the temporal lobe ipsilateral to EEG ictal onsets and not involving the extratemporal regions; true neuronal and synaptic loss and diaschisis can explain these differences in metabolic patterns between 11C-flumazenil and FDG [122].
Good concordance was found between HS on structural MRI and low 11C-flumazenil binding that was confined to the affected hippocampus [123]. Controversy still exists regarding the role of this tracer in the work-up of temporal and extratemporal epilepsy [124,125].
Figure 4.PET-SPECT. PET-SPECT in a 16-year-old male with left medial temporal dysplastic lesion and pharmacoresistant epilepsy; (A) MRI fluid-attenuated inversion recovery showing hyperintense enlarged left medial temporal structures; (B) interictal FDG-PET showing left anterior medial hypometabolism; (C) statistical ictal SPECT coregistered to MRI showing left medial anterior focus. ƒ Courtesy of Dr L Spinelli (Department of Neurology, Geneva University Hospital, Geneva, Switzerland).
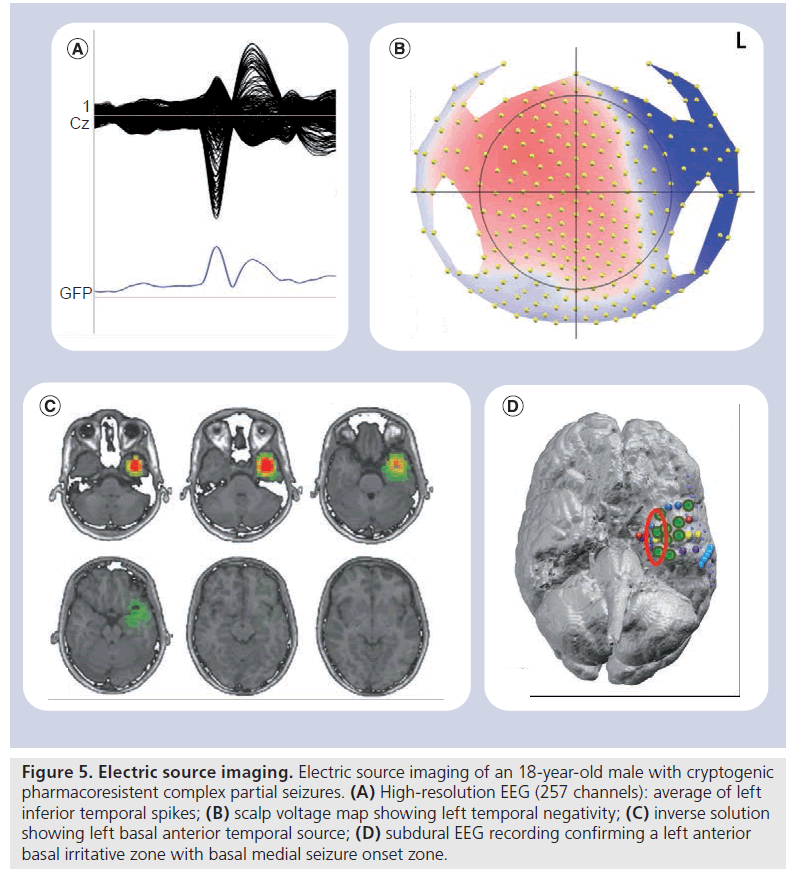
Figure 5.Electric source imaging. Electric source imaging of an 18-year-old male with cryptogenic pharmacoresistent complex partial seizures. (A) High-resolution EEG (257 channels): average of left inferior temporal spikes; (B) scalp voltage map showing left temporal negativity; (C) inverse solution showing left basal anterior temporal source; (D) subdural EEG recording confirming a left anterior basal irritative zone with basal medial seizure onset zone
11C-a-methyl-l-tryptophan is a ligand that reflects the brain serotonin synthesis [126] and an increase of its uptake should indicate the epileptic focus, especially when more than one focus is present, such as in tuberous sclerosis [127]. High AMT uptake in epileptic foci seems to be related to the accumulation of convulsant metabolites in the kynurenine pathway of tryptophan metabolism, especially quinolinic acid [128,129].
In one recent study, increased AMT uptake predicted type IIB cortical dysplasia (with balloon cells) among children with intractable epilepsy and a good surgical outcome, but the subgroup of children with normal histopathology and increased uptake had poor surgical outcome [130]. In another study, up to 25% of children with refractory focal epilepsy and normal structural MRI had an increased uptake in AMT-PET that correlated with epileptic focus [119].
AMT-PET sensitivity is almost 70% while its specifitity is almost 100%, suggesting that a cerebral area uptaking AMT is most likely the epileptic focus that needs to be resected. AMT-PET’s utility is limited as AMT is labeled with 11C, which has a half-life of only 20 min and has to be synthesized on site, in other words, restricted to selected centers [127].
SPECT
SPECT, with PET, is a noninvasive technique for functional imaging, which mainly assesses regional cerebral blood flow (cRBF) through tracer uptake ratios. The basic assumption in epilepsy evaluation is that the increased neuronal activity occurring during seizures is associated with increased cerebral metabolism, and thus with increased cRBF. To assess cRBF with SPECT, radiolabeled tracers such as iodine-123 and technetium-99 are available: the small molecular size and their lipophilicity allow tracers to rapidly cross the intact blood–brain barrier, to be distributed proportionally to blood flow and to be retained in the brain for enough time to permit image acquisition [131]. The most used tracers in epilepsy assessment are 99mTc-hexamethylpropyleneamine-oxime and 99mTc-ethyl-cysteinate-dimer, reaching peak uptake in the brain within 2 min after injection and fixing to brain tissue for 2–4 h without redistribution.
Several studies demonstrated the superiority of ictal SPECT to interictal SPECT, particularly in patients with TLE, to localize or lateralize the epileptic focus [132–134]. However, one of the main limits of ictal SPECT is the poor temporal resolution, which may detect not only the EZ but also the propagation pattern (which is not necessarily removed surgically) even if the tracer is injected immediately [135,136].
Even though its results may indicate the EZ to resect, SPECT is also used for surgical planning of intracerebral electrode placement and to inform on the possible secondary spread of ictal activity [137].
Interictal SPECT localizes or lateralizes the EZ in <50% and today is used only in combination with ictal SPECT (comparison of ictal and interictal SPECT) and MRI (then also known as substraction ictal single-photon emission CT coregistered to MRI [SISCOM]; Figure 4C).
This approach has been proven to be superior to visual assessment only, especially in patients with normal structural MRI and discordant video-EEG [138–141].
SISCOM abnormality localized to the resection site has prognostic value given that it is associated to Engel class I outcome (free of disabling seizure) in patients with nonlesional TLE undergoing anterior temporal lobectomy [142]. Other predictors of Engel class I outcome in this study were the absence of contralateral or extratemporal interictal epileptiform discharges and subtle nonspecific MRI findings in the mesial temporal lobe. Repeated ictal SPECT with SISCOM analysis is helpful for localizing the EZ in patients with partial epilepsy who had a nonlocalized first ictal SPECT; the localizability of ictal SPECT depends on an early injection and a localizing ictal EEG pattern at the time of injection [143].
Localizing value and prognostic value (in terms of postsurgical seizure freedom) of SPECT studies has been demonstrated by a statistical analysis that also included subjects without epilepsy (statistical ictal SPECT coregistered to MRI) to determine whether the ictal-interictal substraction difference (SISCOM) is statistically different from the expected random variation between two SPECT studies [144].
Multimodal coregistration (MRI, FDG-PET or ictal/interictal SPECT) in pediatric pharmacoresistant epilepsy (both extratemporal and temporal) significantly improves focus localization. The surgical outcome was always almost excellent if all localizing techniques showed concordant results [145].
Electric source imaging
Electric source imaging (ESI) is an emerging technique that allows determination of the locations of current sources in the brain, based on EEG recordings. In epilepsy presurgical workup ESI provides 3D images of the source, and thus localizes precisely an EZ in particular when performed with a high number of channels and coupled with the patient’s MRI (Figure 5).
This has recently been shown by Brodbeck et al. in a prospective study including 152 patients with refractory epilepsy (temporal and extratemporal) where the ‘standard’ presurgical work-up (MRI, PET and SPECT) was compared with ESI obtained from high-resolution EEG (128–256 channels) and coregistered with the individual MRI or a template head model. Sensitivity and specificity of ESI was reported as 84 and 88%, respectively, globally superior or equal to structural MRI, PET and ictal/interictal SPECT. Its sensitivity and specificity decreased in patients that were explored with low-resolution ESI (less than 32 channels) and/or with a template head model. No major differences were observed between patients with TLE and those with extratemporal epilepsy undergoing high-resolution EEG/individual MRI ESI, suggesting that this tool can be used in all groups of patients with focal drug-resistant epilepsy [146]. Moreover, nonlesional (temporal- and extratemporal lobe) foci are correctly localized with ESI, as well as those symptomatic of large lesions with heterogenous tissue composition [147,148]. Despite being less widespread than its magnetoencephalography (MEG) source localization counterpart, and extensively validated mostly by one group, this technique has very interesting perspectives in presurgical epilepsy monitoring as it can be carried out at the bedside, with current equipment development allowing high-density long-term recordings. In addition, EEG, contrary to MEG, can be recorded simultaneously to fMRI, and is therefore a more versatile tool for multimodal imaging.
Magnetic source imaging
MEG is another noninvasive method based on neurophysiological signals to study focal epilepsy [149,150]. MEG maps mainly allow visualization of interictal activity from the neocortical areas close to the sensor and are supposedly less sensitive to deep sources, such as mesial temporal and frontal sources, but this is still debated. However, owing to the physics of magnetic currents, MEG does not ‘see’ radial dipoles as they are found in cortical gyri. MEG, like EEG, can distinguish the EZ from propagation sites, owing to its excellent temporal resolution, and help to accurately position intracranial electrodes [151].
In selected cases, MEG can give additional information for intracranial electrodes placements. In a recent study, a few patients (18 out of 77) had additional intracranial electrodes following MEG data and seven of these 18 patients had their EZ detected by electrodes placed thanks to the MEG data [152].
Studies have recently compared the predictive value of the association of FDG-PET, ictal SPECT and magnetic source imaging to intracranial EEG and surgical outcome. Magnetic source imaging showed correlation with intracranial EEG as for localization of epileptic focus, but both PET and ictal SPECT had an additional value for localization: similar to other coregistration studies, there was larger likelihood to benefit from surgery, if all three modalities were concordant [153,154].
Conclusion
The presurgical epilepsy work-up requires a multidisciplinary consensus for defining the EZ localization and tailoring surgical resection in well-selected patients. This consensus comes from a variety of clinical, elecrophysiological and imaging data.
MRI-based techniques and nuclear imaging have revolutionized the TLE epilepsy surgery, allowing better identification of surgical candidates. Advanced neurophysiological methods, such as ESI, EEG–fMRI and MEG, have been found useful for the precise localization of the epileptogenic focus in a number of studies. In recent years, tractography has been introduced and used to map optic pathways to predict visual field defects in TLE surgery. Coregistration allows the fusion of multimodal information (PET, ictal SPECT, MEG or ESI in the patient’s brain) and if all examinations agree, there is a significant chance of postoperative seizure control.
Intracranial EEG evaluation needs to be considered nowadays only in those cases where the side or extent of the EZ is unclear or if its proximity to eloquent cortex cannot be determined by noninvasive methods. Given the availability of these tools, good-to-excellent surgical outcome at 5–10 years is also possible in patients without MRI lesions [155].
Future perspective
The next important steps should be the improvement of existing imaging technologies, such as increasing the spatial resolution, reliable integration of all structural and functional data (both for presurgical decision-making and for image-guided surgery) based on validated algorithms and the development of uniform guidelines to facilitate application, comparison across centers and the ongoing evaluation of clinical yield.

Financial & competing interests disclosure
The work was supported by the Swiss National Science Foundation (33CM30-124089 [MS, SV]; 320030-122073 [KS]). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
*of interest
** of considerable interest
- Polkey C. Report of the National Association of Epilepsy Centers. Presented at: The 3rd European Workshop on Epilepsy Surgery. The Hague Netherlands, 31 August–1 September. Epilepsia (Suppl. 31), (1996).
- Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 45(6), 695–714 (2004).
- Alarcón G, Valentín A, Watt C et al. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J. Neurol. Neurosurg. Psychiatr. 77(4), 474–480 (2006). & Points out why neuroimaging is helpful but not definitive when deciding whether to perform resective surgery for refractory epilepsy.
- Treiman DM. Management of refractory complex partial seizures: current state of the art. Neuropsychiatr. Dis. Treat. 24(6), 297–308 (2010).
- Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 89(2–3), 310–318 (2010).
- Vale FL, Effio E, Arredondo N et al. Efficacy of temporal lobe surgery for epilepsy in patients with negative MRI for mesial temporal lobe sclerosis. J. Clin. Neurosci. 19(1), 101–106 (2012).
- Struck AF, Hall LT, Floberg JM, Perlman SB, Dulli DA. Surgical decision making in temporal lobe epilepsy: a comparison of [18F] FDG-PET, MRI, and EEG. Epilepsy Behav. 22(2), 293–297 (2011).
- McLachlan RS, Nicholson RL, Black S, Carr T, Blume WT. Nuclear magnetic resonance imaging, a new approach to the investigation of refractory temporal lobe epilepsy. Epilepsia 26(6), 555–562 (1985).
- Lehéricy S, Semah F, Hasboun D et al. Temporal lobe epilepsy with varying severity: MRI study of 222 patients. Neuroradiology 39(11), 788–796 (1997).
- Kuzniecky R, de la Sayette V, Ethier R et al. Magnetic resonance imaging in temporal lobe epilepsy: pathological correlations. Ann. Neurol. 22(3), 341–347 (1987).
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nat. Rev. Neurol. 6(10), 537–550 (2010).
- Fauser S, Schulze-Bonhage A, Honegger J et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain 127(Pt 11), 2406–2418 (2004).
- Blümcke I, Vinters HV, Armstrong D, Aronica E, Thom M, Spreafico R. Malformations of cortical development and epilepsies: neuropathological findings with emphasis on focal cortical dysplasia. Epileptic Disord. 11(3), 181–193 (2009).
- Tassi L, Meroni A, Deleo F et al. Temporal lobe epilepsy: neuropathological and clinical correlations in 243 surgically treated patients. Epileptic Disord. 11(4), 281–292 (2009). & Neuropathologic and clinical study of 243 patients with temporal lobe epilepsy, including tumors, cortical abnormalities, hippocampal sclerosis and nonlesional lobe epilepsy.
- Bruton CJ. The Neuropathology of Temporal Lobe Epilepsy. Oxford University Press, Oxford, England (1988).
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 89(3), 499–530 (1966).
- Bronen RA, Fulbright RK, Kim JH, Spencer SS, Spencer DD, al-Rodhan NR. Regional distribution of MR findings in hippocampal sclerosis. Am. J. Neuroradiol. 16(6), 1193–1200 (1995).
- Colombo N, Salamon N, Raybaud C, Ozkara C, Barkovich AJ. Imaging of malformations of cortical development. Epileptic Disord. 11(3), 194–205 (2009).
- Tassi L, Colombo N, Cossu M et al. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain 128(Pt 2), 321–337 (2005).
- Rémi J, Vollmar C, de Marinis A et al. Congruence and discrepancy of interictal and ictal EEG with MRI lesions in focal epilepsies. Neurology 77(14), 1383–1390 (2011).
- Knake S, Triantafyllou C, Wald LL et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology 65(7), 1026–1031(2005).
- Strandberg M, Larsson EM, Backman S, Källén K. Pre-surgical epilepsy evaluation using 3T MRI. Do surface coils provide additional information? Epileptic Disord. 10(2), 83–92 (2008).
- Henry TR, Chupin M, Lehéricy S et al. Hippocampal sclerosis in temporal lobe epilepsy: findings at 7 T¹. Radiology 261(1), 199–209 (2011). & Ultra-high-field MRI study of subregional abnormalities in hippocampal sclerosis, showing Ammon horn atrophy, dentate gyrus atrophy, paucity of hippocampal digitations and malrotation.
- Hanamiya M, Korogi Y, Kakeda S et al. Partial loss of hippocampal striation in medial temporal lobe epilepsy: pilot evaluation with high-spatial-resolution T2-weighted MR imaging at 3.0 T. Radiology 251(3), 873–881 (2009).
- Ugurbil K, Adriany G, Andersen P et al. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn. Reson. Imaging 21(10), 1263–1281 (2003).
- Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc. Natl Acad. Sci. USA 104(28), 11796–11780 (2007).
- Prudent V, Kumar A, Liu S, Wiggins G, Malaspina D, Gonen O. Human hippocampal subfields in young adults at 7.0 T: feasibility of imaging. Radiology 254(3), 900–906 (2010).
- Garbelli R, Zucca I, Milesi G et al. Combined 7-T MRI and histopathologic study of normal and dysplastic samples from patients with TLE. Neurology 76(13), 1177–1185 (2011).
- Madan N, Grant PE. New directions in clinical imaging of cortical dysplasias. Epilepsia 50(Suppl. 9), S9–S18 (2009).
- Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain 115(Pt 4), 1001–1015 (1992).
- Bartolomei F, Khalil M, Wendling F et al. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiologic and volumetric study. Epilepsia 46(5), 677–687 (2005). & Quantitative volumetric MRI study of entorhinal cortex and intracerebral recordings demonstrating the epileptogenicity of the structure in temporal lobe epilepsy.
- Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage 11(6 Pt 1), 805–821 (2000).
- Scanlon C, Mueller SG, Tosun D et al. Impact of methodologic choice for automatic detection of different aspects of brain atrophy by using temporal lobe epilepsy as a model. Am. J. Neuroradiol. 32(9), 1669–1676 (2011). & Overview and comparison of three MRI automated methods for evaluation of structural changes in the brain in temporal lobe epilepsy.
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9(2), 179–194 (1999).
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9(2), 195–207 (1999).
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49(5), 741–757 (2008).
- Focke NK, Yogarajah M, Symms MR, Gruber O, Paulus W, Duncan JS. Automated MR image classification in temporal lobe epilepsy. Neuroimage 59(1), 356–362 (2012).
- Bonilha L, Edwards JC, Kinsman SL et al. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia 51(4), 519–528 (2010).
- Eriksson SH, Thom M, Symms MR et al. Cortical neuronal loss and hippocampal sclerosis are not detected by voxel-based morphometry in individual epilepsy surgery patients. Hum. Brain Mapp. 30(10), 3351–3360 (2009).
- Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Voxel-based morphometry of sporadic epileptic patients with mesiotemporal sclerosis. Epilepsia 51(4), 506–510 (2010).
- Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxelbased morphometry study. Neurology 71(6), 419–425 (2008).
- Bonilha L, Halford JJ, Rorden C, Roberts DR, Rumboldt Z, Eckert MA. Automated MRI analysis for identification of hippocampal atrophy in temporal lobe epilepsy. Epilepsia 50(2), 228–233 (2009).
- Salmenpera TM, Simister RJ, Bartlett P et al. High-resolution diffusion tensor imaging of the hippocampus in temporal lobe epilepsy. Epilepsy Res. 71(2–3), 102–106 (2006).
- Chen Q, Lui S, Li CX et al. MRI-negative refractory partial epilepsy: role for diffusion tensor imaging in high field MRI. Epilepsy Res. 80(1), 83–89 (2008).
- Ashburner J, Hutton C, Frackowiak R, Johnsrude I, Price C, Friston K. Identifying global anatomical differences: deformationbased morphometry. Hum. Brain Mapp. 6(5–6), 348–357 (1998).
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48(2), 371–380 (2009).
- Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Neocortical thinning in ‘benign’ mesial temporal lobe epilepsy. Epilepsia 52(4), 712–717 (2011).
- Hofman PA, Fitt G, Mitchell LA, Jackson GD. Hippocampal sclerosis and a second focal lesion – how often is it ipsilateral? Epilepsia 52(4), 718–721 (2011).
- Widjaja E, Mahmoodabadi SZ, Snead OC 3rd et al. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia 52(9), 1685–1691(2011).
- Huppertz HJ, Wagner J, Weber B, House P, Urbach H. Automated quantitative FLAIR analysis in hippocampal sclerosis. Epilepsy Res. 97(1–2), 146–156 (2011).
- Thivard L, Adam C, Hasboun D et al. Interictal diffusion MRI in partial epilepsies explored with intracerebral electrodes. Brain 129(Pt 2), 375–385 (2006).
- Guye M, Ranjeva JP, Bartolomei F et al. What is the significance of interictal water diffusion changes in frontal lobe epilepsies? Neuroimage 35(1), 28–37 (2007).
- Gross DW. Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52(Suppl. 4), S32–S34 (2011). & Outline about temporal and extratemporal white matter abnormalities in temporal lobe epilepsy studied by diffusion tensor imaging.
- Wehner T, Lapresto E, Tkach J et al. The value of interictal diffusion-weighted imaging in lateralizing temporal lobe epilepsy. Neurology 68(2), 122–127 (2007).
- Gonçalves Pereira PM, Oliveira E, Rosado P. Apparent diffusion coefficient mapping of the hippocampus and the amygdala in pharmacoresistant temporal lobe epilepsy. Am. J. Neuroradiol. 27(3), 671–683 (2006).
- Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn. Reson. Imaging 20(7), 511–519 (2002).
- Kimiwada T, Juhász C, Makki M et al. Hippocampal and thalamic diffusion abnormalities in children with temporal lobe epilepsy. Epilepsia 47(1), 167–175 (2006).
- Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann. Neurol. 57(2), 188–196 (2005).
- Nguyen D, Vargas MI, Khaw N et al. Diffusion tensor imaging analysis with tract-based spatial statistics of the white matter abnormalities after epilepsy surgery. Epilepsy Res doi:10.1016/j. eplepsyres.2011.02.001 (2011) (Epub ahead of print).
- Rodrigo S, Oppenheim C, Chassoux F et al. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur. Radiol. 17(7), 1663–1668 (2007).
- Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbriafornix in temporal lobe epilepsy. J. Neurosci. 30(3), 996–1002 (2010).
- Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 40(2), 728–737 (2008).
- Afzali M, Soltanian-Zadeh H, Elisevich KV. Tract based spatial statistical analysis and voxel based morphometry of diffusion indices in temporal lobe epilepsy. Comput. Biol. Med. 41(12), 1082–1091 (2011).
- Focke NK, Yogarajah M, Symms MR, Gruber O, Paulus W, Duncan JS. Automated MR image classification in temporal lobe epilepsy. Neuroimage 59(1), 356–362 (2012).
- Govindan RM, Makki MI, Sundaram SK, Juhász C, Chugani HT. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res. 80(1), 30–41 (2008).
- Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 82(2–3), 162–170 (2008).
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 47(8), 1360–1363 (2006).
- Thivard L, Lehéricy S, Krainik A et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage 28(3), 682–690 (2005).
- Riley JD, Franklin DL, Choi V et al. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia 51(4), 536–545 (2010).
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia 49(8), 1409–1418 (2008).
- Yogarajah M, Focke NK, Bonelli SB et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain 133(Pt 8), 2348–2364 (2010).
- Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J. Neurol. Neurosurg. Psychiatr. 80(3), 312–319 (2009).
- Powell HW, Parker GJ, Alexander DC et al. MR tractography predicts visual field defects following temporal lobe resection. Neurology 65(4), 596–599 (2005).
- McDonald CR, Hagler DJ Jr, Girard HM et al. Changes in fiber tract integrity and visual fields after anterior temporal lobectomy. Neurology 75(18), 1631–1638 (2010).
- Winston GP, Daga P, Stretton J et al. Optic radiation tractography and vision in anterior temporal lobe resection. Ann. Neurol. 71(3), 334–341 (2012).
- Jackson GD, Connelly A, Duncan JS, Grünewald RA, Gadian DG. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology 43(9), 1793–1799 (1993).
- Woermann FG, Barker GJ, Birnie KD, Meencke HJ, Duncan JS. Regional changes in hippocampal T2 relaxation and volume: a quantitative magnetic resonance imaging study of hippocampal sclerosis. J. Neurol. Neurosurg. Psychiatry 65(5), 656–664 (1998).
- Mueller SG, Laxer KD, Schuff N, Weiner MW. Voxel-based T2 relaxation rate measurements in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia 48(2), 220–228 (2007).
- Kosior RK, Lauzon ML, Federico P, Frayne R. Algebraic T2 estimation improves detection of right temporal lobe epilepsy by MR T2 relaxometry. Neuroimage 58(1), 189–197 (2011).
- Guye M, Le Fur Y, Confort-Gouny S et al. Metabolic and electrophysiological alterations in subtypes of temporal lobe epilepsy: a combined proton magnetic resonance spectroscopic imaging and depth electrodes study. Epilepsia 43(10), 1197–1209 (2002).
- Capizzano AA, Vermathen P, Laxer KD et al. Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. Am. J. Neuroradiol. 23(8), 1359–1368 (2002).
- Mueller SG, Laxer KD, Cashdollar N, Flenniken DL, Matson GB, Weiner MW. Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy. Epilepsia (4), 355–366 (2004).
- Vermathen P, Laxer KD, Matson GB, Weiner MW. Hippocampal structures: anteroposterior N-acetylaspartate differences in patients with epilepsy and control subjects as shown with proton MR spectroscopic imaging. Radiology 214(2), 403–410 (2000).
- Mueller SG, Ebel A, Barakos J et al. Widespread extrahippocampal NAA/ (Cr+Cho) abnormalities in TLE with and without mesial temporal sclerosis. J. Neurol. 258(4), 603–612 (2011).
- Starck G, Vkhoff-Baaz B, Ljungberg M et al. Anterior to posterior hippocampal MRS metabolite difference is mainly a partial volume effect. Acta Radiol. 51(3), 351–359 (2010).
- Wagner J, Weber B, Urbach H, Elger CE, Huppertz HJ. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain 134(Pt 10), 2844–2854 (2011).
- Powell HW, Parker GJ, Alexander DC et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage 36(1), 209–221 (2007).
- Labudda K, Mertens M, Janszky J, Bien CG, Woermann FG. Atypical language lateralisation associated with right frontotemporal grey matter increases – a combined fMRI and VBM study in left-sided mesial temporal lobe epilepsy patients. Neuroimage 59(1), 728–737 (2012).
- Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia 50(6), 1505–1516 (2009).
- Bonelli SB, Thompson PJ, Yogarajah M et al. Imaging language networks before and after anterior temporal lobe resection: results of a longitudinal fMRI study. Epilepsia 53(4), 639–650 (2012).
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 49(8), 1377–1394 (2008).
- Everts R, Harvey AS, Lillywhite L et al. Language lateralization correlates with verbal memory performance in children with focal epilepsy. Epilepsia 51(4), 627–638 (2010).
- Labudda K, Mertens M, Aengenendt J, Ebner A, Woermann FG. Presurgical language fMRI activation correlates with postsurgical verbal memory decline in left-sided temporal lobe epilepsy. Epilepsy Res. 92(2–3), 258–261 (2010).
- Vulliemoz S, Lemieux L, Daunizeau J, Michel CM, Duncan JS. The combination of EEG source imaging and EEG–correlated functional MRI to map epileptic networks. Epilepsia 51(4), 491–505 (2010). & Study on the benefits and limitations of EEG–functional MRI and EEG sourceimaging: coupling both methods allows mapping of the epileptic network with good spatial and temporal resolution.
- Grova C, Daunizeau J, Kobayashi E et al. Concordance between distributed EEG source localization and simultaneous EEG–fMRI studies of epileptic spikes. Neuroimage 39(2), 755–774 (2008).
- Vulliemoz S, Rodionov R, Carmichael DW et al. Continuous EEG source imaging enhances analysis of EEG–fMRI in focal epilepsy. Neuroimage 49(4), 3219–3229 (2010).
- Groening K, Brodbeck V, Moeller F et al. Combination of EEG–fMRI and EEG source analysis improves interpretation of spike-associated activation networks in paediatric pharmacoresistant focal epilepsies. Neuroimage 46(3), 827–833 (2009).
- Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG–fMRI in the preoperative work-up for epilepsy surgery. Brain 130(Pt 9), 2343–2353 (2007).
- Moeller F, Tyvaert L, Nguyen DK et al. EEG–fMRI: adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. J. Neurol. 73(23), 2023–2030 (2010).
- Salek-Haddadi A, Diehl B, Hamandi K et al. Hemodynamic correlates of epileptiform discharges: an EEG–fMRI study of 63 patients with focal epilepsy. Brain Res. 1088(1), 148–166 (2006).
- Al-Asmi A, Bénar CG, Gross DW et al. fMRI activation in continuous and spike-triggered EEG–fMRI studies of epileptic spikes. Epilepsia 44(10), 1328–1339 (2003).
- Grouiller F, Thornton RC, Groening K et al. With or without spikes: localization of focal epileptic activity by simultaneous electroencephalography and functional magnetic resonance imaging. Brain 134(Pt 10), 2867–2886 (2011). & Shows the correlation between epilepsyspecific voltage changes on scalp EEG and hemodynamic changes on functional MRI, helping localization of epileptogenic zone.
- Vulliemoz S, Carmichael DW, Rosenkranz K et al. Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage 54(1), 182–190 (2011).
- Thornton R, Vulliemoz S, Rodionov R et al. Epileptic networks in focal cortical dysplasia revealed using electroencephalographyfunctional magnetic resonance imaging. Ann. Neurol. 70(5), 822–837 (2011).
- Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou-Khalil B. Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia 52(9), 1741–1749 (2011)
- Bettus G, Bartolomei F, Confort-Gouny S
- Bettus G, Ranjeva JP, Wendling F et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One 6(5), E20071 (2011).
- Alkonyi B, Juhász C, Muzik O et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 87(1), 77–87 (2009).
- O’Brien TJ, Miles K, Ware R, Cook MJ, Binns DS, Hicks RJ. The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. J. Nucl. Med. 49(6), 931–937 (2008).
- Lee KK, Salamon N. [18F] fluorodeoxyglucosepositron- emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. Am. J. Neuroradiol. 30(10), 1811–1816 (2009).
- Salamon N, Kung J, Shaw SJ et al. FDG-PET/ MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 71(20), 1594–1601 (2008).
- Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy: a meta-analysis. Seizure 16(6), 509–520 (2007).
- Kurian M, Spinelli L, Delavelle J et al. Multimodality imaging for focus localization in pediatric pharmacoresistant epilepsy. Epileptic Disord. 9(1), 20–31 (2007).
- Vinton AB, Carne R, Hicks RJ et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain 130(Pt 2), 548–560 (2007).
- Carne RP, Cook MJ, MacGregor LR, Kilpatrick CJ, Hicks RJ, O’Brien TJ. “Magnetic resonance imaging negative positron emission tomography positive” temporal lobe epilepsy: FDG-PET pattern differs from mesial temporal lobe epilepsy. Mol. Imaging Biol. 9(1), 32–42 (2007).
- LoPinto-Khoury C, Sperling MR, Skidmore C et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia doi:10.1111/j.1528-1167.2011.03359.x. (2011) (Epub ahead of print).
- Carne RP, O’Brien TJ, Kilpatrick CJ et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain 127(Pt 10), 2276–2285 (2004). & Prospective study showing that PET scan can identify a subgroup of temporal lobe epilepsy patients with normal structural MRI and that benefit from resective surgery.
- Ryvlin P, Bouvard S, Le Bars D et al. Clinical utility of flumazenil-PET versus [18F] fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain 121(Pt 11), 2067–2081 (1998).
- Wakamoto H, Chugani DC, Juhász C, Muzik O, Kupsky WJ, Chugani HT. a-methyl-ltryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatr. Neurol. 39(3), 181–188 (2008).
- Savic I, Persson A, Roland P, Pauli S, Sedvall G, Widén L. In-vivo demonstration of reduced benzodiazepine receptor binding in human epileptic foci. Lancet 2(8616), 863–866 (1988).
- Savic I, Thorell JO, Roland P. [11C] flumazenil positron emission tomography visualizes frontal epileptogenic regions. Epilepsia 36(12), 1225–1232 (1995).
- Henry TR, Frey KA, Sackellares JC et al. In vivo cerebral metabolism and central benzodiazepine-receptor binding in temporal lobe epilepsy. Neurology 43(10), 1998–2006 (1993).
- Koepp MJ, Labbé C, Richardson MP et al. Regional hippocampal [11C]flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain 120 (Pt 10), 1865–1876 (1997).
- Kaneko K, Sasaki M, Morioka T et al. Pre-surgical identification of epileptogenic areas in temporal lobe epilepsy by 123I-iomazenil SPECT: a comparison with IMP SPECT and FDG PET. Nucl. Med. Commun. 27(11), 893–899 (2006).
- Koepp MJ, Hammers A, Labbé C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology 54(2), 332–339 (2000).
- Diksic M, Nagahiro S, Chaly T, Sourkes TL, Yamamoto YL, Feindel W. Serotonin synthesis rate measured in living dog brain by positron emission tomography. J. Neurochem. 56(1), 153–162 (1991).
- Kagawa K, Chugani DC, Asano E et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with a-[11C]methyl-l-tryptophan positron emission tomography (PET). J. Child Neurol. 20(5), 429–438 (2005).
- Chugani DC, Chugani HT, Muzik O et al. Imaging epileptogenic tubers in children with tuberous sclerosis complex using a-[11C] methyl-l-tryptophan positron emission tomography. Ann. Neurol. 44(6), 858–866 (1998).
- Chugani DC. a-methyl-l-tryptophan: mechanisms for tracer localization of epileptogenic brain regions. Biomarkers Med. 5(5), 567–575 (2011).
- Chugani HT, Kumar A, Kupsky W, Asano E, Sood S, Juhász C. Clinical and histopathologic correlates of 11C-a-methyl-ltryptophan (AMT) PET abnormalities in children with intractable epilepsy. Epilepsia 52(9), 1692–1698 (2011).
- Neirinckx RD, Canning LR, Piper IM et al. Technetium-99m d,l-HM-PAO: a new radiopharmaceutical for SPECT imaging of regional cerebral blood perfusion. J. Nucl. Med. 28(2), 191–202 (1987).
- Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. J. Nucl. Med. 40(5), 730–736 (1999).
- Weil S, Noachtar S, Arnold S, Yousry TA, Winkler PA, Tatsch K. Ictal ECD-SPECT differentiates between temporal and extratemporal epilepsy: confirmation by excellent postoperative seizure control. Nucl. Med. Commun. 22(2), 233–237 (2001).
- Zaknun JJ, Bal C, Maes A et al. Comparative analysis of MR imaging, ictal SPECT and EEG in temporal lobe epilepsy: a prospective IAEA multi-center study. Eur. J. Nucl. Med. Mol. Imaging 35(1), 107–115 (2008).
- Van Paesschen W, Dupont P, Sunaert S, Goffin K, Van Laere K. The use of SPECT and PET in routine clinical practice in epilepsy. Curr. Opin. Neurol. 20(2), 194–202 (2007).
- Van Paesschen W. Ictal SPECT. Epilepsia 45(Suppl. 4), S35–S40 (2004).
- Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain 126(Pt 5), 1103–1111 (2003).
- Ahnlide JA, Rosén I, Lindén-Mickelsson Tech P, Källén K. Does SISCOM contribute to favorable seizure outcome after epilepsy surgery? Epilepsia 48(3), 579–588 (2007).
- Kaiboriboon K, Lowe VJ, Chantarujikapong SI, Hogan RE. The usefulness of subtraction ictal SPECT coregistered to MRI in singleand dual-headed SPECT cameras in partial epilepsy. Epilepsia 43(4), 408–414 (2002).
- O’Brien TJ, So EL, Mullan BP et al. Subtraction SPECT co-registered to MRI improves postictal SPECT localization of seizure foci. Neurology 52(1), 137–146 (1999).
- Dupont P, Van Paesschen W, Palmini A et al. Ictal perfusion patterns associated with single MRI-visible focal dysplastic lesions: implications for the noninvasive delineation of the epileptogenic zone. Epilepsia 47(9), 1550–1557 (2006).
- Bell ML, Rao S, So EL et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia 50(9), 2053–2060 (2009).
- Lee JY, Joo EY, Park HS et al. Repeated ictal SPECT in partial epilepsy patients: SISCOM analysis. Epilepsia 52(12), 2249–2256 (2011).
- Kazemi NJ, Worrell GA, Stead SM et al. Ictal SPECT statistical parametric mapping in temporal lobe epilepsy surgery. Neurology 74(1), 70–76 (2010).
- Kurian M, Spinelli L, Delavelle J et al. Multimodality imaging for focus localization in pediatric pharmacoresistant epilepsy. Epileptic Disord. 9(1), 20–31 (2007).
- Brodbeck V, Spinelli L, Lascano AM et al. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain 134(Pt 10), 2887–2897 (2011). & Prospective study using electric source imaging in the presurgical work-up for focal epilepsy, comparing the method with other methods (MRI, PET and SPECT) and showing its clinical utility.
- Brodbeck V, Spinelli L, Lascano AM et al. Electrical source imaging for presurgical focus localization in epilepsy patients with normal MRI. Epilepsia 51(4), 583–591 (2010).
- Brodbeck V, Lascano A, Spinelli L, Seeck M, Michel CM. Accuracy of electrical source imaging of epileptic spikes in patients with large brain lesions. Clin. Neurophysiol. 120, 679–685 (2009).
- Fischer MJ, Scheler G, Stefan H. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain 128(Pt 1), 153–157 (2005).
- Knowlton RC, Elgavish R, Howell J et al. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: a prospective study. Ann. Neurol. 59(5), 835–842 (2006).
- Agirre-Arrizubieta Z, Huiskamp GJ, Ferrier CH, van Huffelen AC, Leijten FS. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain 132(Pt 11), 3060–3071 (2009).
- Knowlton RC, Razdan SN, Limdi N et al. Effect of epilepsy magnetic source imaging on intracranial electrode placement. Ann. Neurol. 65(6), 716–723 (2009).
- Knowlton RC, Elgavish RA, Limdi N et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann. Neurol. 64(1), 25–34 (2008).
- Knowlton RC, Elgavish RA, Bartolucci A et al. Functional imaging: II. Prediction of epilepsy surgery outcome. Ann. Neurol. 64(1), 35–41 (2008).
- de Tisi J, Bell GS, Peacock JL et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378(9800), 1388–1395 (2011).