Mini Review - Interventional Cardiology (2019) Volume 11, Issue 4
Immune senescence and cardiomyopathy associated with obesity
- Corresponding Author:
- Motoaki Sano
Department of Cardiology
Keio University; School of Medicine
Japan
E-mail: msano@a8.keio.jp
Received Date: August 12, 2019 Accepted Date: August 26, 2019 Published Date: September 02, 2019
Abstract
Obesity, diabetes, and metabolic syndrome are common comorbidities in patients with heart failure with preserved ejection fraction. The molecular mechanisms by which these comorbidities promote LV diastolic dysfunction are complex. This review summarizes research performed in mice showing that excessive saturated fatty acid intake causes LV diastolic dysfunction by accelerating immune senescence and increasing cardiomyocyte membrane stiffness, as well as discussing preventive strategies.
Keywords
Osteopontin ; Immune senescence ; Obesity-induced cardiomyopathy ; Diastolic; dysfunction ; Monounsaturated fatty acids ; Sirt-1 ; Membrane fluidity
OPN, A Biomarker of Aging
Chronic inflammation is involved in many; age-associated chronic disorders, such as; diabetes, cardiovascular disease (CVD),; and heart failure, a phenomenon known as; “inflamm-aging”. Discovery of targets that; suppress “inflamm-aging” could lead to the; development of anti-aging drugs. We have; focused on osteopontin (OPN), since a high; circulating OPN level is associated with an; increased risk of cardiovascular death or; heart failure [1]. Typically, the blood level; of OPN increases with age. Surprisingly,; healthy centenarians have lower OPN levels; than healthy controls in their 70 s [2]. This; observation suggests that lower OPN levels; are associated with successful aging. OPN; activates the immune system and plays an; important role in the wound healing process.; After myocardial infarction, OPN is produced; by M2-like macrophages, and it induces; phagocytosis of dead cells and reparative; fibrosis that promotes scar formation at; the infarct site [3]. However, sustained and; uncontrolled OPN production causes chronic; systemic low-grade inflammation, leading to; development of various diseases associated; with aging. Thus, OPN is a potential; biomarker and target for personalized antiaging; therapies.
Senescence-associated T cells are a major; source of OPN in the elderly
In elderly persons, OPN is constitutively; secreted by senescent CD4 T cells [4]. The; proportion of CD4 T cells showing high; surface expression of programmed cell; death 1 (PD-1) increases with age. These; PD-1hi CD4 T cells are senescent cells,; not functionally inactivated cells. As their; senescence-associated secretory phenotype; (SASP), senescent CD4 T cells show high; secretion of OPN. On the other hand,; senescent CD4 T cells lose their ability to; maximize antipathogenic immune responses,; while suppressing nonessential immune; responses. Accordingly, the immune system; becomes unbalanced as the proportion of; senescent T cells increases. Senescent T cells; showing high secretion of OPN are known; as “senescence-associated T cells”, and these; cells are a major source of circulating OPN; in elderly persons. Because the increase of; senescence-associated T cells unbalances; the immune system, it is considered to be; the basis of immune senescence, which is characterized by impairment of acquired immunity, a; predisposition to inflammation, increased susceptibility; to autoimmune disease.
Visceral adiposity accelerates immune senescence
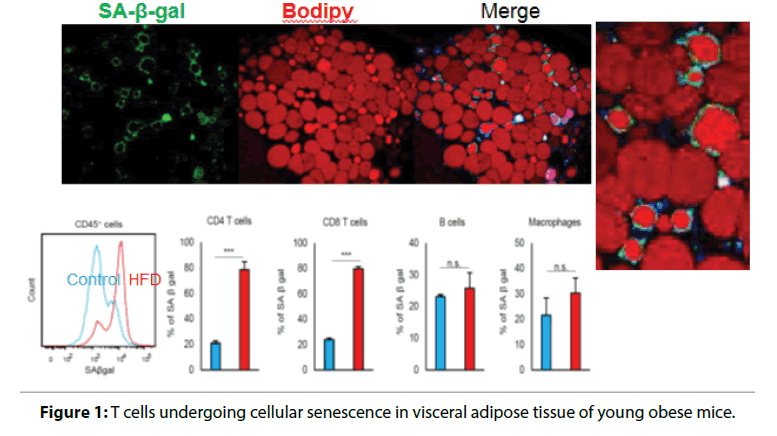
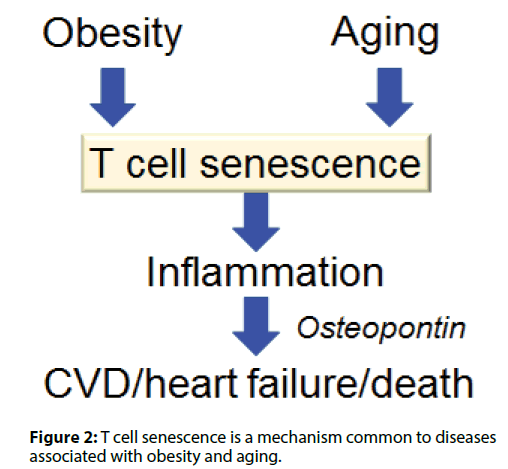
Visceral adiposity accelerates aging by enhancing; inflammation and increases the risk of CVD and heart; failure. We have found that T cells undergo cellular; senescence in obese visceral adipose tissue (VAT) [5,6].; When mice were fed either a high fat diet (HFD) or a; control diet containing less fat, the HFD caused weight; gain, fat deposition in VAT, impaired glucose tolerance,; and insulin resistance. F4/80+ CD11b+macrophages; are increased in obese VAT, indicating a shift of; macrophage polarity to the proinflammatory; phenotype. Macrophages were localized to crown-like; structures in VAT, which are a histologic hallmark of; chronic inflammation, and circulating OPN levels; were increased in HFD-fed obese mice. Figure 1 shows; identification of cells undergoing senescence in the VAT; of obese mice by detection of senescence-associated; β-galactosidase activity. The senescence-associated; β-galactosidase-positive cells were found to be T cells; invading the region around necrotic adipocytes. The; HFD induced accumulation of PD-1hi CD4+ T cells; in VAT, and more than half of VAT CD44 high CD4+; T cells expressed PD-1 in 18-week-old HFD-fed mice.; Senescent T cells in VAT showed the same phenotypic; profile as senescent T cells from aged mice, including; predominance of OPN production, positivity for; senescence-associated β-galactosidase, and elevated; γ-H2AX expression, indicating greater genetic stress.; These results suggested that visceral adiposity is linked; to T cell senescence independently of aging. We found; that CD153 expression defines a unique PD-1hi CD4+; T cell population with senescent features and high OPN secretion. These cells did not exist in the adipose tissue; of young lean mice. To confirm whether CD153+ PD-; 1hi CD4+ T cells produce OPN in the VAT of HFDfed; obese mice, we investigated EGFP-Spp1 knockin; reporter mice fed the HFD. In this mouse model,; cells with high Spp1 gene transcriptional activity can; be identified as GFP-positive cells. Among CD4+ T; cells in the VAT, GFP was almost exclusively expressed; by the PD-1hi cell population, in which expression; of GFP and CD153 were closely correlated. Thus,; CD153+PD-1hi CD4+T cells are the main source of; OPN in the VAT of HFD-fed mice, which implies that; T cell senescence is a common mechanism underlying; diseases associated with obesity and aging (Figure 2).
Figure 1: T cells undergoing cellular senescence in visceral adipose tissue of young obese mice.
Figure 2: T cell senescence is a mechanism common to diseases associated with obesity and aging.
Anti-aging vaccine
In order to eliminate senescence-associated T cells,; a vaccine was developed by targeting an antigen; specifically expressed on the surface of these T cells.; When this vaccine was administered to obese diabetic; mice and induced the production of cytotoxic; antibodies targeting senescence-associated T cells, the; number of these T cells in VAT was decreased, along; with improvement of VAT inflammation and glucose; tolerance. This anti-aging vaccine is a potential tool for; promotion of productive aging.
Differences between obese persons with/without; obesity-associated diseases
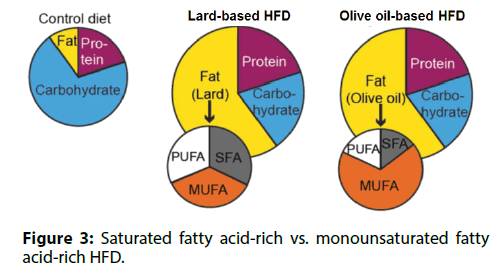
Many overweight or obese persons are healthy. We; investigated whether different types of dietary fat; influenced the susceptibility of T cells to senescence.; Mice were fed either a saturated fatty acid-rich, lardbased; HFD or a monounsaturated fatty acid-rich, olive; oil-based HFD (Figure 3). These two diets had the same; total calories and the same fat content, resulting in equal levels of obesity. Compared to mice fed the lard-based; HFD, mice fed the olive oil-based HFD had less VAT; inflammation and lower insulin levels. In mice receiving; the olive oil-based HFD, senescence-associated T; cells were not detected in VAT and the circulating; OPN level remained low. Based on these findings, we; concluded that the appearance of senescence-associated; T cells in VAT, chronic VAT inflammation, and high; circulating OPN levels are induced by a saturated; fatty acid-rich HFD, but are less likely to occur with a; monounsaturated fatty acid-rich HFD.
Decrease of unsaturated membrane phospholipids; is correlated with diastolic dysfunction
The mechanisms leading to cardiac dysfunction are; multifactorial, and may include generation of excess; ROS by the mitochondria due to fatty acid overload or; accumulation of harmful lipid intermediate metabolites; such as ceramide and diacylglycerol. However, whether; changes in the fatty acid composition of membrane; phospholipids are involved in the development of; cardiac dysfunction associated with fatty acid overload; has not been examined.
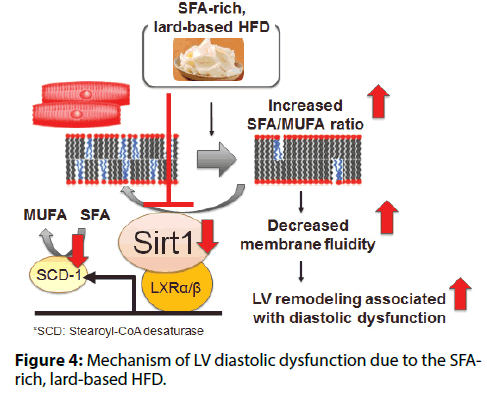
In mice, intake of a lard-based HFD caused cardiac; hypertrophy, interstitial fibrosis, and cardiomyocyte; death, while such changes were rare in mice fed an; olive oil-based HFD [7]. The lard-based HFD caused; diastolic dysfunction, while the olive oil-based HFD; did not. Both HFDs caused a similar increase in the; expression of various genes involved in transcriptional; regulation of mitochondrial function (Tfam, Pparα,; Pgc1α, and Nrf1) and TG turnover (Pnpla2/Atgl,; and Dgat1), as well as PPARα target genes involved; in fatty acid uptake and mitochondrial oxidation; (Cpt1, Cd36, and Acsl1). Both HFDs caused a similar; increase of the total TG, DAG, and ceramide content; in cardiomyocytes. However, there was a difference in; the fatty acid composition of membrane phospholipids.; The lard-based HFD increased the SFA/MUFA ratio of; membrane phospholipids, while this change was not; observed in mice receiving the olive oil-based HFD.; Generally, membrane fluidity decreases when the SFA/; MUFA ratio of membrane phospholipids increases,; leading to impairment of membrane protein function.; Thus, the lard-based HFD increased the SFA/MUFA; ratio in cardiomyocytes, leading to LV remodeling and; diastolic dysfunction (Figure 4).
Sirt1 prevents membrane phospholipid; unsaturation and diastolic dysfunction due to; saturated fatty acid overload
The lard-based HFD was associated with reduced Sirt1; expression in cardiomyocytes, while the olive oil-based; HFD was not. Therefore, we examined the role of; cardiac Sirt1 in cardiomyocyte-specific Sirt1-KO mice; [8]. In mice fed a lard-based HFD, we found that Sirt1; deficiency in cardiomyocytes decreased the expression of; SCD-1 (a rate-limiting enzyme for synthesis of MUFA; from SFA), increased the membrane SFA/MUFA ratio,; and exacerbated LV diastolic dysfunction. Next, we investigated whether activation of Sirt1 by nicotinamide; mononucleotide (NMN) could reverse membrane; phospholipid unsaturation and diastolic dysfunction; in animals on a lard-based HFD. Administration of; NMN increased Sirt1 activity and Scd1 expression,; thereby reducing the membrane SFA/MUFA ratio; and restoring LV diastolic function. Sirt1 regulates; stearoyl-CoA desaturase (SCD-1) expression and thus; contributes to maintaining a low SFA/MUFA ratio by; promoting conversion of SFA to MUFA. The lard-based; HFD impaired this counterregulatory mechanism by suppressing Sirt1 expression, thus increasing the SFA/; MUFA ratio and worsening diastolic dysfunction.
Conclusion
Visceral fat obesity due to SFA overload accelerates the; process of immune senescence. An anti-aging vaccine; targeting senescent T cells has the potential to support; productive aging. Excessive intake of SFA directly; affects the fatty acid composition of cardiomyocyte; membranes and induces diastolic dysfunction. Balanced; intake of MUFA or activation of Sirt1 can ameliorate; diastolic dysfunction due to SFA overload.
References
- Abdalrhim AD, Marroush TS, Austin EE, et al. Plasma osteopontin levels and adverse cardiovascular outcomes in the PEACE trial. PLoS One. 11(6): e0156965 (2016).
- Sanchis-Gomar F, Santos-Lozano A, Pareja-Galeano H, et al. Galectin-3, osteopontin and successful aging. Clin Chem Lab Med. 54(5): 873-7 (2016).
- Shirakawa K, Endo J, Kataoka M, et al. IL (Interleukin)-10-STAT3-galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation. 138(18): 2021-35 (2018).
- Shimatani K, Nakashima Y, Hattori M, et al. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc Natl Acad Sci. 106(37): 15807-12 (2009).
- Shirakawa K, Yan X, Shinmura K, et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 126(12): 4626-39 (2016).
- Shirakawa K, Endo J, Katsumata Y, et al. Negative legacy of obesity. PLoS One. 12(10): e0186303 (2017).
- Yamamoto T, Endo J, Kataoka M, et al. Decrease in membrane phospholipids unsaturation correlates with myocardial diastolic dysfunction. PLoS One. 13(12): e0208396 (2018).
- Yamamoto T, Endo J, Kataoka M, et al. Sirt1 counteracts decrease in membrane phospholipid unsaturation and diastolic dysfunction during saturated fatty acid overload. J Mol Cell Cardiol. 133: 1-11 (2019).