Case Report - Clinical Practice (2021) Volume 18, Issue 6
Immune Thrombocytopenia associated with severe COVID-19 Infection
- Corresponding Author:
- Rasha Awawdeh
Department of Internal Medicine
Mediclinic Parkview Hospital
Dubai, United Arab Emirates
E-mail: Rasha.Awawdeh@mediclinic.ae - McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2, e437-445 (2020).
- Chammard TB, Schepers K, Breurec S, et al. Severe Thrombocytopenia after Zika Virus infection, guadeloupe, 2016. Emerg Infect Dis. 23, 696-698 (2017).
- Rashmi MV, Hamsaveena. Hematological and biochemical markers as predictors of dengue infection. Malays J Pathol. 37, 247-251 (2015).
- Yang M, Ng MH, Li CK. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology. 10, 101-105 (2005).
- Al-Tawfiq JA, Hinedi K, Abbasi S, et al. Hematologic, hepatic and renal function changes in hospitalized patients with Middle East respiratory syndrome coronavirus. Int J Lab Hematol. 39, 272-278 (2017).
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. New England J Med. 382, 1708-1720 (2020).
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395, 507-513 (2020).
- Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta. 507, 174-180 (2020).
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46, 846-848 (2020).
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 99, 1205-1208 (2020).
- Gupta GP, Massague J. Platelets and metastasis revisited a novel fatty link. J Clin Invest. 114, 1691-1693 (2004).
- Jagerschmidt A, Fleury V, Leory MA, et al. Human thrombopoietin structure-function relationships: identification of functionally important residues. Biochem J. 333, 729-734 (1998).
- Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 98, 2720-2725 (2001).
- Henry BM, de Oliveira S, Benoit S, et al. Lippi, Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 58, 1021-1028 (2020).
- Levesque V, Millaire E, Carrier FM, et al. Severe immune thrombocytopenic purpura in critical COVID-19. Intern J Hematol. 112, 746-750 (2020).
- Chen W, Yang B, Li Z, et al. Sudden severe thrombocytopenia in a patient in the recovery stage of COVID-19. Lancet Haematol. 7, e624 (2020).
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med. 381, 1708-1720 (2020).
- Chen W, Li Z, Yang B, et al. Delayed phase thrombocytopenia in patients with coronavirus disease 2019 (COVID-19). Br J Haematol. 190, 179-184 (2020).
- Sahu KK, Siddiqui AD, Rezaei N, et al. Challenges for management of immune thrombocytopenia during COVID-19 pandemic. J Med Virol. 92, 2277-2282 (2020).
- Pavord S, Thachil J, Hunt BJ, et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br J Haematol. 189, 1038-1043 (2020).
- Patel T, Stanton N, Gkikas I, et al. Severe thrombocytopenia secondary to COVID 19. Br Med J Case Rep. 13, e237645 (2020).
Abstract
Serious hematological complications of COVID-19 are well recognized and are often related to dysfunction of coagulation. Several clinical and laboratory degrees of thrombocytopenia have been reported in association with COVID-19, varying from mild cases to severe cases with fatal outcomes. Extreme thrombocytopaenia may correlate with the severity of the infection and several mechanisms have been proposed to account for the low platelet count in this pandemic. We present a case of a 37-year-old male admitted via our emergency department with a ten-day history of classic symptoms of acute lower respiratory tract infection associated with anosmia and ageusia. He had a positive nasal swab PCR for COVID-19. His platelet count was initially normal, 168 × 103/μL (150-400 103/μL) but was followed by a gradual reduction throughout hospitalization to 10 × 103/μL. His thrombocytopaenia was initially managed with steroids and intravenous immunoglobulin therapy. Due to poor response to these treatments, Romiplostim was added and resulted in a slow increase in platelet count to 124 × 103/μL on the day of discharge. Platelet transfusion was not considered because the patient had no bleeding. The increase of platelet count and response to therapy coincided with clinical improvement from COVID-19.
Keywords
COVID-19, immune thrombocytopenia, dyspnoea
Introduction
The clinical presentation of COVID-19 ranges from asymptomatic to severe illness and fatal outcomes. The most common symptoms include fever, dyspnoea, and non-productive cough. COVID-19 has been documented to induce systemic coagulation abnormalities, including pulmonary microvascular thrombosis [1]. ITP has been previously been reported following several viral infections including hepatitis B and C, cytomegalovirus, varicella-zoster, human immunodeficiency virus, Zika Virus [2], dengue [3], SARS-COV [4], and MERS-CoV [5]. In COVID-19, the most common haematological findings are lymphopaenia [6], neutrophilia [7], and eosinopaenia with mild thrombocytopenia [8] and, less frequently, thrombocytosis [9]. There are three mechanisms hypothesized for thrombocytopaenia in COVID-19. Firstly, direct infection of bone marrow cells by the virus and inhibition of platelet synthesis. Secondly, platelet destruction by the immune system and finally, platelet aggregation in the lungs, resulting in microthrombi and platelet consumption [10].
Case Presentation
A 37-year-old male presented to the emergency department with a ten-day history of classic symptoms of acute lower respiratory tract infection, anosmia, and ageusia. He had no history of pyrexias. He had a positive nasal swab PCR for COVID-19. The patient had no significant past medical history or drug history. He was a non-smoker and did not consume alcohol.
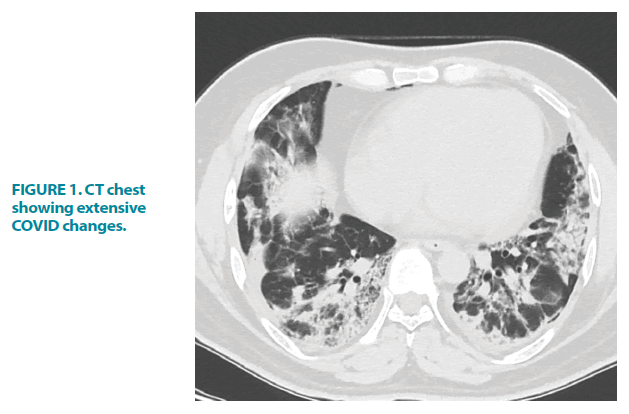
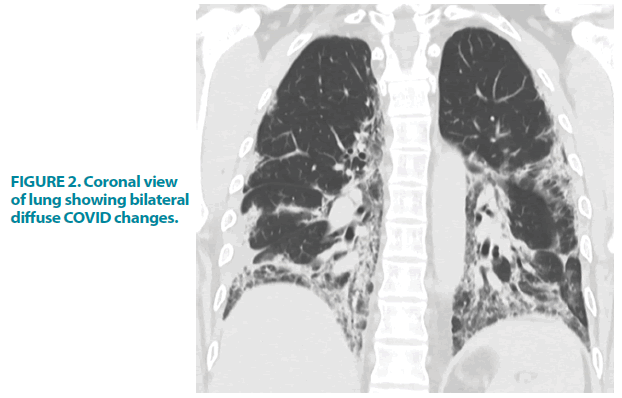
On admission, he was found to be dyspnoeic and hypoxic with an O2 saturation of 88%. This normalized to 7 liters of oxygen/min via a non-rebreathe mask. Throughout his admission, there were neither clinical signs of active bleeding from the skin/ mucus membrane nor lymphadenopathy suggestive of haematological malignancy or other illnesses. Chest X-ray and HRCT scan demonstrated moderate to severe COVID-19 pneumonia FIGURE 1 and FIGURE 2.
Figure 2: Coronal view of lung showing bilateral diffuse COVID changes.
Treatment for his COVID-19 infection included corticosteroid (Dexamethasone 6 mg IV once daily), thromboprophylaxis (Enoxaparin 30 mg SC twice daily), and antiviral (Remdesivir over a course of five days). On the 5th day of admission, there was a gradual decline of the platelets count from normal, 168 × 103/μL (150-400 × 103/ μL), to 38 × 103/μL mandating an increase in IV dexamethasone to 4 mg TID, applied pneumatic compression and cessation of Enoxaparin. The response was very poor with a further decrease in platelet count to 16 × 103/μL over 2 days. Therefore, a three-day course of intravenous immunoglobulin was administered (0.4 mg/ kg daily). There was a negligible response and the platelet count dropped further to 10 × 103/ μL. Romiplostim (250 μg SC on day 1 followed by 500 μg SC on day 2) was initiated and there was a gradual increase in platelets count from 14 × 103/μL to 30 × 103/μL after one week. However, a dip of the platelet counts to 24 × 103/μL occurred which resulted in a further single dose of Romiplostim of 500 μg SC. This resulted in a continuous rise in the platelet count to 120×103/μL at discharge (TABLE 1).
| Drugs | Platelets | WBC | Neutrophil | Lymphocyte | Hb | D-dimers | Ferritin |
|---|---|---|---|---|---|---|---|
| 150.0-450.0 | 4.0-11.0 | 1.80-7.70 | 1.0-4.80 | 13.0-17.5 | 0.0-0.5 | 22-275 | |
| 103/uL | 103/uL | 103/uL | 103/uL | g/dL | ug/mL FEU | ng/mL | |
| Dexamethasone 6mg od | 167 | 8.4 | 7.22 | 0.358 | 15.9 | 0.58 | 1735 |
| Dexamethasone 4mg TID Stop Enoxaparin | 38 | 16 | 14.89 | 0.415 | 14.7 | 0.41 | 1662 |
| IVIG; 0.4 g/kg od for 6 days | 16 | 15.3 | 14.02 | 0.45 | 14.7 | 0.42 | 2151 |
| Romiplostim | 10 | 14.5 | 13.38 | 0.341 | 12.7 | NA | 2067 |
| 2 days later | 14 | 14.8 | 12.83 | 0.331 | 12.6 | NA | 2746 |
| 4 days later | 30 | 10.7 | 9.37 | 0.529 | 11.5 | NA | NA |
| Romiplostim High dose | 24 | 10.6 | 9.41 | 0.519 | 11.4 | NA | 3346 |
| On discharge Day | 120 | 5.9 | 4.6 | 0.973 | 11.1 | 0.36 | 4221 |
TABLE 1. Lab results.
Although the platelet count was extremely low, the patient had no bleeding issues and the haemoglobin was stable throughout the course of the illness. Therefore, platelet transfusion was not required.
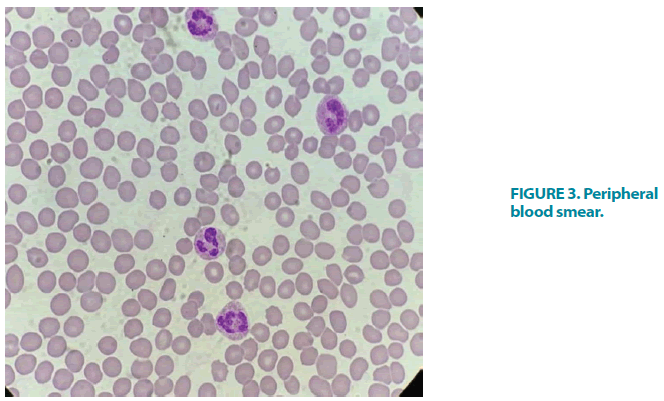
A blood film showed megakaryocytes (FIGURE 3). The patient had hy perferritinaemia, but the degree of lymphopaenia, leukocytosis, and neutrophilia correlated with platelet count better than inflammatory markers. D-dimer levels were almost normal as were PT, PTT, INR, and fibrinogen. Other metabolic blood tests demonstrated newly diagnosed T2DM with HbA1c of 7.8%, normal renal function, and a slight increase in liver enzymes, mainly alanine aminotransferase and aspartate aminotransferase. Further tests were performed to exclude other causes of secondary thrombocytopaenia including DIC, collagen, vascular disease, viral hepatitis, HIV, and other viruses. Hepatitis B and C serology, HIV, and collagen vascular screen were negative. The abdominal US demonstrated the normal size of both the liver and the spleen and no abdominal mass or visible lymphadenopathy.
Discussion
The term “thrombopoietin” was first coined in 1958 to describe the humoral substance responsible for increasing platelet production [11]. Thrombopoietin, the primary regulator of platelet production, is an acidic glycoprotein produced primarily in the liver, kidney, and bone marrow. The biochemistry and structureactivity relationships of thrombopoietin have been carefully evaluated, as have the binding sites to its receptor, the product of the cellular protooncogene c-Mpl [12]. During the acute phase of an inflammatory stimulus, the IL-6 mediator affects thrombopoietin production by increasing thrombopoietin transcription from the liver and leads to a reactive thrombocytosis [13].
Leukocytosis, lymphopenia, and thrombocytopenia are associated with severe COVID-19 infection [14]. The vast majority of severe thrombocytopenia cases involve patients presenting with severe infection [15] and multiple cases of ITP secondary to COVID-19 have been reported [16]. Mild thrombocytopenia has been observed in up to one-third of patients with COVID-19, with even higher rates in patients with severe disease (57.7%) compared with non-severe disease (31.6%) [17]. The lateonset of mild thrombocytopenia (mean time for nadir count from illness onset 28.3 days) for a short duration (mean 4.3 days) has also been reported [18]. However, the majority did not have any severe degree of thrombocytopenia (<20 × 109/L or a sudden drop >50% over 24 hrs-48 hrs) as can be seen in patients with immune thrombocytopenia irrespective of inciting event [19]. The discontinuation of heparin and intermittent pneumocompression has been recommended in the interim guidance in cases with platelet counts <30 × 109/L or 30 × 103/μL [20].
There are multiple mechanisms of severe thrombocytopenia are triggered in severe COVID-19 infection. In the patient we present, their response to steroids was poor and platelets count was almost the same. However, there is an even greater paucity of evidence of immunoglobulin therapy in these cases regarding their effectiveness [21]. It was added to steroid but unfortunately, it was not effective and platelets counts were static in the presence of severe infection. The same has been reported elsewhere although steroids were stopped after 14 days due to concerns over increased risk of infection and mortality [21].
The third line of treatment initiated in the patient we present was Romiplostim daily SC injection for 2 days. Although there was no immediate improvement in platelet count, they started rising 2 days after the second dose of Romiplostim from 14 × 103/μL to 30 × 103/ μL followed by a dip to 24 × 103/μL requiring a third single high dose. This resulted in the continuous slow increase in platelet count over a period of 2 weeks until it finally reached 120 × 103/μL.
Our clinical observation demonstrated the degree of thrombocytopenia correlated with the clinical severity of the COVID-19 infection. The response to therapy and the increase of the platelet count was correlated to clinical improvement and the normalization of the leucocyte, neutrophil, and lymphocytes counts.
Conclusion
Severely ill COVID-19 patients are at high risk of developing significant thrombocytopenia and this may, in turn, lead to complications in form of serious bleeding. Physicians should be aware of early recognition and management of this condition to prevent further morbidity and reduce mortality.