Review Article - Interventional Cardiology (2009) Volume 1, Issue 1
Impact of bleeding complications on outcomes after percutaneous coronary interventions
- Corresponding Author:
- John P Vavalle MD
Duke University Medical Center
Division of Cardiovascular Medicine
Box 31356, Durham, NC 27710-000, USA
Tel: +1 919 681 6197
Fax: +1 919 681 9842
E-mail: jv18@notes.duke.edu
Abstract
Keywords
bivalirudin, bleeding, mortality, percutaneous coronary intervention, transfusion, transradial
Percutaneous coronary interventions (PCIs) are an important part of the treatment for acute ischemic heart disease. Over the last several years, improvements in techniques, instrumentation and anticoagulation strategies have greatly reduced ischemic complications and major adverse cardiac events associated with PCI [1]. With this has come a greater emphasis on understanding the impact of bleeding that results from coronary interventions. Previously underappreciated, it is now widely recognized that there is a stepwise increase in both short- and intermediate-term mortality as bleeding severity worsens [2]. Bleeding has now also become an integral part of evaluating new anticoagulants for use in the cardiac catheterization laboratory.
Bleeding associated with PCI is known to portend significant adverse events and increased mortality [3–6]. However, fully understanding the impact that bleeding has on outcomes presents a number of challenges. For example, there is a lack of consistency among clinical definitions used to define bleeding. Furthermore, bleeding data collected from clinical trials differ from that collected from clinical registries because of the nature of the data collections and varying definitions used. Together, these factors make it challenging to fully understand the impact of bleeding on outcomes.
A number of pharmacologic and procedural strategies can be employed to reduce the risk of bleeding associated with PCI. Many of these have been studied and validated in prospective clinical trials [7–9]. This article will discuss the challenges of defining bleeding events in the literature and review strategies to reduce bleeding risk. In addition, the impact of bleeding and transfusions on outcomes after PCI will be discussed. Ideas for future study to better understand the importance of PCI-related bleeding will conclude this article.
Bleeding definitions used in PCI trials & registries
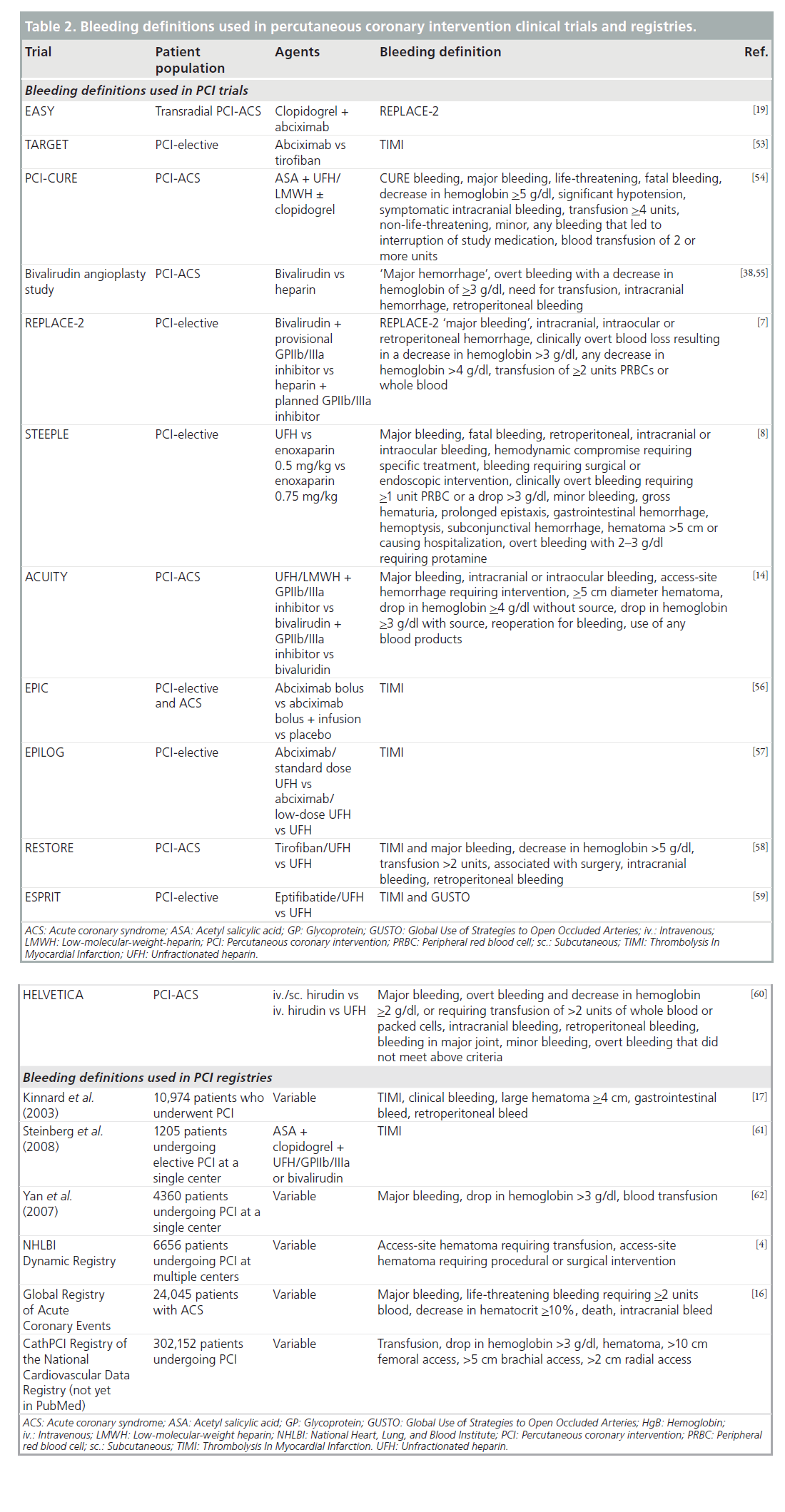
One of the obstacles to fully understanding the impact of bleeding after PCI is the inconsistency in definitions used to define a bleeding event. A number of definitions used to scale the severity of bleeding events have been developed and the reported bleeding rate incidence has been shown to be highly dependent upon the definitions used [10]. Steinhubl et al. analyzed bleeding data from 13 large trials evaluating antithrombotic drugs in acute coronary syndrome (ACS) in over 178,000 patients. They concluded that it is ‘undoubtedly true’ that variations in definitions used to define major bleeding have led to differences in reported rates [11].
▪ Bleeding definitions used in clinical trials
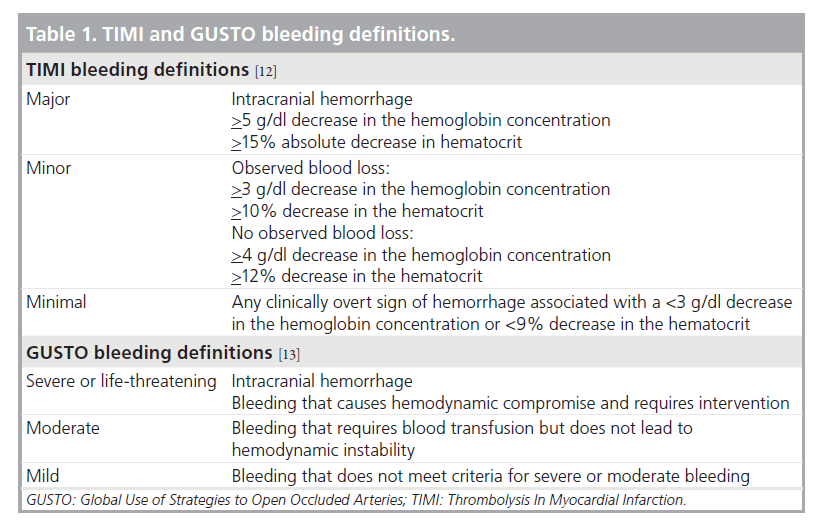
Two commonly used def initions in the past were the Thrombolysis In Myocardial Infarction (TIMI) and Global Use of Strategies to Open Occluded Arteries (GUSTO) bleeding definitions (Table 1) [12,13]. The TIMI scale uses decreases in hemoglobin or hematocrit and intracranial hemorrhage to classify bleeding as minimal, minor or major. The GUSTO scale defines clinical events that stratify bleeding episodes into mild, moderate or severe. While some studies have used either the GUSTO or TIMI definition, others have used both, and yet others have combined selected elements of both scales. Furthermore, some studies have developed their own criteria to define bleeding events, such as in the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events II (REPLACE-2), the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY), the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE), and the Safety and Efficacy of Enoxaparin in Percutaneous Coronary Intervention Patients (STEEPLE) trials [7,8,14,15]. In the 13 trials analyzed by Steinhubl, nine of them used their own definitions, other than TIMI or GUSTO [11].
Table 2 provides a listing of some of the bleeding definitions used in PCI clinical trials and registries. This table illustrates the wide spectrum of bleeding definitions used and demonstrates why bleeding rates can vary so widely based solely on the definitions applied. Very broad definitions will capture a larger number of bleeding events compared with very narrow definitions and will lead to a higher reported incidence of bleeding events. Even within a single clinical trial, bleeding rates may vary depending on the definitions used. For instance, in the REPLACE-2 study, bleeding episodes were reported using the TIMI and REPLACE-2 definitions. This study compared bivalirudin with provisional glycoprotein (GP)IIb/IIIa inhibitor (GPI) versus heparin with planned GPIIb/IIIa in patients undergoing elective or urgent PCI. When the TIMI definitions are used, no difference in TIMI major bleeding is noted between the two arms (0.9 vs 0.6%; p = 0.30); however, when the REPLACE-2 definitions are applied there is significantly less major bleeding in the bivalirudin group (4.1 vs 2.4%; p = <0.001) [7].
Another example of this is found in the STEEPLE trial, comparing two different doses of intravenous enoxaparin with unfractionated heparin (UFH) in patients undergoing PCI. Using the STEEPLE major bleeding definition, there is significantly more bleeding in the UFH group than in either enoxaparin group. If one uses the TIMI major bleeding definition, there is no difference between any of the groups. Furthermore, if one applies the GUSTO moderate or severe definitions, there is a difference in bleeding rates between the UFH group and the low-dose enoxaparin group, but no difference between the enoxaparin groups or between the UFH group and high-dose enoxaparin group [8].
▪ Bleeding definitions used in registries
Another confounder in delineating an accurate rate of bleeding complications comes from the variations in the source data. For example, clinical trial data that report bleeding rates are very different from registry data for several reasons. First, registry data can more accurately represent a ‘real world’ demographic of patients, and often include patients with more comorbidities who are often not candidates for clinical trials. Second, the way in which bleeding events are captured in clinical trials differs from how these events are noted in registries. In clinical trials, patients are often prospectively evaluated for bleeding events by study coordinators or clinicians, while bleeding events reported from registry data are often found through retrospective chart review. Owing to this difference in identifying bleedings, the rate of bleedings in registries are usually based on data elements that are readily identifiable from chart review such as transfusions or surgical interventions [16]. Therefore, variation in patient populations, data capture and definitions all likely lead to differences in sensitivity of detecting bleeding events.
Kinnard et al. reported a three-hospital registry experience on 10,974 patients undergoing PCI from 1991 to 2000 on the incidence and predictors of bleeding after PCI. They used the TIMI bleeding definition and noted major bleeding in 5.4% and minor bleeding in 12.7%, with a blood transfusion given to 5.4% of patients. The majority of bleeding events in both the major and minor bleeding groups were related to vascular access-site hematomas. They also reported the independent risk factors for bleeding, after adjustment for confounders: age, procedural hypotension, intra-aortic balloon pump use, chronic renal insufficiency, systemic hypertension history, and the use of abciximab, which were all independently associated with in-hospital bleeding events [17].
The National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry included a cohort of 6656 patients undergoing PCI enrolled at multiple centers, of which 97% had femoral access. Access-site hematomas requiring blood transfusions occurred in 1.8% of these patients. Older age, lower BMI, female sex, history of renal, cerebrovascular, peripheral vascular or pulmonary disease, and hypertension were significantly associated with hemorrhage [4].
The Global Registry of Acute Coronary Events (GRACE) analyzed data from 24,045 patients with ACS and found the overall major bleeding rate to be 3.9%. Right-heart catheter and PCI were independently associated with an increased risk of bleeding. Among the patients in the registry who had PCI, female sex, advanced age and renal insufficiency were associated with increased bleeding risk. Among all major bleeds recorded in this registry, nearly a quarter (23.8%) of them were vascular access site bleeds [16].
Data from over 300,000 patients undergoing PCI from the National Cardiovascular Data Registry have been used to develop a risk model to predict the risk of in-hospital bleeding after PCI. Bleeding was defined as transfusion, a drop in hemoglobin greater than 3 g/dl, or an accesssite hematoma greater than 10, 5 or 2 cm in the femoral, brachial or radial site, respectively (Table 2). With this definition, the incidence of bleeding was relatively low (2.5%). Significant predictors of bleeding included female sex, age, renal insufficiency, prior PCI, cardiogenic shock, emergent/urgent PCI and chronic obstructive pulmonary disease [18].
As outlined above, the reported incidence of bleeding depends highly on several factors: the data source (clinical trial vs registry), the patients, concomitant medical and procedural strategies (e.g., antithrombotic regimens, vascular access site), and the bleeding definition used. Despite these differences, there are some consistencies across studies. In patients undergoing PCI, the primary site of bleeding is the vascular access site. Older patients, females and patients with renal insufficiency are consistently identified as being at high risk for bleeding complications. These data are valuable in determining strategies to reduce bleeding risk, as will be discussed later.
Impact of bleeding on outcomes
A number of studies have now demonstrated the association between bleeding complications after PCI and adverse outcomes [2–4,6,7,17,19]. Specifically, adverse cardiac ischemic events and death correlate in a stepwise fashion with the severity of the bleeding complications in both the short and intermediate term [2,17].
Data from Kinnaird and colleagues demonstrated that bleeding after PCI was associated with a longer hospital stay (8.9 vs 3.1 days; p < 0.001), and higher in-hospital and 1‑year mortality. The rates of in-hospital mortality for major, minor and no bleeding were 7.5 versus 1.8 versus 0.6%; p < 0.001; and rates for 1‑year mortality were 17.2 versus 9.1 versus 5.5%, respectively. Using multivariate regression analysis, TIMI major bleeding after PCI was an independent predictor of in-hospital mortality [17], but was not associated with 1‑year mortality. Blood transfusion after PCI was associated with both in-hospital and 1‑year mortality. Similarly, Rao et al. examined the relationship between in-hospital GUSTO bleeding and 30‑day and 6‑month mortality in 26,452 patients with ACS from four large randomized control trials. Among patients with periprocedural bleeding, there was a stepwise increase in the risk of 30‑day and 6‑month mortality. The adjusted hazard ratio (HR) for 30‑day mortality was 1.3 for mild bleeding (95% CI: 0.9–1.8), 3.7 for moderate bleeding (95% CI: 2.8–4.9) and 16.5 for severe bleeding (95% CI: 12.0–22.8). For 6‑month mortality, the adjusted HR for mild bleeding was 1.1 (95% CI: 0.9–1.4), for moderate bleeding was 2.6 (95% CI: 2.1–3.3) and for severe bleeding was 10.5 (95% CI: 8.0–13.7) [2].
This finding was corroborated by findings from the NHLBI Dynamic Registry. Patients experiencing access-site hematomas requiring transfusion were nine-times more likely to die within the hospital (1.2 vs 9.9%; OR: 9.32; 95% CI: 4.93–17.63) and 4.5-times more likely to die within 1 year (4.7 vs 18.8%; HR: 4.46; 95% CI: 2.83–7.02) [4]. In the REPLACE-2 trial, which compared UFH with planned GPIIb/IIIa to bivalirudin plus provisional GPIIb/IIIa, 3.2% of the 6010 patients experienced major bleeding. Mortality rates at 30 days, 6 months and 1 year were all significantly higher in those with major hemorrhage compared with those without. Major bleeding was found to be an independent predictor of 1‑year mortality with an OR of 2.66 (95% CI: 1.44–4.92; p = 0.002) [5].
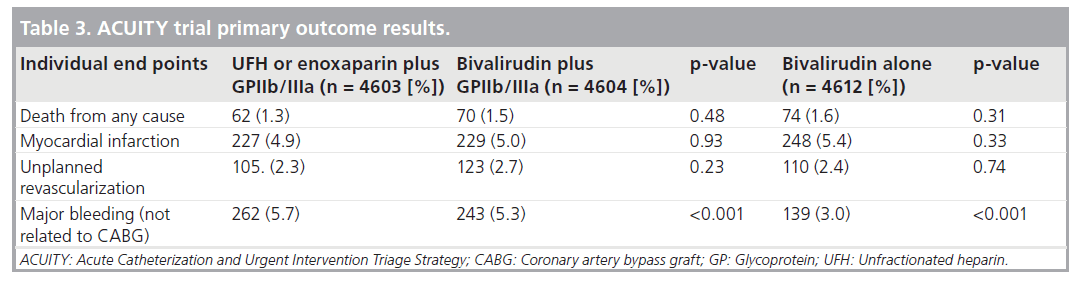
The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial compared bivalirudin alone versus bivalirudin plus a GPI or heparin plus a GPIIb/IIIa in the management of ACS [14]. Major bleeding, according to the definitions defined for this study and listed in Table 2, occurred in 644 patients out of 13,819 (4.7%). An analysis on the impact of major bleeding on 30-day clinical outcomes and mortality from this trial are consistent with other studies in showing a higher rate of 30‑day mortality in those with major bleeding versus those without (7.3 vs 1.2%; p < 0.0001) [6].
Furthermore, major bleeding was found to be the strongest independent predictor of 30‑day mortality (OR: 7.55; 95% CI: 4.68–12.18; p < 0.0001). Patients with major bleeding also experienced higher rates of myocardial infarction (MI), unplanned revascularization, stent thrombosis, thrombocytopenia and longer lengths of hospital stay.
Analysis of the 1‑year mortality in the ACUITY study population comparing those with major bleeding to those without showed higher rates of death at 1 year for those experiencing a major bleed. This was true across all age stratifications but was most pronounced in the oldest age group (>75 years) with 1-year mortality of 23.0% for those with a major bleed versus 7.9% for those without (p < 0.0001) [20].
A recently published risk model derived from the ACUITY data also demonstrates the increased mortality associated with major bleeding [21]. Using the 13,819 patients enrolled in that trial, a multivariable Cox regression model was developed to relate independent predictors of 1‑year mortality. Both MIs occurring after randomization and major bleeding were independently associated with increased mortality. While MI had a much higher impact than bleeding on the mortality risk in the days immediately after the event, that risk decreased rapidly over time. Alternatively, the increased risk of death from major bleeding or transfusions did not significantly decline over time and was associated with a higher risk of death at 30‑days and 1 year than MI.
A comparison of fondaparinux versus enoxaparin in the treatment of ACS was performed in the Organization to Assess Strategies for Ischemic Syndromes (OASIS)-5 trial [22]. In this study, a significant reduction in bleeding was observed with fondaparinux, which translated into a significant reduction in mortality. This mortality benefit persisted until the end of follow-up at 180 days (HR: 0.89; 95% CI: 0.80–1.00; p = 0.05).
The Early Discharge After Transradial Stenting of Coronary Arteries (EASY) trial enrolled 1348 patients undergoing transradial stenting [19]. They found independent predictors of major bleeding (as defined by the REPLACE-2 definition) to be creatinine clearance of less than 60 ml/min, procedure duration greater than 1 h, and sheath size greater than or equal to 6 F. Those who had a major bleeding event had higher 30‑day (11 vs 0%; p < 0.001) and 1‑year (16 vs 0.6%; p < 0.001) mortality. The composite end point of death, MI or target vessel revascularization was higher in patients with major bleeding at 30 days, 6 months and 1 year.
Taken together, all of these studies indicate a strong, consistent, dose-dependent relationship between bleeding complications after PCI and short- and long-term adverse outcomes including death, MI, target vessel revascularization, stent thrombosis and stroke. While none of these studies can prove causality between bleeding and mortality, which is likely true only at the extremes (i.e., intracranial hemorrhage or severe bleeding), they do suggest that strategies that reduce bleeding risk can improve outcomes from PCI.
Potential mechanisms underlying the association between bleeding & outcomes
A number of hypotheses exist as to why bleeding is associated with adverse events and mortality. The most obvious explanations involve the hypovolemia, anemia, hypotension and diminished oxygen-carrying capacity that results from acute blood loss. This, by itself, does not fully explain the higher rates of ischemic complications in patients who bleed. In the ACUITY data set, for example, there were nearly six-times as many stent thromboses in those who bled [6]. These may arise from the early discontinuation of antiplatelet or antithrombotic drugs after bleeding is discovered. In addition, those with major bleeding often have longer hospital stays with more invasive procedures, as well as blood transfusions, which can increase the chances of an adverse outcome.
Another potential issue is the role of blood transfusion. If bleeding correlates with an increased risk of morbidity and mortality, one might assume that the transfusion of blood products would mitigate that risk. Studies indicate that this is not the case [23,24]. In fact, the transfusion of blood products in patients with ACS or undergoing PCI is associated with adverse outcomes. Rao and colleagues performed a pooled analysis of 24,112 patients enrolled in three large international clinical trials of ACS to determine the association between transfusion and 30‑day mortality [24]. After adjustment for confounders including the propensity to receive blood, transfusion was associated with an increased risk for both mortality and the composite of death or MI with an adjusted HR of 3.94 (95% CI: 3.26–4.75; p < 0.001) for 30‑day death and 2.92 (95% CI: 2.55–3.35; p < 0.001) for death or MI.
As mentioned above, blood transfusions were also found to be an independent predictor of inhospital and 1-year mortality, regardless of bleeding category (major, minor and none) in the study published by Kinnaird et al. Specifically, for those who experienced major bleeding, the in-hospital mortality for those receiving a blood transfusion was 10.6% compared with just 5.1% for those with a major bleed not receiving a blood transfusion. There was also a link between the number of units transfused and 1‑year mortality with the OR of 1‑year death being 1.47 per unit transfused (95% CI: 1.36–1.55; p < 0.001) [17].
The reasons why blood transfusions correlate to a higher risk of adverse outcomes are unclear. A number of mechanisms have been proposed and include shifts in the oxyhemoglobin dissociation curve, increases in systemic inflammation [25,26], and the lack of nitric oxide in packed red cells [27], all of which lead to paradoxical decreases in tissue oxygenation as transfusion is used to increase hemoglobin. It should be noted that all data linking transfusion to adverse outcomes in patients with ischemic heart disease are retrospective and subject to unmeasured confounding. However, the consistency of the findings suggests that routine use of transfusion is best avoided in patients undergoing PCI provided that they are not symptomatic from anemia or actively bleeding. Perhaps more telling is the fact that transfusion of blood does not necessarily correlate with major clinical bleeding. Blood transfusions have been shown to poorly correlate with bleeding severity. In fact, many transfusions are given to those with clinically mild or no bleeds, while many with severe bleeding by definition do not receive transfusions. Moscucci and others have shown that perhaps up to 64% of blood transfusions are given inappropriately, according to current guidelines [28].
Strategies to reduce bleeding risk
▪ Pharmacologic strategies
Unfractionated heparin has traditionally been the antithrombin of choice for PCI, with or without the addition of a GPI. If a GPIIb/IIIa is used, the target activated clotting time (ACT) is between 200 and 250 s. If no GPIIb/IIIa is used in conjunction with UFH, the desired ACT during the intervention is between 250 and 300 s [29]. ACT above 350 s has not only been demonstrated to increase the risk of bleeding, but also increase ischemic complications such as MI, death and revascularization [30].
Since UFH and drugs that are derived from it are associated with platelet activation, the use of a GPI during PCI reduces periprocedural ischemic complications compared with UFH alone [31], especially in patients with ACS undergoing PCI [32]. However, this comes at the expense of increased bleeding, mostly at the vascular access site [33]. Indeed, the use of GPIs has been shown to be an independent risk factor for developing vascular access-site bleeding requiring a transfusion in several observational studies [4,16].
One strategy to reduce, but not eliminate, the increased risk of bleeding with the addition of a GPI to heparin is to dose both agents appropriately. The magnitude of misdosing of anticoagulants and antiplatelet agents has been underscored by a report from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) registry [34]. Elderly patients with ACS were more likely to be overdosed with UFH, low-molecular-weight heparin and GPI, which was associated with a significant increase in the risk of bleeding [34]. This issue is also true for women with ACS, and up to 25% of the increased risk of bleeding observed in females is attributable to overdosing of antithrombotic agents [35]. Therefore, it seems reasonable that appropriate dosing based on weight and renal clearance is an important step to reducing bleeding risk.
Enoxaparin is an alternative to UFH that has been studied for the management of ACS and in PCI. When administered subcutaneously in ACS patients undergoing an early invasive strategy, enoxaparin was noninferior to UFH and was associated with an increased risk for some measures of bleeding [36]. Enoxaparin can also be administered intravenously and has been studied in the setting of elective PCI [8]. The STEEPLE trial randomly assigned 3528 patients to intravenous enoxaparin at 0.5 or 0.75 mg/kg, or weight-adjusted UFH to be given at the initiation of the procedure. The primary outcome was protocol-defined noncoronary artery bypass grafting-related major bleeding occurring within 48 h (Table 2). This was lowest in the enoxaparin 0.5 mg/kg arm (5.9 vs 6.5% in the 0.75 mg/kg arm vs 8.5% in the UFH arm; p = 0.051 for comparison between the enoxaparin arms and p = 0.01 for comparison between the 0.5 mg/kg enoxaparin and UFH arms). MI was also lowest in this arm; however, the mortality was higher, leading to early discontinuation of enrollment into the 0.5 mg/kg enoxaparin arm. Although it appears that intravenous enoxaparin is associated with less bleeding compared with UFH, the inability to measure its effect at the bedside and the higher mortality rate in the low-dose enoxaparin arm of STEEPLE have limited its widespread acceptance for PCI [37].
Bivalirudin, a direct thrombin inhibitor, has consistently been shown to reduce bleeding complications compared with UFH or enoxaparin, with or without GPI. The first trial with bivalirudin in PCI was the Bivalirudin Angioplasty Trial (BAT), which used a higher dose than that currently used in clinical practice [38]. The BAT trial randomly assigned 4312 patients with unstable angina undergoing balloon angioplasty to either bivalirudin or high-dose UFH. The rate of both ischemic complications (7.9 vs 6.2%; p = 0.039) and major bleeding (9.3 vs 3.5%; p < 0.001) was lower in the bivalirudin group [38].
Modern dosing of bivalirudin was evaluated in the REPLACE-2 trial, which randomized 6010 patients undergoing elective or urgent PCI [7] to UFH with planned GPIIb/IIIa or bivalirudin with provisional GPIIb/IIIa. The bivalirudin strategy was statistically noninferior to the UFH plus GPI strategy with respect to the primary end point of 30‑day death, MI, target vessel revascularization or major bleeding. There was no difference in ischemic end points between the two arms; there was, however, a 41% relative reduction in major bleeding events in the bivalirudin group (2.4 vs 4.1%; p < 0.001). In this study, the most common site of major bleeding was the vascular access site, occurring with an incidence of 2.5% in the heparin plus GPIIb/IIIa group and 0.8% in the bivalirudin group.
The ACUITY study evaluated the role of bivalirudin in moderate- or high-risk ACS [14]. In the ACUITY trial, 13819 patients were assigned to one of three anticoagulant strategies: UFH or enoxaparin plus GPIIb/IIIa, bivalirudin plus GPIIb/IIIa, or bivalirudin alone. The primary end point was again a quadruple composite of death, MI, urgent target vessel revascularization and major bleeding. Bivalirudin monotherapy was superior to either of the GPI arms with respect to the quadruple composite end point, a difference that was driven by a substantial reduction in major bleeding without any significant differences among the other individual end points at 30 days (Table 3) [14].
The HORIZONS-AMI trial evaluated the use of bivalirudin in 3602 patients with ST-segment elevation MI undergoing primary PCI, compared with UFH plus a GPIIb/IIIa. The primary end points were major bleeding and a combined adverse clinical event rate that included death, reinfarction and target-vessel revascularization for ischemia and stroke. At 30 days, there were significantly fewer net adverse clinical events in the bivalirudin group (9.2%) as compared with the heparin plus GPIIb/IIIa group (12.1%; p = 0.005). As seen in the ACUITY trial, there were also large differences in the major bleeding rates between the two groups, 4.9% for bivalirudin and 8.3% for heparin plus GPIIb/IIIa (p < 0.001). This translated into a significant reduction in cardiovascular death for the bivalirudin group, as compared with the heparin plus GPIIb/IIIa group (1.8 vs 2.9%; p = 0.03) [39].
Performing percutaneous interventions with only antiplatelet therapy and no anticoagulation therapy has also been evaluated in the Coronary Interventions Antiplatelet-based Only study [40]. This was a double-blind, randomized, prospective study in 700 patients on aspirin and thienopyridine undergoing elective PCI of an uncomplicated lesion assigned to receive unfractionated heparin (70–100 UI/kg) or no heparin (heparinized flushes were allowed). No patients received GPIIb/IIIa. Bleeding events were rare in both groups, but were significantly reduced in the noheparin arm when using the STEEPLE definition (1.7 vs 0.0%; p = 0.048). Interestingly, there were also no significant differences in the rates of acute MI, urgent lesion revascularization or major adverse cardiac events at 30 days, but the percentage of patients with postprocedural creatine kinase isoenzyme elevations were significantly lower in the no-heparin arm (1.7 vs 3.1%; p < 0.05). The Coronary Interventions Antiplatelet-based Only (CIAO) trial suggests that uncomplicated PCI in low-risk patients can be performed safely with very little anticoagulation and aggressive antiplatelet therapy, which can significantly reduce the risk of bleeding.
Procedural methods
Just as important to the reduction of bleeding events is an understanding of ways to reduce bleeding through appropriate vascular access techniques. When using the femoral artery approach, proper location of arterial puncture and sheath insertion can significantly reduce bleeding events, including retroperitoneal hematomas. Arteriotomy in the common femoral artery above the bifurcation, but below the inferior epigastric artery, is associated with the lowest bleeding rate [41]. The use of fluoroscopy to identify the middle third of the femoral head can help to puncture into the femoral artery at the appropriate location [42,43]. Once access is gained, the use of smaller sized sheaths (5 F) are associated with reduced bleeding [44].
Changing access site altogether from the femoral artery to the radial artery may be the single most effective procedural method to reduce bleeding. In a number of studies, transradial PCI appears to be associated with lower rates of bleeding, access site complications and transfusions [44,45]. In the EASY trial, for example, transradial PCI in a cohort of 1348 patients had only 19 (1.4%) experience major bleeding [19]. This was in the setting of all patients receiving aspirin, clopidogrel, UFH at 70 U/kg and abciximab. It is unclear how much further this could be reduced if the radial approach is used in conjuction with the substitution of an agent such as bivalirudin for UFH plus GPIIb/IIIa. A meta-analysis of 12 randomized trials showed an 80% reduction in vascular access complications when using radial access, at the expense of a lower procedural success rate [45]. This is in contrast to a randomized trial by Mann and colleagues comparing radial access to femoral access in 142 patients with ACS [46]. As in the meta-analysis, bleeding events in this study were lower (0 vs 4%; p < 0.01) in the radial group; however, procedural success was identical in both groups. In another randomized study of 900 patients undergoing balloon angioplasty, radial access was compared with both brachial artery and femoral artery access [47]. The radial approach had the lowest incidence of vascular complications (0% radial, 2.3% brachial and 2.0% femoral) with no difference in procedural success. Data from three combined provincial registries, including 32,822 patients in the Mortality Benefit of Reduced Transfusion After Percutaneous Coronary Intervention Via the Arm or Leg (MORTAL) study, showed that the radial approach was associated with lower rates of transfusion and a significant reduction in 30‑day mortality (OR: 0.71; 95% CI: 0.61–0.82; p < 0.001) and 1‑year mortality (OR: 0.83; 95% CI: 0.71–0.98; p < 0.001) compared with the femoral approach [48]. Challenges to adopting the radial approach include higher operator radiation exposure, inability to obtain access, arterial spasm in the radial and brachial arteries that occasionally occurs, and the potential for radial artery occlusion [46,49,50]. Despite the benefits of transradial PCI, data from a large registry demonstrate that the radial approach is rarely used in the USA [51].
Future perspective
Much research still needs to be done to fully understand the factors that increase one’s risk for bleeding and the mechanisms responsible for translating that bleeding into worse outcomes. Risk models for bleeding, such as that developed from the National Cardiovascular Data Registry (NCDR) and REPLACE-1 and 2 trials, should continue to be refined and employed prior to catheterization to identify those at highest risk for bleeding [18,52]. Interventional devices continue to evolve rapidly, and the emphasis should be on developing technology that minimizes bleeding risk, such as allowing for smaller arteriotomy size. Newer antithrombin and antiplatelet drugs are currently being studied and hope to provide better anticoagulation effect with reduced bleeding risk. Lastly, a greater focus on providing exposure to transradial PCI during interventional training may lead to an expansion in the use of the radial approach and lead to greater reductions in postprocedure bleeding.
Conclusion
Percutaneous coronary interventions are commonly performed as the accepted practice for the treatment of coronary artery disease. Owing to its invasive nature in conjunction with the use of antithrombotic and antiplatelet agents, bleeding remains a major clinical concern. Differences in definitions used to define bleeding events, and the different rates observed in clinical trials versus retrospective registries, make this risk hard to exactly delineate. Regardless, any amount of bleeding is now known to portend significant increases in the risk of ischemic complications such as MI and stroke, as well as death.
Understanding this association, and minimizing the bleeding incidence, is central to improving outcomes associated with coronary interventions. Pharmacological strategies such as appropriate dosing of UFH, or using bivalirudin, can significantly reduce bleeding risk. Given that the majority of bleeding complications in patients undergoing PCI are related to the vascular access site, the use of the radial approach is an important part of any strategy that seeks to reduce periprocedual bleeding. Both clinical trials and registry data demonstrate an association between strategies that reduce bleeding risk and improve survival.
Financial & competing interests disclosure
John Vavalle has no disclosures relevant to this manuscript. Sunil Rao receives research funding from Cordis Corporation, Momenta Pharmaceuticals and Portola Pharmaceuticals, and receives honoraria for consulting or speaking for Sanofi-Aventis, Bristol Myers Squibb and The Medicines Company. This manuscript was prepared without external funding.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Definitions used for bleeding
▪ Establishing the incidence of percutaneous coronary intervention (PCI)-related bleeding is difficult owing to differences in definitions used to define bleeding events and reporting systems used to capture them.
Linking bleeding to outcomes
▪ The mechanisms that underlie the adverse long- and short-term outcomes in patients who experience bleeding complications is not fully understood but likely involves hypotension, tachycardia, myocardial ischemia and systemic inflammation.
Outcomes associated with bleeding
▪ Blood transfusions are independently associated with increased morbidity and mortality.
▪ Bleeding complications from PCI are associated with significant increases in ischemic complications and death.
Methods to reduce bleeding risk
▪ Alterations in pharmacotherapy, such as the use of bivalirudin in place of unfractionated heparin and/or GPIIb/IIIa has been shown to lessen the risk of bleeding.
▪ Procedural strategies that employ careful vascular access, smaller sized catheters and the use of radial artery access have been shown to reduce bleeding.
Conclusions
▪ Reductions in PCI-related bleeding may lead to improved long-term outcomes and survival.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Singh M, Rihal CS, Gersh BJ et al.: Twenty-five-year trends in in-hospital and long-term outcome after percutaneous coronary intervention: a single-institution experience. Circulation 115, 2835–2841 (2007).
- Rao SV, O’Grady K, Pieper KS et al.: Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am. J. Cardiol. 96, 1200–1206 (2005).
- Fuchs S, Kornowski R, Teplitsky I et al.: Major bleeding complicating contemporary primary percutaneous coronary interventions-incidence, predictors, and prognostic implications. Cardiovasc. Revasc. Med. 10, 88–93 (2009).
- Yatskar L, Selzer F, Feit F et al.: Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc. Interv. 69, 961–966 (2007).
- Feit F, Voeltz MD, Attubato MJ et al.: Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am. J. Cardiol. 100, 1364–1369 (2007).
- Manoukian SV, Feit F, Mehran R et al.: Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J. Am. Coll. Cardiol. 49, 1362–1368 (2007).
- Lincoff AM, Bittl JA, Harrington RA et al.: Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 289, 853–863 (2003).
- Montalescot G, White HD, Gallo R et al.: Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N. Engl. J. Med. 355, 1006–1017 (2006).
- Brener SJ, Moliterno DJ, Lincoff AM et al.: Relationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary intervention. Circulation 110, 994–998 (2004).
- Rao SV, O’Grady K, Pieper KS et al.: A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J. Am. Coll. Cardiol. 47, 809–816 (2006).
- Steinhubl SR, Kastrati A, Berger PB: Variation in the definitions of bleeding in clinical trials of patients with acute coronary syndromes and undergoing percutaneous coronary interventions and its impact on the apparent safety of antithrombotic drugs. Am. Heart J. 154, 3–11 (2007).
- Chesebro JH, Knatterud G, Roberts R et al.: Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 76, 142–154 (1987).
- The GUSTO Investigators: An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N. Engl. J. Med. 329, 673–682 (1993).
- Stone GW, McLaurin BT, Cox DA et al.: Bivalirudin for patients with acute coronary syndromes. N. Engl. J. Med. 355, 2203–2216 (2006).
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK: Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
- Moscucci M, Fox KA, Cannon CP et al.: Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur. Heart J. 24, 1815–1823 (2003).
- Kinnaird TD, Stabile E, Mintz GS et al.: Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am. J. Cardiol. 92, 930–935 (2003).
- Mehta SK, Frutkin FA, Lindsey JB et al.: Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ. Cardiovasc. Intervent. DOI: 10.1161/ CIRCINTERVENTIONS.108.846741 (2009) (Epub ahead of print).
- Bertrand OF, Larose E, Rodes-Cabau J et al.: Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am. Heart J. 157, 164–169 (2009).
- Lopes RD, Alexander KP, Manoukian SV et al.: Advanced age, antithrombotic strategy, and bleeding in non-ST-segment elevation acute coronary syndromes: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J. Am. Coll. Cardiol. 53, 1021–1030 (2009).
- Mehran R, Pocock SJ, Stone GW et al.: Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur. Heart J. 30, 1457–1466 (2009).
- The OASIS Investigators: Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N. Engl. J. Med. 354, 1464–1476 (2006).
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM: Blood transfusion in elderly patients with acute myocardial infarction. N. Engl. J. Med. 345, 1230–1236 (2001).
- Rao SV, Jollis JG, Harrington RA et al.: The relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 292, 1555–1562 (2004).
- Welch HG, Meehan KR, Goodnough LT: Prudent strategies for elective red blood cell transfusion. Ann. Intern. Med. 116, 393–402 (1992).
- Fransen E, Maessen J, Dentener M, Senden N, Buurman W: Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest 116, 1233–1239 (1999).
- Rao SV, Eikelboom JA, Granger CB et al.: Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 28, 1193–1204 (2007).
- Moscucci M, Ricciardi M, Eagle KA et al.: Frequency, predictors, and appropriateness of blood transfusion after percutaneous coronary interventions. Am. J. Cardiol. 81, 702–707 (1998).
- Smith SC Jr, Feldman TE, Hirshfeld JW Jr et al.: ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J. Am. Coll. Cardiol. 47, E1–E121 (2006).
- Chew DP, Bhatt DL, Lincoff AM et al.: Defining the optimal activated clotting time during percutaneous coronary intervention: aggregate results from 6 randomized, controlled trials. Circulation 103, 961–966 (2001).
- Kong DF, Hasselblad V, Harrington RA et al.: Meta-analysis of survival with platelet glycoprotein IIb/IIIa antagonists for percutaneous coronary interventions. Am. J. Cardiol. 92, 651–655 (2003).
- Kastrati A, Mehilli J, Neumann FJ et al.: Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 295, 1531–1538 (2006).
- The PURSUIT Trial Investigators: Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes: platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy. N. Engl. J. Med. 339, 436–443 (1998).
- Alexander KP, Chen AY, Roe MT et al.: Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA 294, 3108–3116 (2005).
- Alexander KP, Chen AY, Newby LK et al.: Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation 114, 1380–1387 (2006).
- The SYNERGY Trial Investigators: Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 292, 45–54 (2004).
- O’Neill WW: Risk of bleeding after elective percutaneous coronary intervention. N. Engl. J. Med. 355, 1058–1060 (2006).
- Bittl JA, Chaitman BR, Feit F, Kimball W, Topol EJ: Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: final report reanalysis of the Bivalirudin Angioplasty Study. Am. Heart J. 142, 952–959 (2001).
- Stone GW, Witzenbichler B, Guagliumi G et al.: Bivalirudin during primary PCI in acute myocardial infarction. N. Engl. J. Med. 358, 2218–2230 (2008).
- Stabile E, Nammas W, Salemme L et al.: The CIAO (Coronary Interventions Antiplatelet-based Only) Study: a randomized study comparing standard anticoagulation regimen to absence of anticoagulation for elective percutaneous coronary intervention. J. Am. Coll. Cardiol. 52, 1293–1298 (2008).
- Sherev DA, Shaw RE, Brent BN: Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc. Interv. 65, 196–202 (2005).
- Fitts J, Ver Lee P, Hofmaster P, Malenka D: Fluoroscopy-guided femoral artery puncture reduces the risk of PCI-related vascular complications. J. Interv. Cardiol. 21, 273–278 (2008).
- Garrett PD, Eckart RE, Bauch TD, Thompson CM, Stajduhar KC: Fluoroscopic localization of the femoral head as a landmark for common femoral artery cannulation. Catheter Cardiovasc. Interv. 65, 205–207 (2005).
- Cantor WJ, Mahaffey KW, Huang Z et al.: Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter Cardiovasc. Interv. 69, 73–83 (2007).
- Agostoni P, Biondi-Zoccai GG, de Benedictis ML et al.: Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J. Am. Coll. Cardiol. 44, 349–356 (2004).
- Mann T, Cubeddu G, Bowen J et al.: Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J. Am. Coll. Cardiol. 32, 572–576 (1998).
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R: A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J. Am. Coll. Cardiol. 29, 1269–1275 (1997).
- Chase AJ, Fretz EB, Warburton WP et al.: Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart 94, 1019–1025 (2008).
- Lange HW, von Boetticher H: Randomized comparison of operator radiation exposure during coronary angiography and intervention by radial or femoral approach. Catheter Cardiovasc. Interv. 67, 12–16 (2006).
- Stella PR, Kiemeneij F, Laarman GJ et al.: Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet. Cardiovasc. Diagn. 40, 156–158 (1997).
- Rao SV, Ou FS, Wang TY et al.: Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 1, 379–386 (2008).
- Nikolsky E, Mehran R, Dangas G et al.: Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur. Heart J. 28, 1936–1945 (2007).
- Topol EJ, Moliterno DJ, Herrmann HC et al.: Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N. Engl. J. Med. 344, 1888–1894 (2001).
- Mehta SR, Yusuf S, Peters RJ et al.: Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358, 527–533 (2001).
- Bittl JA, Strony J, Brinker JA et al.: Treatment with bivalirudin (Hirulog®) as compared with heparin during coronary angioplasty for unstable or postinfarction angina. Hirulog Angioplasty Study Investigators. N. Engl. J. Med. 333, 764–769 (1995).
- The EPIC Investigators: Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N. Engl. J. Med. 330, 956–961 (1994).
- The EPILOG Investigators: Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N. Engl. J. Med. 336, 1689–1696 (1997).
- The RESTORE Investigators: Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty: randomized Efficacy Study of Tirofiban for Outcomes and Restenosis. Circulation 96, 1445–1453 (1997).
- The ESPRIT Investigators: Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet 356, 2037–2044 (2000).
- Serruys PW, Herrman JP, Simon R et al.: A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. Helvetica Investigators. N. Engl. J. Med. 333, 757–763 (1995).
- Steinberg DH, Shah P, Kinnaird T et al.: Bleeding risk and outcomes of bivalirudin versus glycoprotein IIb/IIIa inhibitors with targeted low-dose unfractionated heparin in patients having percutaneous coronary intervention for either stable or unstable angina pectoris. Am. J. Cardiol. 102, 160–164 (2008).
- Yan BP, Gurvitch R, Duffy SJ et al.: An evaluation of octogenarians undergoing percutaneous coronary intervention from the Melbourne Interventional Group registry. Catheter Cardiovasc. Interv. 70, 928–936 (2007).
▪ This review of data from the National Heart, Lung, and Blood Institute (NHLBI) provides important insights into the significance of vascular access-site hematomas and transfusions in worse clinical outcomes.
▪ The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-2 study highlights the importance of anticoagulant choice in the setting of percutaneous coronary intervention (PCI) to reduce bleeding risk.
▪▪ This analysis of a large cohort of acute coronary syndrome (ACS) patients demonstrated the frequency of dosing errors in antiplatelet and antithrombotic agents and the correlating impact on bleeding and outcomes.
▪▪ Provides a systematic overview of randomized trials comparing radial access and femoral access for coronary angiography and interventions. It provides insights into the risks and benefits of each approach.
▪▪ Recent publication, this analysis from the National Cardiovascular Data Registry demonstrates the reduction in bleeding rates with radial access as compared to the femoral approach. It also highlights how few transradial interventions are currently being performed in the USA.