Review Article - Imaging in Medicine (2011) Volume 3, Issue 3
Impact of preoperative endoscopic ultrasound in surgical oncology
Sascha S Chopra1 & Michael Hünerbein†1Department of General & Transplantation Surgery, Charité Campus Virchow-Clinic, Berlin, Germany
- Corresponding Author:
- Michael Hünerbein
Department of Surgery & Surgical Oncology
Helios Hospital Berlin, 13122 Berlin, Germany
Tel: +49 309 417 1480
Fax: +49 309 417 1404
E-mail: michael.huenerbein@ helios-kliniken.de
Abstract
Endoscopic ultrasound (EUS) has a strong impact on the imaging and staging of solid tumors within or in close proximity of the upper GI tract. Technological developments during the last two decades have increased the image quality and allowed very detailed visualization of local tumor spread and lymph node affection. Current indications for EUS of the upper GI tract encompass the differentiation between benign and malignant lesions, the staging of esophageal, gastric and pancreatic cancer, and the procurement of a biopsy specimen through fine-needle aspiration. Various technical innovations during the past two decades have increased the diagnostic quality and have simultaneously strengthened the role of EUS in the clinical setting. This article will give a compressed summary on the current state of EUS and possible further technical developments.
Keywords
3D imaging ▪ elastosonography ▪ endoscopic ultrasound ▪ miniprobes ▪ oncologic surgery
Conventional endoscopic ultrasound
■ Linear versus radial systems
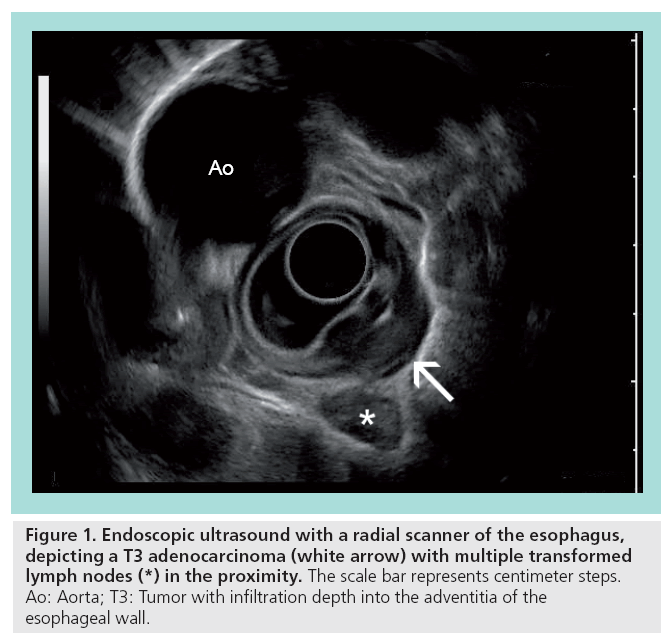
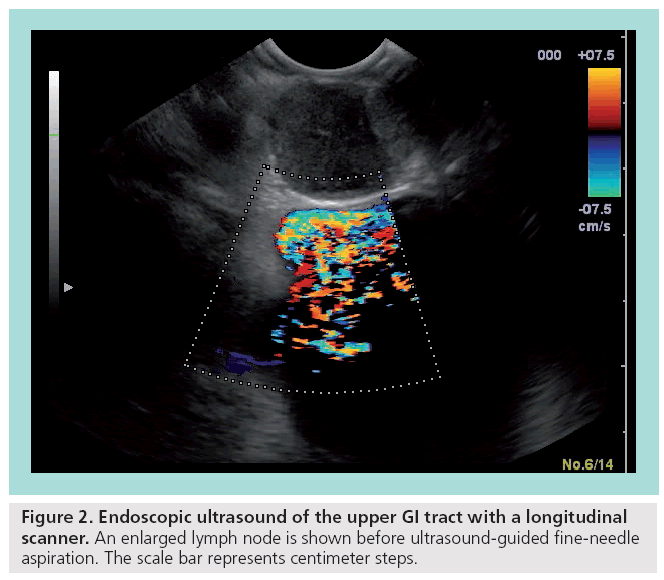
Endoscopic ultrasound (EUS) with f lexible endoscopes is an important diagnostic and therapeutic tool, especially for the local staging of gastrointestinal (GI) cancers, the differentiation between benign and malignant tumors, and interventional procedures, such as biopsies and stent applications. Flexible endoscopy was primarily based on radial scanners, which are attached at the tip of an endoscope. The usual 5–12-MHz scanners produce a single-plane 360° image in the immediate proximity. Most studies investigating the role of flexible EUS have been conducted with these radial scanners (Figure 1). Later on, a linear (longitudinal) ultrasound array was introduced and entered the clinical setting. In accordance with the radial scanner, it uses comparable frequencies between 5 and 10 Hz. A major advantage is the possibility to allow fineneedle biopsies under real-time guidance with complete visualization of the needle (Figure 2). In addition, the linear array allows duplex and power Doppler sonography. It remains controversial whether the radial scanner offers an increased anatomical orientation compared with the linear arrays.
Figure 1: Endoscopic ultrasound with a radial scanner of the esophagus, depicting a T3 adenocarcinoma (white arrow) with multiple transformed lymph nodes (*) in the proximity. The scale bar represents centimeter steps. Ao: Aorta; T3: Tumor with infiltration depth into the adventitia of the esophageal wall.
■ Miniprobes
The introduction of miniaturized ultrasound probes, which can be applied through the working channel of flexible endoscopes, has expanded the possibilities of EUS. It is now possible to direct the so-called ‘miniprobes’ into the biliary system or the pancreatic duct in order to obtain high-resolution radial ultrasound images locally. Present miniprobes show a diameter of 2–3 mm and operate with frequencies between 12 and 30 MHz. The main drawbacks of these devices are the limited durability and the decreased depth of penetration (~2 cm). In order to simplify the access into the common biliary duct, different miniprobes have been developed, which can be introduced via a guidewire. Linghu et al. have shown that intraductal ultrasound is potentially beneficial for the detection of stones in the common bile duct [1]. Compared with endoscopic retrograde cholangiopancreatography and conventional transabdominal ultrasound, intraductal ultrasound offered the highest sensitivity of 97% (endoscopic retrograde cholangiopancreatography: 81%; ultrasound: 45%) [1]. Further indications for intraductal ultrasound include the staging and surgical assessment of cholangiocarcinoma and pancreatic tumors. Next to intraductal analysis, miniprobes allow the visualization of stenotic tumors in the upper and lower GI tract, which are not accessible with standard EUS equipment.
■ Techniques of EUS fine-needle aspiration & biopsies
Facing competition from different imaging modalities, EUS fine-needle aspiration (FNA) remains an important component of diagnostic EUS. Different studies have shown the efficacy and safety of EUS-guided FNA in the clinical setting. For example, Chhieng et al. conducted a retrospective evaluation of 80 patients with 103 intramural and extramural GI lesions, with an overall accuracy of 81% without major complications [2]. The needle usually applied is a 22-G needle, although the range encompasses 19–25 G. It is generally accepted that EUS FNA of pancreatic masses, submucosal lesions and lymph nodes above and below the diaphragm is extremely safe compared with other tissuesampling techniques. Studies describe a risk profile similar to conventional endoscopy [3]. A multicenter review of approximately 500 EUSguided FNAs with a broad variety of indications showed a morbidity rate of 0.5%, without mortality [4]. Levy et al. tested and compared the more recently developed Tru-Cut® biopsy needles with the conventional FNA system [5]. The Tru-Cut needle obtained tissue samples in 19 patients with known or suspected mass lesions or lymphadenopathy. The authors describe a higher accuracy with the EUSguided Tru-Cut biopsy needle compared with EUS FNA. The advantage of the EUS-guided Tru-Cut biopsy needle appears to be a potential decrease in the number of biopsies required. In addition, availability of a histological core sample in contrast to a cytopathology specimen has diagnostic advantages in the evaluation of neoplastic lesions.
■ Contrast-enhanced ultrasound
The introduction of contrast agents has changed the diagnostic potential of Doppler ultrasonography dramatically. Since the concentration of the contrast agent can be determined as a function of time, a measure for the actual blood flow can now be obtained that provides quantitative information. By using these technological advances, it is now possible to assess blood flow in very small vessels that feed normal or abnormal tissues, and to assess changes in flow and vascularity that occur in response to therapeutic efforts. A study by Napoleon et al. evaluated the effects of contrast-enhanced EUS in 35 patients with pancreatic lesions [6]. Using the second-generation contrast agent SonoVue® (Bracco, Milan, Italy), they analyzed the microvascular pattern and compared the results with the final diagnosis based on FNA or surgery. Contrast-enhanced EUS achieved a sensitivity and specificity of 89 and 88%, respectively, for the diagnosis of pancreatic adenocarcinoma. The authors conclude that the contrast agents during EUS are potentially helpful in the differentiation of unclear pancreatic lesions.
■ Elastography
Elastography has been developed as a new EUS technique that differentiates the tissue stiffness in a way similar to palpation. The prototypic elasticity imaging technique consists of a device for generating shear waves in tissues: it is an EUSbased method for imaging propagation of these waves, and provides an algorithm for processing the wave images to generate quantitative images depicting tissue stiffness. During the examination, a sequence of ultrasonic images is acquired while the tissue is slightly compressed by the ultrasound probe [7]. Using numerical analysis of image pairs for the acquired sequence, the tissue strain that represents the spatial elasticity distribution of a specific cross-section of the organ is calculated. Elastograms of tumor specimen show focal areas of high shear stiffness. Preliminary results suggest that ultrasound elastography has the potential to detect malignant tissue areas, which are not shown in the B-mode image [8]. König et al. have shown that elastography may be a helpful tool during ultrasound-guided biopsies of the prostate gland [9].
Larino et al. used elastosonography to evaluate abdominal and mediastinal lymph nodes for detecting the presence of malignancy. They used a different elastosonographic pattern to differentiate between malign and benign lymph nodes. From 63 lymph nodes in 57 consecutive patients, 26 were classified as being blue-predominant and 23 green-predominant, while 14 showed a heterogeneous pattern. After FNA in all cases and clinical follow-up, it was shown that most of the malignant nodes were blue-predominant (n = 24) or heterogeneous (n = 7). In this series, the probability of a malignancy in a greenpredominant node was 0%. The authors conclude that elastosonography may be helpful in the differential diagnosis of possibly malignant lymph nodes.
The same group evaluated the accuracy of elastosonography in a series of 57 consecutive patients with solid pancreatic lesions [10]. Elastography was performed in representative areas from the mass and soft-tissue reference areas and a strain ratio was calculated (quotient: reference area/mass). FNA was performed in all cases. The strain ratio was significantly higher among patients with pancreatic malignant tumors compared with those with inflammatory masses. The sensitivity and specificity of strain ratio for detecting pancreatic malignancies were 100 and 92.9%, respectively. The authors conclude that EUS elastography is useful for the differential diagnosis of solid pancreatic lesions.
■ 3D imaging
The major reason for interest in 3D-EUS is related to the limitations of 2D viewing of 3D anatomy. Since only discrete 2D images can be assessed by the operator at any given time, no direct information is available on the longitudinal extent of the tumor and its spatial relationships. Consequently, a series of transverse images must be integrated by the observer to produce a mental impression of the real anatomy. This necessitates repeated movement of the scan plane over the region of interest, which can be time consuming and painful for the patient. However, it remains difficult to obtain a spatial impression of the tumor and its location in relation to relevant structures.
Advantages of 3D ultrasound include allowing the physician to evaluate arbitrary planes not available with 2D ultrasound, to improve assessment of complex anatomic situations by 3D display, to measure organ dimensions and volumes, and to standardize the ultrasound examination procedures. It must be emphasized that the quality of 3D images is dependent on the resolution of the probe used to acquire the individual 2D images. Currently, only probes with 10 MHz or, even better, 16 MHz, as described by Santoro and Fortling, can be considered to provide adequate resolution [11]. Other factors influencing the quality of the images include the number of acquired scan planes and the acquisition time (motion artifacts).
3D ultrasound images are based on multiple serial sections [12]. The ultrasound data can be obtained either by tracking of a conventional transducer or by a mechanical approach. The mechanical approach involves standardized withdrawal of a conventional probe or a special volume probe using a sweeping transducer.
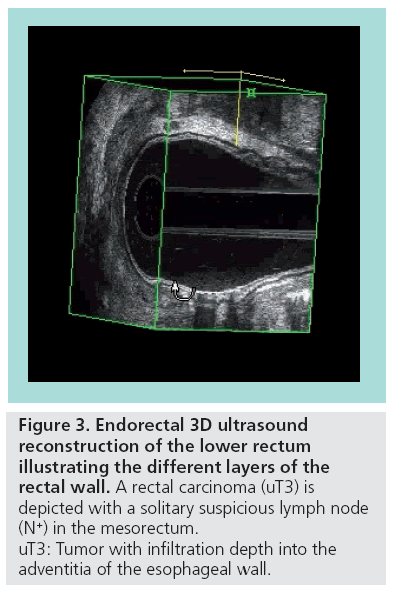
The volume data are digitally stored, which allows a real-time re-examination at any time, without loss of information. 3D image analysis can be performed using various display modes. The section display depicts the region of interest in three orthogonal planes simultaneously; this is particularly valuable for the assessment of small structures (e.g., lymph nodes). The volume display visualizes data as a 3D view, which improves the understanding of spatial relations between tumors and anatomic structures (Figure 3). Volume rendering allows reconstruction of life-like 3D projections.
Figure 3: Endorectal 3D ultrasound
reconstruction of the lower rectum
illustrating the different layers of the
rectal wall. A rectal carcinoma (uT3) is
depicted with a solitary suspicious lymph node
(N+) in the mesorectum.
uT3: Tumor with infiltration depth into the
adventitia of the esophageal wall.
Limited experience with this technique has been gained with transrectal EUS. In a pilot study, Mueller et al. performed 3D endorectal ultrasound in three patients with rectal cancer [13]. A comprehensive study involving 100 patients has demonstrated encouraging results of 3D endorectal ultrasound in the evaluation of rectal cancer [14]; 3D ultrasound facilitated the interpretation of the ultrasound scans and improved the diagnostic confidence in approximately 60% of the examinations.
■ Image postprocessing
The 3D data can be displayed in multiple planes (multiplanar reformatting) or as a 3D reconstruction. The data can be subjected to rendering algorithms that display only selected pixels depending on the brightness. Various rendering modes are available, such as maximum, minimum or transparency mode, or combinations thereof. Although these modes are helpful to enhance some information, other details may be lost. It is very important to realize that too much manipulation of the data can destroy information, and may lead to confusion and misinterpretation of the data. Similarly to conventional EUS, this new technique requires training and experience. 3D reconstructions may closely resemble the real 3D anatomy and can therefore significantly improve the assessment of the normal and pathologic anatomy. Complex information on the exact location, extent and relation of the tumor to relevant structures can be visualized in a single 3D image. Although hard copies are valuable, interactive manipulation of the data on the computer will enhance the ability of the surgeon to assess critical details.
Interactive analysis of the 3D data, also referred to as virtual operation planning, allows the data to be displayed according to the clinical requirements. Various 3D views, including rotating cine loops, can be visualized. Selected structures can be marked by colors and measurements can be made. Computer animation techniques can be performed to simulate surgical procedures (e.g., tumor resections). It seems likely that these new diagnostic tools will be increasingly used in the future to facilitate planning of operations and surgical training.
■ Clinical impact of 3D-EUS
Until now, only a limited number of studies are available that have investigated the clinical relevance of 3D endorectal ultrasound [15,16].
Comparable preliminary experience has also been reported for 3D endoanal ultrasound imaging of perianal fistulas and sphincter defects. West et al. performed preoperative 3D endoanal ultrasound and endoanal MRI in 40 patients with symptoms of a perianal fistula and a visible external opening [17]. The results were separately assessed by experienced observers. Fistulas were described according to the following characteristics: classification of the primary fistula tract according to Parks, location of the internal opening, presence of secondary tracts and fluid collections. The methods agreed in 88% of cases for the primary fistula tract, 90% for the location of the internal opening, 78% for secondary tracts and 88% for fluid collections.
Endoanal & endorectal ultrasonography
Endoscopic ultrasound remains the most sensitive imaging modality for the evaluation of the rectum and the anal canal. While a majority of publications deal with the staging of rectal cancer, endorectal ultrasound plays also a major role in the diagnosis of benign anorectal disease, especially fistula and sphincter defects. Recently, several new ultrasound techniques have been developed that could significantly improve the diagnostic value of endorectal ultrasound. These new methods include power Doppler sonography, a variety of harmonic imaging techniques, electronic compounding and pulse-sequencing methods that improve the signal-to-noise relationship, as well as structural conspicuity.
Preoperative staging and treatment planning of rectal cancer has been significantly improved by EUS. This technique enables clinicians to evaluate locoregional tumor spread accurately and provides the criteria to select the appropriate management strategies. Depending on the tumor stage, different treatment concepts including local excision, radical resection and multimodal therapy are available for rectal cancer [18,19].
Various imaging methods, including CT and MRI, have been used for preoperative staging of rectal cancer. It has been shown that extensive tumor spread can be accurately visualized with CT; however, the accuracy of this technique in assessing small lesions has been disappointing. Despite encouraging early results, MRI has not been significantly superior to CT because of the limited resolution of conventional magnetic resonance techniques. Recently, it has been shown that high-resolution images of the rectal wall can be obtained by endorectal MRI [20]. However, it remains difficult to assess the value of this new method exactly, because only preliminary data from studies involving limited numbers of patients are available.
Currently, transrectal ultrasound is the most sensitive technique for staging of rectal carcinoma. Several authors have reported accuracy rates of more than 85% in the assessment of the tumor infiltration depth and more than 75% in the assessment of lymph node involvement [21]. However, there have been some well-recognized problems of conventional transrectal ultrasound. Interpretation of the ultrasound images is difficult and requires much experience. Obstructing tumors cannot be examined, owing to the inability to pass the probe across the tumor. It remains difficult to establish the clinical relevance of pararectal lesions in follow-up examinations.
■ Miniprobe EUS
Recently, miniprobes have been developed, which can be introduced through the instrument channel of endoscopes. Miniprobe EUS of colorectal tumors can be carried out during routine colonoscopy using ultrathin probes with a diameter of 6 Fr, and a conventional ultrasound unit. The high-frequency transducer (12.5–30 MHz) of the mechanical probe provides 360° high-resolution images of the intestinal wall.
At present, we have only limited experience with miniprobe EUS in colorectal cancer. Hamada et al. have performed miniprobe EUS on 33 patients with colorectal cancer using a 15-MHz miniprobe [22]. The accuracy of the miniprobe for the depth of invasion and the nodal status was 82 and 87%, respectively [22]. We have obtained comparable results in a group of 63 patients [23]. The number of patients with positive lymph nodes in both studies was small and may not yet allow valid assessment of lymph node staging with miniprobes.
In our experience, miniprobe EUS proved to be particularly valuable for the staging of stenotic rectal tumors, which are not accessible to conventional probes. Correct assessment of the infiltration depth was obtained in 87% of the cases. In most patients, T3 or T4 carcinomas were diagnosed. High-resolution miniprobe EUS also seems helpful to determine the indication for endoscopic resections of broadbased rectal polyps. Many surgeons believe that endoscopic treatment of broad-based adenoma is inappropriate because occult cancer may be found in approximately 30% of the specimens. In spite of apparently complete snare resection, local recurrence, lymph node metastases or both will be observed in 10–20% of patients. Therefore, a reliable method for the detection of invasive cancer is essential to avoid inadequate endoscopic treatment of broad-based polyps with carcinoma. Hizawa et al. performed EUS in 60 patients with colorectal tumors confined to mucosa or submucosa using a flexible colonoscope (7.5 MHz). The accuracy for the detection of early cancer was only 77% [24]. In our experience, miniprobe ultrasonography with a 12.5-MHz transducer provided a correct diagnosis in 96% of the broad-based polyps [23]. Notably, EUS revealed T1 tumors in two patients, although endoscopic biopsy had suggested adenoma with dysplasia. The combined accuracy of EUS and biopsy in the detection of invasive carcinoma was 100%.
■ Transrectal biopsy
Local recurrence represents a significant problem in 15–25% of patients who have undergone apparently curative resection of rectal cancer. More than 80% of local recurrences are perianastomotic or pelvic recurrences, which are not accessible to endoscopic biopsy.
Radiological methods, including CT and immunoscintigraphy, have been used with limited success to diagnose recurrent rectal cancer. In the meantime, postoperative EUS is routinely used by most of the colorectal surgeons in the USA. However, one major problem of EUS is the inability to make a tissue-specific diagnosis because early recurrence is often indistinguishable from postoperative changes [25].
Therefore, it is essential to obtain histological confirmation of suspicious perirectal lesions. Beynon et al. described a transperineal approach to the biopsy of perirectal lesions using a 360° radial scanner [26]. This technique proved to be difficult because the pathway of the needle could not be visualized. Furthermore, the procedure had to be performed under general anesthesia. More recently, Milsom et al. used a longitudinally oriented endorectal probe for preoperative biopsy of lymph nodes [27]. This study suggested that real-time ultrasound-guided biopsy of perirectal lymph nodes is safer and more accurate. Diagnostic material was obtained in 18 out of 26 patients (70%). However, it was necessary to perform a second examination with a conventional radial scanner because the longitudinal scan plane was not suitable for accurate diagnostic evaluation.
In the meantime, transrectal ultrasoundguided biopsy can be obtained by attaching a special targeting device to a conventional ultrasound probe. It has been shown that this method significantly improves the specificity of EUS in the postoperative follow-up of rectal cancer [28]. Diagnostic tissue samples can be obtained in more than 90% of patients.
Negative biopsies are particularly reassuring for the surgeon and the patient because there is clearly a tendency to overestimate benign perirectal lesions by postoperative EUS. EUSguided biopsy may avoid overtreatment of patients with suspicious endosographic lesions. However, it must be considered that false-negative biopsies may occur and caution is necessary. On the basis of histopathology findings, the treatment will be changed in approximately 30% of the patients [29]. EUS-guided biopsy improves the diagnosis of recurrent rectal cancer and may obviate the need for extensive imaging studies and diagnostic surgical procedures.
Conclusion
Based on technological innovations, EUS continues to develop and remains an important diagnostic tool in the clinical setting. In order to remain competitive against the evolving techniques of CT and MRI, new technical ideas need to be developed and incorporated into existing EUS technology.
Future perspective
Endoscopic ultrasound as an imaging tool for diagnostic procedures is currently challenged by the evolving techniques of contrast-enhanced magnetic resonance and CT imaging. EUS will remain a precious instrument for interventional procedures such as transluminal biopsies or the placement of internal drains.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Linghu EQ, Cheng LF, Wang XD et al.: Intraductal ultrasonography and endoscopic retrograde cholangiography in diagnosis of extrahepatic bile duct stones: a comparative study. Hepatobiliary Pancreat. Dis. Int. 3(1), 129–132 (2004).
- Chhieng DC, Jhala D, Jhala N et al.: Endoscopic ultrasound-guided fine-needle aspiration biopsy: a study of 103 cases. Cancer 96(4), 232–239 (2002).
- Silvestri GA, Hoffman BJ, Bhutani MS et al.: Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann. Thorac. Surg. 61(5), 1441–1445 (1996).
- Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM: Endosonographyguided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 112(4), 1087–1095 (1997).
- Levy MJ, Jondal ML, Clain J, Wiersema MJ: Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest. Endosc. 57(1), 101–106 (2003).
- Napoleon B, Alvarez-Sanchez MV, Gincoul R et al.: Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy 42(7), 564–570 (2010).
- Sommerfeld HJ, Garcia-Schurmann JM, Schewe J et al.: [Prostate cancer diagnosis using ultrasound elastography. Introduction of a novel technique and first clinical results]. Urologe A 42(7), 941–945 (2003).
- Lorenz A, Ermert H, Sommerfeld HJ, Garcia-Schurmann M, Senge T, Philippou S: [Ultrasound elastography of the prostate. A new technique for tumor detection]. Ultraschall. Med. 21(1), 8–15 (2000).
- König K, Scheipers U, Pesavento A, Lorenz A, Ermert H, Senge T: Initial experiences with real-time elastography guided biopsies of the prostate. J. Urol. 174(1), 115–117 (2005).
- Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez- Munoz JE: Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology 139(4), 1172–1180 (2010).
- Santoro GA, Fortling B: The advantages of volume rendering in three-dimensional endosonography of the anorectum. Dis. Colon Rectum 50(3), 359–368 (2007).
- Ivanov KD, Diavoc CD: Three-dimensional endoluminal ultrasound: new staging technique in patients with rectal cancer. Dis. Colon Rectum 40(1), 47–50 (1997).
- Mueller MP, Stamos MJ, Cavaye DM, Kopchok GE, Laas TE, White RA: Three-dimensional transrectal ultrasound: preliminary patient evaluation. J. Laparoendosc. Surg. 2(5), 223–227 (1992).
- Hunerbein M, Schlag PM: Threedimensional endosonography for staging of rectal cancer. Ann. Surg. 225(4), 432–438 (1997).
- Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G: The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis. Colon Rectum 52(11), 1837–1843 (2009).
- Watanabe M, Kida M, Yamada Y, Saigenji K: Measuring tumor volume with three-dimensional endoscopic ultrasonography: an experimental and clinical study (including video). Endoscopy 36(11), 976–981 (2004).
- West RL, Zimmerman DD, Dwarkasing S et al.: Prospective comparison of hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging of perianal fistulas. Dis. Colon Rectum 46(10), 1407–1415 (2003).
- Rau B, Wust P, Hohenberger P et al.: Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a Phase II clinical trial. Ann. Surg. 227(3), 380–389 (1998).
- Hohenberger W, Matzel KE, Stadelmaier U: Possibilities of extensive surgery. Recent Results Cancer Res. 146, 59–65 (1998).
- Vogl TJ, Pegios W, Mack MG et al.: Accuracy of staging rectal tumors with contrast-enhanced transrectal MR imaging. AJR Am. J. Roentgenol. 168(6), 1427–1434 (1997).
- Rosch T: Endoscopic ultrasonography. Endoscopy 26(1), 148–168 (1994).
- Hamada S, Akahoshi K, Chijiiwa Y, Sasaki I, Nawata H: Preoperative staging of colorectal cancer by a 15 MHz ultrasound miniprobe. Surgery 123(3), 264–269 (1998).
- Hunerbein M, Totkas S, Ghadimi BM, Schlag PM: Preoperative evaluation of colorectal neoplasms by colonoscopic miniprobe ultrasonography. Ann. Surg. 232(1), 46–50 (2000).
- Hizawa K, Suekane H, Aoyagi K, Matsumoto T, Nakamura S, Fujishima M: Use of endosonographic evaluation of colorectal tumor depth in determining the appropriateness of endoscopic mucosal resection. Am. J. Gastroenterol. 91(4), 768–771 (1996).
- Hizawa K, Aoyagi K, Suekane H, Mibu R, Yao T, Fujishima M: Suture granuloma in rectal anastomosis mistaken for locally recurrent cancer. J. Clin. Gastroenterol. 23(1), 78–79 (1996).
- Beynon J, Mortensen NJ, Foy DM, Channer JL, Rigby H, Virjee J: Preoperative assessment of mesorectal lymph node involvement in rectal cancer. Br. J. Surg. 76(3), 276–279 (1989).
- Milsom JW, Czyrko C, Hull TL, Strong SA, Fazio VW: Preoperative biopsy of pararectal lymph nodes in rectal cancer using endoluminal ultrasonography. Dis. Colon Rectum 37(4), 364–368 (1994).
- Hunerbein M, Totkas S, Moesta KT, Ulmer C, Handke T, Schlag PM: The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery 129(2), 164–169 (2001).
- Lohnert MS, Doniec JM, Henne-Bruns D: Effectiveness of endoluminal sonography in the identification of occult local rectal cancer recurrences. Dis. Colon Rectum 43(4), 483–491 (2000).