Special Report - Interventional Cardiology (2013) Volume 5, Issue 5
In case of procedure failure: facilitating future success
- Corresponding Author:
- William M Wilson
Edinburgh Heart Centre
Royal Infirmary of Edinburgh
51 Little France Crescent, Edinburgh, EH41 5SA,
Scotland, UK
E-mail: wmclwilson@me.com
Abstract
Current chronic total occlusion (CTO) success rates are approximately 80–85% in the hands of skilled operators [1]. The most common mode of failure is an inability to cross an occlusion with a coronary guidewire with reasons to abandon a case, including futility, reaching contrast or radiation dose thresholds, patient tolerability, time pressures on catheter laboratory use or significant procedural complications. If primary case failure looks likely, it is important to understand the ‘failure mode’ and identify modifiable contributors to failure, which may then be addressed to facilitate future procedural success. Prospective actions during the index procedure can potentially lead to subsequent spontaneous recanalization and/or enhance the success rates of a future attempt.
Keywords
chronic total occlusion, mode of failure, preplasty, procedural failure
Preplasty: the role of the investment procedure
Current chronic total occlusion (CTO) success rates are approximately 80–85% in the hands of skilled operators [1]. The most common mode of failure is an inability to cross an occlusion with a coronary guidewire with reasons to abandon a case, including futility, reaching contrast or radiation dose thresholds, patient tolerability, time pressures on catheter laboratory use or significant procedural complications. If primary case failure looks likely, it is important to understand the ‘failure mode’ and identify modifiable contributors to failure, which may then be addressed to facilitate future procedural success. Prospective actions during the index procedure can potentially lead to subsequent spontaneous recanalization and/or enhance the success rates of a future attempt.
We will introduce this concept of ‘preplasty’ or ‘investment procedure’, where the initial procedure acts to facilitate future success.
▪ Common failure modes
It is important to accurately assess the mode of failure; it is not as simple as “failure of the guidewire to enter the distal true lumen.” Common modes of failure are described below. The probability of each mode of failure can often be predicted by careful preprocedure angiographic assessment.
Antegrade
▪ Failure to penetrate the proximal cap
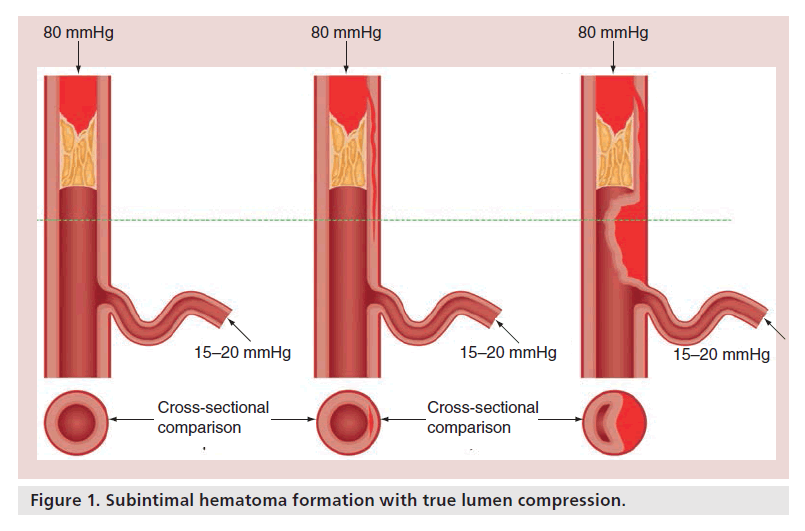
Histological analysis of CTOs has demonstrated that the proximal cap of the occlusion is usually the most calcified component and, thus, the most difficult to breach [2,3]. As such, considerable time (and, therefore, radiation use) can be consumed by efforts to modify/cross the proximal cap. Furthermore, unsuccessful attempts to cross the occluded segment can result in areas of subintimal hematoma, especially when aggressive strategies are required that involve a significant amount of force, so that when distal luminal re-entry is attempted, the chances of success will be reduced by compression of the true lumen (Figure 1). Examples of aggressive strategies employed in the setting of an inability to penetrate the cap include deliberate balloon rupture in an attempt to disrupt the cap or, alternatively, deliberate preocclusion access to the subintimal space to effectively bypass the cap (Figure 2). When procedural failure appears probable, it is vital that the proximal cap is modified, by balloon dilatation, where possible to permit future equipment passage.
▪ Inability to deliver equipment over a successfully placed guidewire
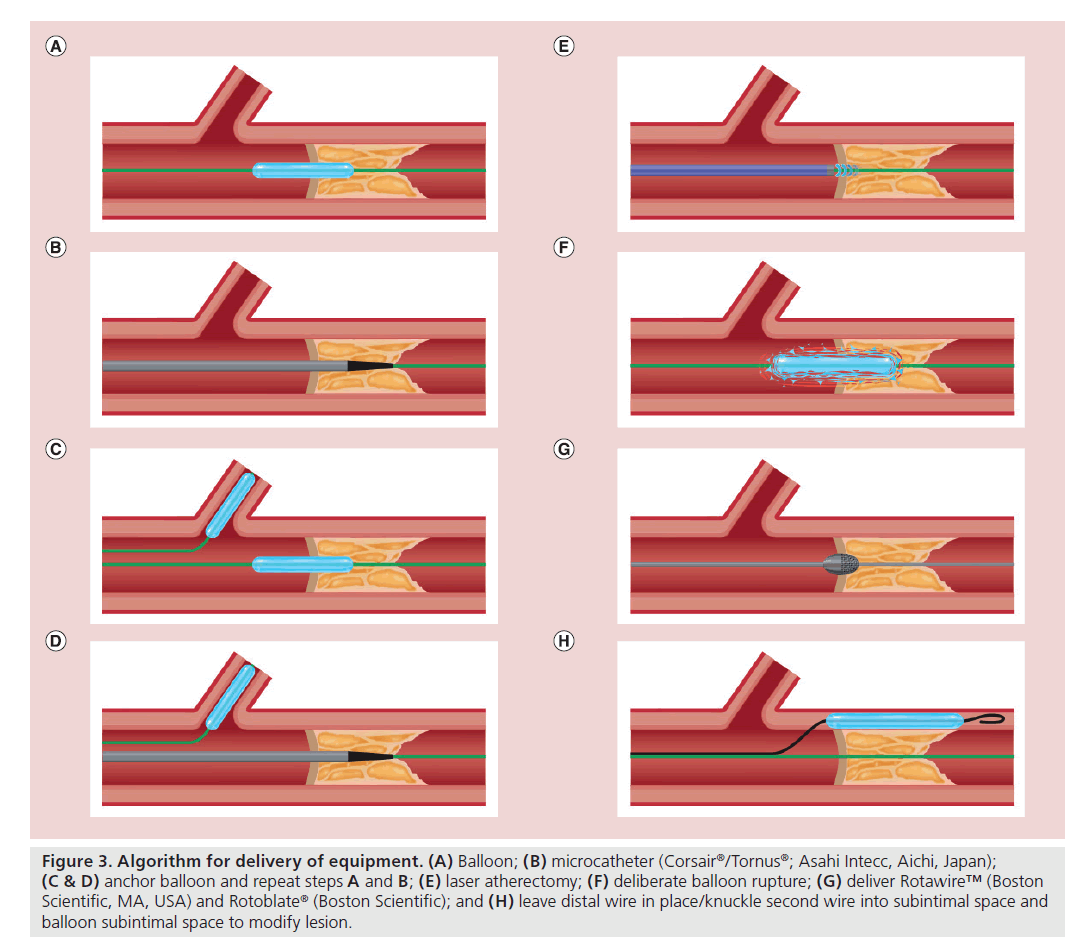
While uncommon (<5% of cases), the inability to pass any ‘equipment’ after successful wiring of the distal true lumen is a recognized failure mode. An algorithmic approach to resolving this issue should be adopted (Figure 3). The initial attempt is often made with a low-profile balloon; balloons with diameters of 1.5 mm or less are available and have one middle radiographic marker, which facilitates deliverability (compared with two end markers on larger balloons).
Figure 3. Algorithm for delivery of equipment. (A) Balloon; (B) microcatheter (Corsair®/Tornus®; Asahi Intecc, Aichi, Japan); (C & D) anchor balloon and repeat steps A and B; (E) laser atherectomy; (F) deliberate balloon rupture; (G) deliver Rotawire™ (Boston Scientific, MA, USA) and Rotoblate® (Boston Scientific); and (H) leave distal wire in place/knuckle second wire into subintimal space and balloon subintimal space to modify lesion.
A microcatheter may afford better penetration than a small balloon, especially when tapered and delivered into the lesion with rapid clock-wise or anticlockwise rotation. An anchor balloon or GuideLiner® (Vascular Solutions, MN, USA) catheter can then be used to increase penetration power. If this still proves unsuccessful, more aggressive strategies can be considered, such as deliberate balloon rupture, which may compromise initial procedural success, but can facilitate success on a repeat procedure.
▪ Failure of re-entry from subintimal space into distal true lumen
A further failure mode is passage of the antegrade wire into the subintimal space and failure to redirect into the distal true lumen. Specialized technologies, with demonstrated efficacy [4], have evolved to deal with this issue (Crossboss® catheter and Stingray® balloon; BridgePoint system, Boston Scientific, MA, USA). Alternative techniques, which include parallel wiring and intravascular ultrasound-guided re-entry, have been plagued with procedural complexity, poor reproducibility and limited applicability. An issue pertinent to all re-entry techniques is the necessity for a relatively healthy and disease-free distal vessel that does not involve a major bifurcation.
▪ Subintimal hemorrhage/loss of distal perfusion pressure
Expansion of the subintimal space may lead to subintimal hematoma and compression of the true lumen, which, in conjunction with consequent loss of visualization, will reduce the success of distal lumen re-entry (Figure 1). The expansion of the subintimal space occurs with exposure of the subintimal space to systemic pressure, but can be further adversely expanded by antegrade contrast injection into this space. This should, therefore, be avoided during such procedures, but this can be challenging if collateral filling is primarily ipsilateral. The visibility of the distal vessel filling should be viewed as a balance between external compression (by subintimal hemorrhage), distal tissue resistance and collateral filling pressures. Certain adverse anatomical features, such as significant proximal calcium or vessel tortuosity, may require more aggressive maneuvers, such as large wire knuckles or balloon expansion, to cross the occluded segment with consequent expansion of the subintimal space and compression of the distal true lumen.
Retrograde
▪ Retrograde dissection re-entry
The retrograde approach for CTO percutaneous coronary intervention (PCI) is an important component of the ‘hybrid’ strategy and there are reasons why a retrograde approach may be preferred. The distal cap is often tapered and softer than the proximal cap, thus distal cap penetration is usually less problematic, while re-entry to the true lumen through the use of retrograde dissection re-entry is usually uncomplicated. Predictive factors of failure of retrograde dissection re-entry include the presence of significant calcification within the occluded segment, significant vessel tortuosity, attempting to re-enter on a curve or the presence of a large diameter vessel.
The common issue is the inability to connect the antegrade and retrograde subintimal spaces. Strategies to increase success rates include use of a larger diameter balloon or use of a GuideLiner catheter (Vascular Solutions) on the antegrade wire that can assist in ‘capture’ of the retrograde wire and microcatheter.
Balloon dilatation on the antegrade wire (i.e., reverse controlled antegrade and retro-grade subintimal tracking) usually serves to expand the subintimal space within which both wires lie. If, however, the wires lie in different planes (i.e., antegrade wire subintimal and retrograde wire ‘true lumen’), balloon dilatation is required not only to expand the space, but also to create a connection, usually by way of causing a dissection. This can be particularly challenging in areas of high or calcific plaque burden.
▪ Failure to negotiate retrograde collaterals
The primary ‘failure mode’ for retrograde approaches is usually an inability to wire retro-grade collaterals or to cross these collaterals with a microcatheter after successful wiring. Even in the hands of experienced CTO operators under-taking cases preselected for retrograde PCI, the rate of failure to negotiate retrograde collaterals is approximately 20% [5,6].
Case examples
▪ Case one: PCI right coronary artery CTO requiring staged antegrade & retrograde approaches
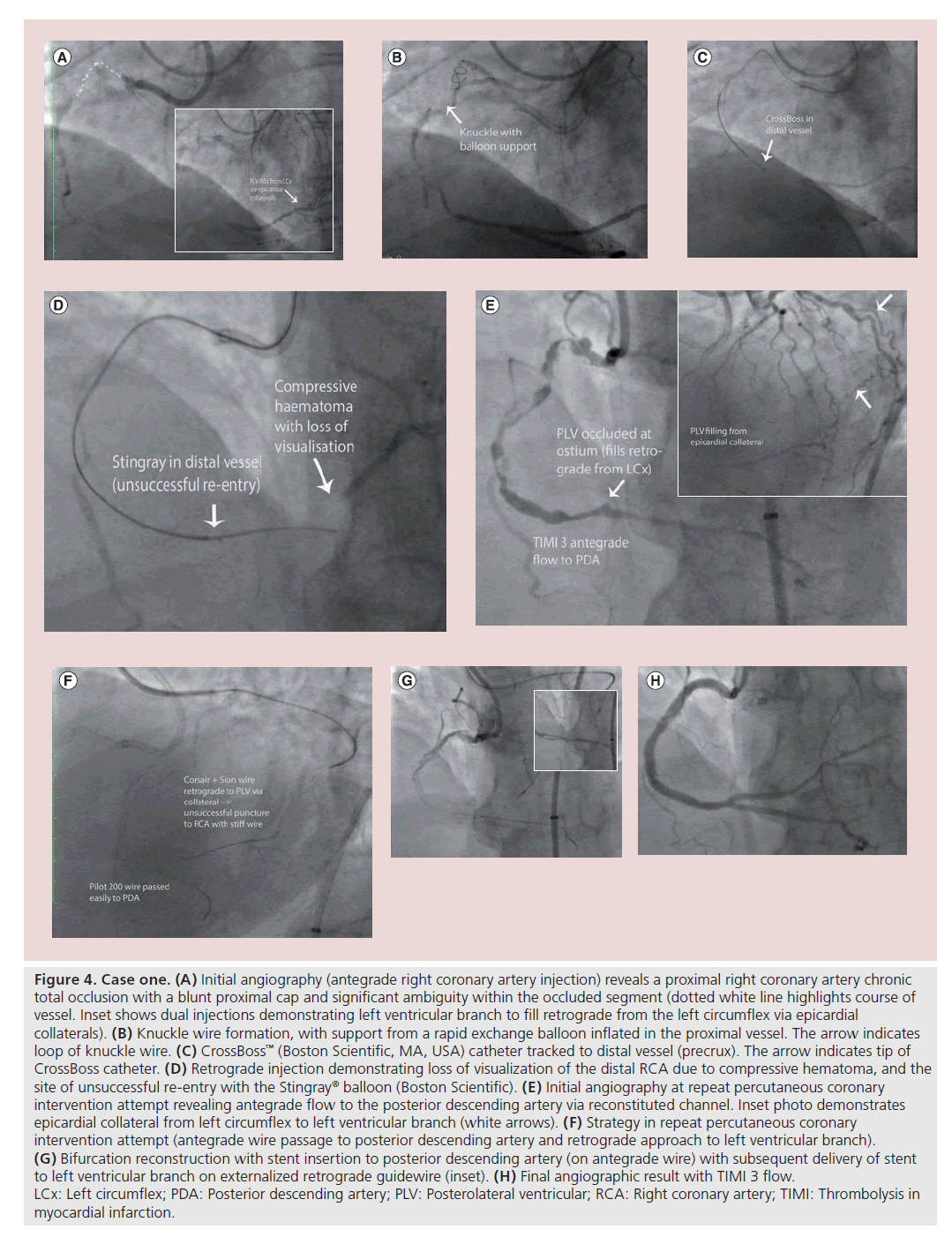
An 80-year-old retired welder with a history of hypertension and gout presented with a 2-year history of Canadian class 3 stable angina. He had no history of a myocardial infarction, but his ECG revealed anterior Q waves and echo-cardiography revealed moderate left ventricular systolic dysfunction with an akinetic distal anterior wall. Diagnostic angiography demonstrated an occluded mid-left anterior descending artery, minor left circumflex disease and an occluded proximal right coronary artery (RCA), which filled in a retrograde fashion via tortuous epicardial circumflex collaterals (Figure 4A). As the viability of the anterior wall was questionable, the patient was brought forward for elective PCI to the RCA CTO. The RCA occlusion was characterized by coronary cardiac CT and with dual injections at PCI; it was demonstrated to be 20–30 mm in length with significant tortuosity and a blunt proximal cap. There were suboptimal ‘interventional’ collaterals (Figure 4A). A radial and femoral approach was employed with an 8 F AL1 guide as the antegrade RCA guide. The proximal cap proved difficult and time consuming to cross (owing to heavy calcification, as well as anatomic ambiguity relating to the presence of multiple small atrial branches that arose within the occluded segment). Eventually, having punctured the proximal cap with a high gram force stiff wire, a knuckle could be created with a Pilot 200 wire (Abbott Vascular, CA, USA; with the support of an inflated rapid exchange balloon proximally) (Figure 4B). A CrossBoss™ catheter (Boston Scientific) was then used to extend the subintimal track (Figure 4C) and controlled re-entry was attempted via a Stingray® balloon (Boston Scientific) in the mid-vessel and then in the distal vessel unsuccessfully. The failure was likely related to loss of visualization secondary to compressive subintimal hematoma (likely created by the excessive force required to negotiate the proximal and mid-vessel with a large knuckle) (Figure 4D). Owing to the long procedure time (~3 h) with a high radiation dose (dose–area product 20,320 cGycm2 and skin dose 3.8 Gy), it was elected to abandon the case without complication. Prior to case completion, balloon angioplasty was undertaken to the proximal cap of the CTO vessel to facilitate future PCI attempts.
Figure 4. Case one. (A) Initial angiography (antegrade right coronary artery injection) reveals a proximal right coronary artery chronic total occlusion with a blunt proximal cap and significant ambiguity within the occluded segment (dotted white line highlights course of vessel. Inset shows dual injections demonstrating left ventricular branch to fill retrograde from the left circumflex via epicardial collaterals). (B) Knuckle wire formation, with support from a rapid exchange balloon inflated in the proximal vessel. The arrow indicates loop of knuckle wire. (C) CrossBoss™ (Boston Scientific, MA, USA) catheter tracked to distal vessel (precrux). The arrow indicates tip of CrossBoss catheter. (D) Retrograde injection demonstrating loss of visualization of the distal RCA due to compressive hematoma, and the site of unsuccessful re-entry with the Stingray® balloon (Boston Scientific). (E) Initial angiography at repeat percutaneous coronary intervention attempt revealing antegrade flow to the posterior descending artery via reconstituted channel. Inset photo demonstrates epicardial collateral from left circumflex to left ventricular branch (white arrows). (F) Strategy in repeat percutaneous coronary intervention attempt (antegrade wire passage to posterior descending artery and retrograde approach to left ventricular branch). (G) Bifurcation reconstruction with stent insertion to posterior descending artery (on antegrade wire) with subsequent delivery of stent to left ventricular branch on externalized retrograde guidewire (inset). (H) Final angiographic result with TIMI 3 flow. LCx: Left circumflex; PDA: Posterior descending artery; PLV: Posterolateral ventricular; RCA: Right coronary artery; TIMI: Thrombolysis in myocardial infarction.
The patient experienced ongoing angina and underwent a planned repeat PCI procedure 3 months later. The initial RCA angiogram at this procedure demonstrated the RCA to have reconstituted with thrombolysis in myocardial infarction (TIMI) 3 flow to the distal vessel (posterior descending artery only), partly via a subintimal track. The posterolateral ventricular (PLV) branch was occluded at its ostium and filled retrogradely via the epicardial collaterals (Figure 4E). A jacketed wire was passed easily within 5 min of screening time to the distal posterior descending artery; however, there was some ambiguity with regard to the ostium of the PLV and length of the occlusion. It was elected to salvage this vessel in a retrograde fashion (Figure 4F). Direct puncture of the retrograde PLV wire to the distal RCA was unsuccessful, but retrograde dissection re-entry in the distal RCA afforded externalization of the PLV wire. The artery was then reconstructed with stent insertion from the RCA ostium to distal RCA, including a ‘minicrush’ two-stent strategy at the bifurcation (Figure 4G). A potential concern with stent insertion in this setting with subintimal tracking at the bifurcation is loss of a large side branch (such as the PLV); the ‘minicrush’ was employed as it obviates the need to rewire through a dissected plane as one would need to do with other techniques. An excellent final result was achieved with TIMI 3 flow (Figure 4H). This procedure was uncomplicated and took 150 min with a radiation dose of 24,020 cGycm2 and a contrast load of 325 ml. The patient remains well and free of angina at 1 year.
▪ Case two: PCI proximal RCA CTO
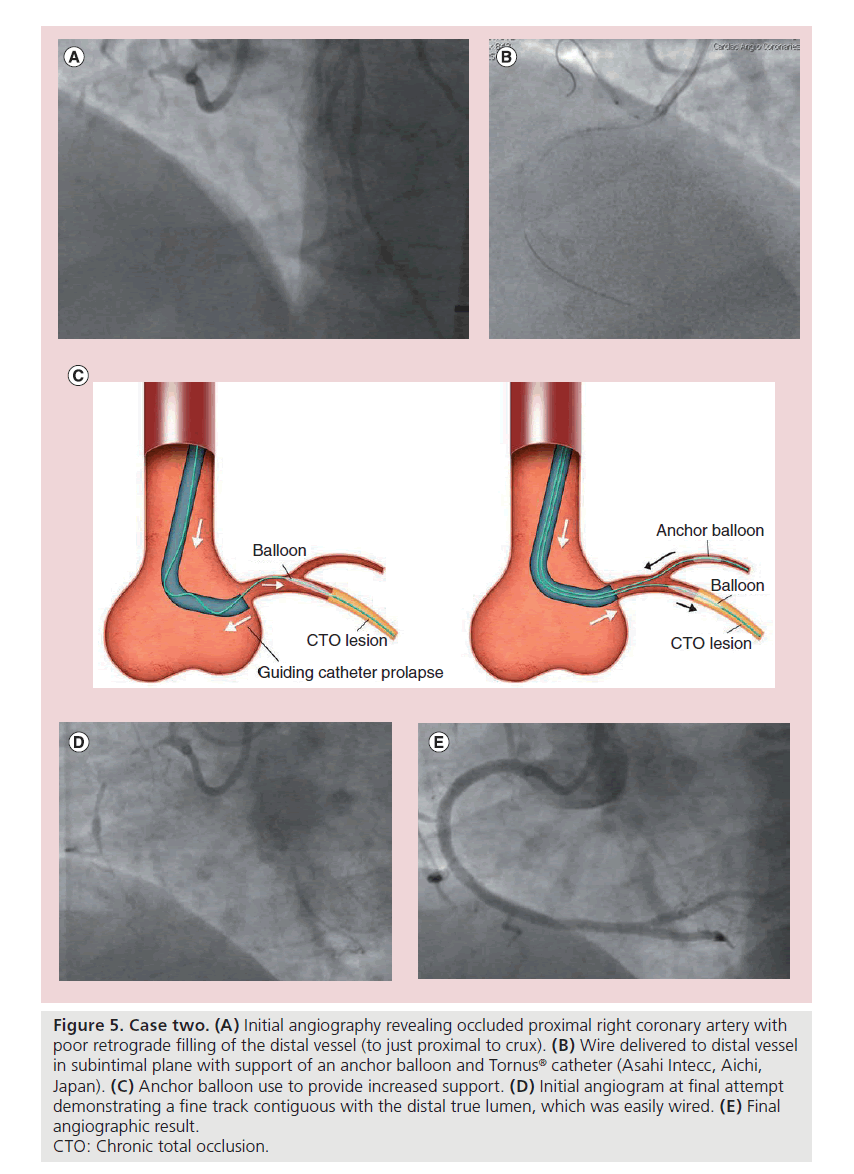
A 50-year-old obese male with hypertension and dyslipidemia presented with Canadian class 3 angina, refractory to optimal medical therapy. Diagnostic angiography revealed minor disease in the left system with a proximal RCA CTO. An unsuccessful ad hoc PCI attempt was made, with the failure mode being an inability to cross the proximal cap. A second PCI attempt was made 4 weeks later. The initial angiogram with dual injections is shown in Figure 5A; antegrade dissection and re-entry was the primary strategy. The proximal lesion was heavily calcified and proved very difficult to cross with high gram tipload stiff wires. Deliberate rupture of a 1.5 mm rapid exchange balloon at the proximal cap finally disrupted the proximal cap, thus allowing wire passage into the occluded segment, which was also heavily calcified. A 2.6 F Tornus® catheter (Asahi Intecc, Aichi, Japan) with the support of an anchor balloon in an 8 F system was required to cross the lesion (Figure 5B & C) and finally a CrossBoss catheter could be delivered to the distal vessel; however, re-entry with the Stingray guidewire and balloon was unsuccessful, pre-dominantly owing to loss of distal visualization. The procedure was abandoned with a neutral outcome, with a procedure and fluoroscopy time of 185 and 67 min, respectively, a contrast dose of 375 ml and a radiation dose of 20,447 cGycm2. Prior to case cessation, balloon angioplasty with a 2.5 mm diameter balloon was performed at the proximal cap to consolidate the lesion modification achieved. A repeat PCI attempt was made 4 months later in the face of ongoing angina. Initial angiography revealed a track contiguous with the distal vessel (Figure 5D). This was easily wired with a jacketed wire within minutes and the vessel reconstructed with three overlapping drug-eluting stents (Figure 5E). Procedural time was short (90 min) and contrast/radiation doses were low (240 ml and 22 min, respectively) in the second case. An excellent final result was achieved and the patient remains free of angina.
Figure 5. Case two. (A) Initial angiography revealing occluded proximal right coronary artery with
poor retrograde filling of the distal vessel (to just proximal to crux). (B) Wire delivered to distal vessel
in subintimal plane with support of an anchor balloon and Tornus® catheter (Asahi Intecc, Aichi,
Japan). (C) Anchor balloon use to provide increased support. (D) Initial angiogram at final attempt
demonstrating a fine track contiguous with the distal true lumen, which was easily wired. (E) Final
angiographic result.
CTO: Chronic total occlusion.
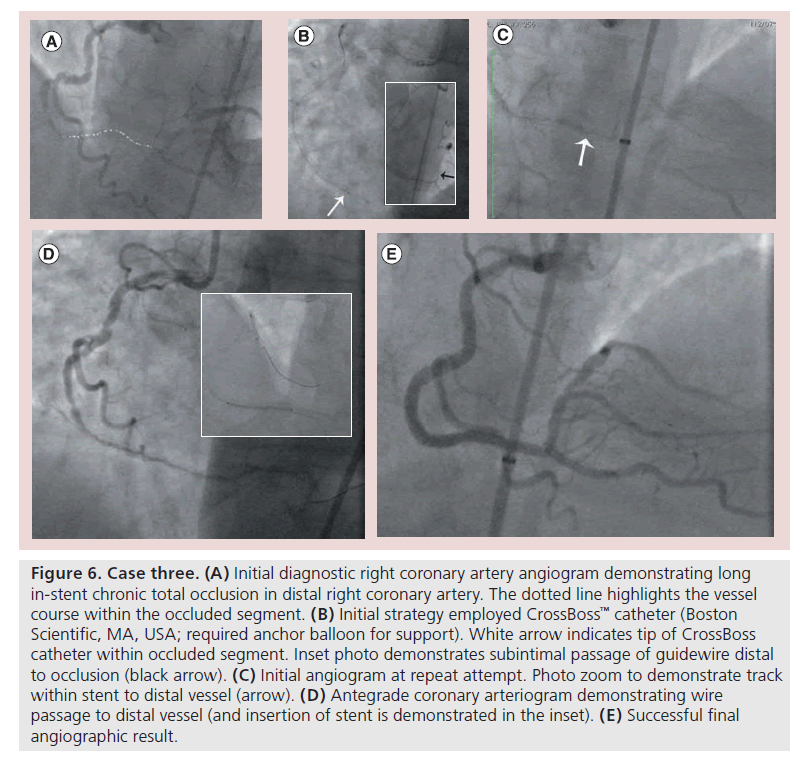
▪ Case three: PCI distal RCA CTO (occlusive in-stent restenosis)
A 65-year-old diabetic male represented with Canadian class 3 stable angina, having undergone PCI to the distal RCA 8 years prior (two overlapping bare stents). Diagnostic angiography revealed occlusive in-stent restenosis; the occluded segment was 50 mm in length and involved the entire length of stents with maximal diameter 2.5 mm at the index PCI (Figure 6A). The CrossBoss catheter has emerged as a useful tool in the setting of occlusive in-stent restenosis, often tracking from lumen to lumen as it is contained by the stent struts, and thus affording direct wiring of the distal vessel [7]. In this instance, however, the CrossBoss catheter stalled half way through the occluded stented segment despite the support of an anchor balloon. Wire passage through the CrossBoss catheter was difficult and tracked subintimally distal to the stents (Figure 6B). The case was abandoned at this juncture, owing to long procedure time and high-contrast and -radiation loads.
Figure 6. Case three. (A) Initial diagnostic right coronary artery angiogram demonstrating long in-stent chronic total occlusion in distal right coronary artery. The dotted line highlights the vessel course within the occluded segment. (B) Initial strategy employed CrossBoss™ catheter (Boston Scientific, MA, USA; required anchor balloon for support). White arrow indicates tip of CrossBoss catheter within occluded segment. Inset photo demonstrates subintimal passage of guidewire distal to occlusion (black arrow). (C) Initial angiogram at repeat attempt. Photo zoom to demonstrate track within stent to distal vessel (arrow). (D) Antegrade coronary arteriogram demonstrating wire passage to distal vessel (and insertion of stent is demonstrated in the inset). (E) Successful final angiographic result.
Repeat elective angiography 6 weeks later revealed an antegrade channel where the CrossBoss had tracked with TIMI 2 flow to the distal vessel (Figure 6C). The distal vessel was wired easily with a jacketed wire and reconstruction of the vessel undertaken with an excellent final result (Figure 6D & E).
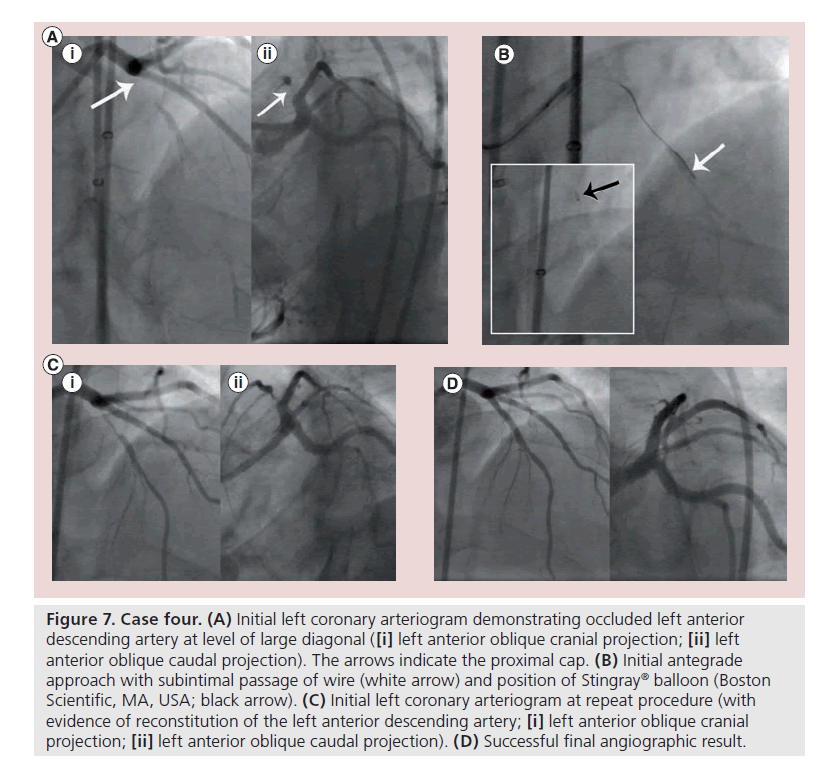
▪ Case four
A previously well 49-year-old man presented with stable angina. Angiography revealed an occluded mid-left anterior descending artery (Figure 7A). A previous limited attempt had been made by another operator, with the mode of failure being an inability to cross the proximal cap. A repeat attempt was undertaken 2 weeks later after the patient presented emergently with further chest pain. An initial antegrade wire-based strategy was successful in crossing the proximal cap, but resulted in subintimal passage of a Pilot 200 wire (Figure 7B), whose track was dilated with a Corsair® microcatheter (Asahi Intecc). Attempted distal re-entry using a Stingray balloon was unsuccessful, mainly hindered by poor visualization of the distal vessel (Figure 7B: inset). A retrograde approach was unsuccessful and the procedure was abandoned in light of a high- contrast load and time constraints.
Figure 7. Case four. (A) Initial left coronary arteriogram demonstrating occluded left anterior descending artery at level of large diagonal ([i] left anterior oblique cranial projection; [ii] left anterior oblique caudal projection). The arrows indicate the proximal cap. (B) Initial antegrade approach with subintimal passage of wire (white arrow) and position of Stingray® balloon (Boston Scientific, MA, USA; black arrow). (C) Initial left coronary arteriogram at repeat procedure (with evidence of reconstitution of the left anterior descending artery; [i] left anterior oblique cranial projection; [ii] left anterior oblique caudal projection). (D) Successful final angiographic result.
A third planned elective attempt was made 3 months later. Initial angiography revealed a new channel contiguous with the distal vessel (Figure 7C). The channel was wired easily with a jacketed guidewire after which angioplasty and stent insertion was undertaken (Figure 7D) with a short procedural time, low contrast dose (180 ml) and low radiation exposure (skin dose 0.6 Gy).
Discussion
Success rates for PCI of CTOs have improved in recent years, with technical success rates of greater than 80% recently reported in unselected and complex CTO patients [8–10].
Modes of failure include an inability to cross the lesion with a guidewire, an inability to re-enter the true lumen in the case of subintimal ante-grade or retrograde wire passage, or an inability to deliver a balloon or stent across the lesion.
▪ When to abandon a case?
The decision to abandon a case can be difficult, but is best made according to clear, predefined criteria. A clear appreciation of the mechanism for failure may allow one to identify, and there-fore, modify the case limiter, thereby increasing the chances of subsequent procedural success. Specific circumstances may dictate case cessation.
Radiation
Prolonged radiation exposure can be associated with skin injury [11]. Higher x-ray doses are associated with higher BMI, longer fluoroscopy times and increasing numbers of cine frames, as well as technical factors such as collimation, focus–detector distance and image intensifier field size [12]. An increased sensitivity to radiation-induced skin injury has been described in patients with connective tissue disease, diabetes or in the setting of previous radiation injury [13,14].
Radiation limits should be predefined and a case should be abandoned if success is not imminent when these thresholds are soon to be breached. Air kerma radiation skin dosages of >5 Gy or dose–area products of >17,000 cGycm2 are associated with skin injury and should be avoided. Fluoroscopy time is an imprecise measure of total radiation exposure, but nonethe-less, in general, fluoroscopy times of more than 60 min should be avoided [8]. It should be remembered though that 60 min of fluoroscopy in a thin patient will result in a lower air kerma dose than that in an obese person. Radiation dose can be minimized by reducing fluoroscopy dosages (e.g., by reducing frame rate to 7.5 frames/s), as well as utilization of nonmagnified viewing, coning and fluoroscopy storage (in lieu of cine acquisition) [1].
Contrast
The risk of contrast-induced nephropathy is proportional to the contrast dose and risk is increased with pre-existing renal dysfunction, diabetes, anemia, reduced left ventricular function and concurrent diuretic use [15]. A general rule of thumb is to avoid dosages of contrast greater than six-times the creatinine clearance, thus a procedure should be abandoned if more than 600 ml has been used in a nondiabetic patient with normal renal function if success is not in sight. Preoperative hydration and minimization of contrast dosages are the best preventative measures [16].
Subintimal hematoma
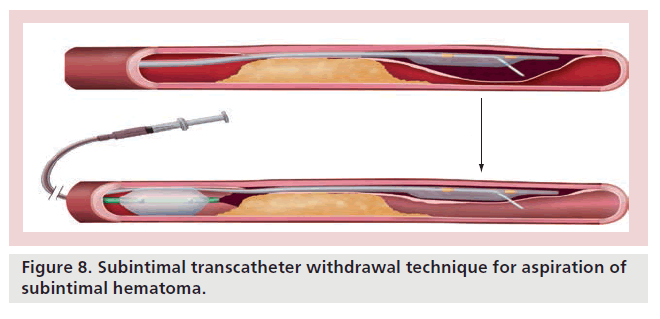
Use of the subintimal plane is increasing as part of a hybrid strategy and can afford rapid progress to allow one to establish a ‘base of operations’ quickly [1]. Poor management of this space can, however, result in intramural coronary hematoma with loss of distal vessel visualization or the formation of a long dissection plane, which can compromise distal branches. Subintimal hematoma formation may also drastically reduce the probability of success through compromising the success of re-entry techniques. The subintimal transcatheter withdrawal (STRAW) technique has developed as a potential solution in this instance, whereby true lumen compression is relieved by aspiration of subintimal hematoma through a microcatheter or over-the-wire balloon (Figure 8).
Complications
In general, the occurrence of a clinically relevant complication during a CTO procedure requires prompt and proper treatment and is a sufficient reason to stop the procedure. Perforation may be a consequence of intrawall routing of the wire and balloon, wire-mediated damage of channels connecting to the vasa vasorum, or inadvertent balloon inflation in a small side branch. Small, localized perforations (Ellis type 1 or 2), where extravasation of contrast is seen but does not clear, do not necessarily mandate case cessation, but larger perforations with hemodynamic instability requiring pericardiocentesis do.
Operator fatigue
It is important to recognize operator limitations and be aware of the impact that procedural fatigue can have on one’s concentration, decision-making and dexterity.
▪ Repeat PCI attempt?
When any of the above reasons dictate cessation of a PCI procedure, one must assess whether success is probable at repeat attempt (either in the present operator’s hands or in those of a more experienced operator). If so, one should ensure any progress made in the current setting is consolidated. In many cases (such as case one and two in this series), this pertains to the proximal cap, where much of the procedure time may have been consumed, and ‘consolidation’ may simply involve balloon angioplasty at this site. Balloon angioplasty in cases one and two led to a favorable healing response over time such that at the second procedure, a tract had formed to the distal vessel, thus little time needed to be spent renegotiating the proximal cap. Indeed, in case two, the wire was passed to the distal vessel in minutes.
Dissection propagation may compromise distal vessels, such as the posterior left ventricular branch in case one; indeed, most of the procedural time in the repeat attempt in case one was consumed in securing this branch in a retrograde manner. One mooted concern with balloon modification of the proximal cap is associated propagation of distal dissection. We feel that the risk of this is low owing to the absence of flow through the occlusion, with the tamponade effect of subintimal hemorrhage preventing further propagation. It should be emphasized, however, that there is no rationale to image the vessel by antegrade injection of contrast after balloon angioplasty at the proximal cap. This is unlikely to be helpful and more likely to be harmful. It is not usually necessary to dilate the entire CTO tract, rather just the proximal cap.
Even in the absence of ‘active’ lesion modification with balloon angioplasty, passage of microcatheters, such as the CrossBoss and Corsair catheters, past the distal cap can themselves lead to ‘passive’ lesion modification, which can facilitate future success. This was evident in cases three and four. The decision to stop in case four was based predominantly upon futility and high contrast load/procedure time. There were poor interventional collaterals in this case, thus all reasonable options had been exhausted. It would have been reasonable to dilate the track where the CrossBoss and distal wire had been (to the end of the occluded stents); however, it was interesting to see that the CrossBoss (3 F) track itself was enough to allow formation of a track that was contiguous with the distal vessel. In case four, the track created by the Corsair catheter and Stingray balloons communicated with the distal vessel at the time of repeat intervention.
To our knowledge, spontaneous recanalization as described in these four cases has not been previously reported. These cases demonstrate that in the case of failure where a subintimal track has been created, repeat angiography may be worthwhile to assess for potential vessel reconstitution. In general, repeat attempts should be deferred for at least 4 weeks to allow healing of the vessel wall and resolution of wall hematoma. Staging procedures in this fashion serves to avoid excess contrast and radiation loads; however, one must be cognizant of the cumulative x-ray dose, in particular, when planning repeat procedures.
Success in the second attempt may also be improved through a better understanding of the anatomy if ambiguity has been resolved (as in case one). This is particularly so if mode of failure in the initial case is understood.
Conclusion
Procedural failure in the setting of CTO PCI can have many modes. In the case of successful negotiation of the proximal cap, but subintimal passage of the guidewire and failed re-entry, repeat angiography in 4–6 weeks may be reasonable to assess for vessel reconstitution. Prior to case abandonment, actions to consolidate any lesion modification in the current procedure may facilitate future success.
Future perspective
Planning is critical to maximize procedural success, but at present is limited in its ability to accurately predict procedural complexity, and it is anticipated that further advances will occur in this area. We expect further technique modifications to address the primary failure mode for antegrade failure, in particular to assist in crossing the proximal cap and in reducing the degree of subintimal hemorrhage. It is also anticipated that advances in wire technology may be able to reduce the failure rate in crossing contralateral collaterals.
Financial & competing interests disclosure
JC Spratt has consultancy agreements with Boston Scientific and Abbott Vascular. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Procedural failure in chronic total occlusion percutaneous coronary intervention can have many modes.
▪ Common failure modes for antegrade chronic total occlusion percutaneous coronary intervention include failure to penetrate the proximal cap, failure to deliver equipment over a guidewire or failure to re-enter the distal lumen when subintimal.
▪ The most common retrograde percutaneous coronary intervention failure mode is an inability to wire collaterals.
▪ Case cessation prior to success should be considered in the setting of breaching radiation or contrast thresholds, loss of visualisation due to subintimal hematoma, the presence of significant complications or due to futility.
▪ A clear appreciation of the mechanism for failure can increase chances of future procedural success.
▪ Lesion modification in the first procedure, in particular of the proximal cap, may facilitate subsequent procedural success.
▪ In the setting of subintimal tracking but failed re-entry, repeat angiography at 6 weeks is worthwhile to assess for spontaneous vessel reconstitution.
References
- Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 5, 367–379 (2012).
- Katsuragawa M, Fujiwara H, Miyamae M, Sasayama S. Histologic studies in percutaneous transluminal coronary angioplasty for chronic total occlusion: comparison of tapering and abrupt types of occlusion and short and long occluded segments. J. Am. Coll. Cardiol. 21, 604–611 (1993).
- Srivasta SS, Edwards WD, Boos CM et al. Histologic correlates of angiographic chronic total occlusions: influence of occlusion duration on neovascular channel patterns and intimal plaque composition. J. Am. Coll. Cardiol. 29, 955–963 (1997).
- Whitlow PL, Burke MN, Lombardi WL et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs trial. J. Am. Coll. Cardiol. Interv. 5, 393–401 (2012).
- Igarashi Y, Yamane M, Fujita T et al. Clinical results of retrograde approach in PCI for CTOs: comparison between 2009 and 2010 with multicenter registry data in Japan. J. Am. Coll. Cardiol. 59, E1524 (2012).
- Rathore S, Katoh O, Matsuo H et al. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries. Procedural outcomes and predictors of success in contemporary practice. Circ. Cardiovasc. Interv. 2, 124–132 (2009).
- Wilson W, Walsh S, Hanratty C, Strange J, Hill J, Spratt J. A novel approach to the management of occlusive in-stent restenosis (ISR). EuroIntervention (2013) (In Press).
- Grantham JA, Marso SP, Spertus J, House J, Holmes DR Jr, Rutherford BD. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc. Interv. 2, 479–486 (2009).
- Thompson CA, Jayne JE, Robb JF et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions: an early US experience. JACC Cardiovasc. Interv. 2, 834–842 (2009).
- Sianos G, Barlis P, Di Mario C et al. European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the EuroCTO club. EuroIntervention 4(1), 84–92 (2008).
- Suzuki S, Furui S, Isshili T et al. Radiation exposure to patient’s skin during percutaneous coronary intervention for various lesions, including chronic total occlusion. Circ. J. 70, 44–48 (2006).
- Journy N, Sinnio-Tellier S, Maccia C et al. Main clinical, therapeutic and technical factors related to patient’s maximum skin dose in interventional cardiology procedures. Br. J. Radiol. 85, 433–442 (2012).
- Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am. J. Roentgenol. 17, 3–11 (2001).
- Suzuki S, Furui S, Isshiki T et al. Factors affecting the patient’s skin dose during percutaneous coronary intervention for chronic total occlusion. Circ. J. 71, 229–233 (2007).
- Mehran R, Aymong ED, Nikolsky E et al. A simple risk score for prediction of contrastinduced nephropthy after percutaneous coronary intervention. J. Am. Coll. Cardiol. 44(7), 1393–1399 (2004).
- Tepel M, Aspelin P, Lameire N. Contrastinduced nephropathy. Circulation 113, 1799–1806 (2006).