Research Article - Pharmaceutical Bioprocessing (2016) Volume 4, Issue 6
In Vitro Antioxidant Properties of Edible Marine Algae Sargassum swartzii, Ulva fasciata and Chaetomorpha antennina of Kerala Coast
- Corresponding Author:
- G
Muraleedhara Kurup
Department of Biochemistry
University of Kerala, Karyavattom
Thiruvananthapuram, Kerala, India
E-mail: gmkbio@gmail.com
Abstract
Keywords
aoxidative stress, antioxidants, Sargassum swartzii, Ulva fasciata, Chaetomorpha antennina
Introduction
Oxidative stress induced by the free radicals has been gained a vital importance as it forms the root cause of about 200 human diseases [1]. Free radicals are highly reactive molecules with unpaired electrons and are produced during various cellular processes [2]. They represent an essential part of metabolism and aerobic life. Many of the reactive oxygen species (ROS) and reactive nitrogen species (RNS) are grouped under free radicals. These include superoxide anions (O2.), hydroxyl radical (.OH), singlet oxygen, hydrogen peroxide (H2O2), ferric ion, nitric oxide (NO) and so on [3]. ROS and RNS are produced from both endogenous (inflammation, mental stressand cancer) and exogenous (pollutants, drugs and radiations) sources [4]. Under normal conditions ROS participate in many physiological functions such as protecting body from invading pathogens, regulates calcium concentration and act as second messengers [5]. They participate in the signal transduction of cytokine and tyrosine receptor, serine/threonine kinases and G protein-coupled receptors [3]. They are also essential in development and differentiation process [6].
Several degenerative changes in the cells and tissues due to oxidative stress can lead to many deadly diseases [7]. The oxidative stress damages nucleic acids, lipids and proteins in our body, alters cellular functions and finally results in apoptosis or necrosis [5]. Also it is responsible for the progression of diseases like diabetics, rheumatoid arthritis, myocardial infarction, cancer, post-ischemic perfusion injury, autoimmune pathologies, cardiovascular, neurodegenerative and inflammatory diseases [8]. Antioxidants can grant protection from oxidative damages and prevent the onset of many chronic diseases [9]. They are naturally present in our body (endogenous) and the additional supplementation can be done through the diet (exogenous). Natural antioxidants like ascorbic acid (vitamin C), α-tocopherol, and carotenoids are readily absorbed through diet [10]. Butyl hydroxyanisole (BHA) and butyl hydroxytoluene (BHT) are synthetic antioxidants but are proven to cause major side effects such as cancer [11]. The current trends in the pharmaceutical industries are the exploration of natural antioxidants to resolve such issues. Plants have been widely investigated as the potent source of antioxidants. The dietary antioxidants such as α-tocopherol, ascorbic acid, carotenoids, amino acids, peptides, proteins, flavonoids and other phenolic compounds were proven effective in boosting antioxidant mechanism. The role of naturally occurring peptides in the biological system is well-established [12]. Peptides capable of developing synthetic vaccines against diseases are reported [13].
The marine world is also a rich source of bioactive molecules [14]. Among the marine organisms, seaweeds are well-explored for various bioactive compounds such as secondary metabolites, dietary fiber, minerals, lipids, proteins, omega-3 fatty acids, essential amino acids, polysaccharides and vitamins [15]. These compounds impart numerous bioactivities such as anti-oxidative, anti-inflammatory, antimicrobial and anti-cancer potential to these algae [16]. Among different algal derived compounds, sulfated polysaccharides (SPS) gained a crucial attention by the pharmaceutical industries. They are complex group of molecules with varying structure and properties. SPS are present in brown, green and red marine algae [17]. The development of a standardized algal polysaccharide based product is challenging, as their structure and properties are influenced by seasonal and climatic variations. Their high molecular weight and low bioavailability also form hurdles [18].
Despite of these difficulties, scientists have succeeded in elucidating several biological significances for algal derived sulfated polysaccharides.SPS have been hailed for the antioxidant, antitumor, immunomodulatory, anti-inflammation, anticoagulant and antimicrobial activities [18]. Fucoidan, a sulfated polysaccharide from Undaria pinnatifida was proven effective against hypersensitivity reactions by reducing the concentrations of both IL-4 and IL-13. Anti coagulant activity is the most studied property of marine sulfated polysaccharides and is similar to that of natural antioxidant heparin [19]. Cytotoxicity of marine sulfated polysaccharides against various cancer cell lines such as HeLa, HepG2, MCF-7 and melanoma B16were also well proven [21]. Studies show that, the sulfate content and the molecular weight play key roles in the bioactivities of these sulfated polysaccharides [17].
Sargassum swartzii (brown), Ulva fasciata and Chaetomorpha antennina (green) are the three main macro algae found in South Kerala coast. Sargassum swartzii was exploited for its larvicidal [22], anti HIV-1, anti-inflammatory and analgesic activities [23]. Anti-microbial, haemolytic [24] and anti-cancer [25] potentials of Ulva fasciata are also well studied. Chaetomorpha antennina is proven to possess anti-bacterial and antioxidant activity [26]. In the present study, we aimed to compare the antioxidant potentials of SPS from these three sources. We also have thrown light to correlate the chemical composition and the antioxidant potential among the three SPS.
Materials and Methods
Chemicals
All chemicals used in this study were of analytical grade obtained from Sisco Research Laboratories (SRL), Mumbai, Sigma-Aldrich, New Delhi.
Collection of seaweeds
Samples of Sargassum swartzii, Ulva fasciata and Chaetomorpha antennina were collected from Vizhinjam coast of Kerala, (Lat. 80 22’ N; Long. 760 59’ E on the west coast of India) during the month of January-February.
Extraction and isolation of crude sulfated polysaccharides
The collected seaweeds were washed in tap water, dried under shade, powdered and stored in airtight containers. Crude sulfated polysaccharides were isolated from all the samples using cold acidic extraction method [21]. The samples were decolorized and defatted by soaking and continuous stirring in acetone: methanol solvent mixture (7:3) and then stirred in 1N HCl for two days and then filtered. These steps were repeated twice and the filtrates were pooled and stored at 40C overnight. Then the polysaccharides were precipitated using absolute ethanol and lyophilized to obtain crude sample.
Determination of chemical composition.
The compositions of sulfated polysaccharide moieties such as total carbohydrates, sulfate, uronic acid, sulfated polysaccharide, fucose and xylose present in all extracts were determined.
Total carbohydrate content was estimated by the phenol–sulfuric acid method as described by Pham Duc Thinh et al., [27]. 5% phenol and concentrated sulfuric acid were added to the test sample (10mg/ml), incubated for 20 min and optical density (OD) was read at 490 nm. Dextrose was used as standard.
The sulfate content was determined by barium chloride–gelatin method using potassium sulfate as standard [28].The reaction mixture was prepared by adding 2g barium chloride to a solution of 0.6g gelatin in 200ml water which was kept overnight at 40C. To the test solution (10mg/ml) 4% TCA and 1ml chloride – gelatin solution was added and optical density was read at 360 nm after 15 min incubation.
Uronic acid content of the extract was estimated by carbazole method using glucoronic acid as standard [29].The test sample (10mg/ml) was heated in a boiling water bath for 10 min with 0.025M borax. Then 0.1% carbazole (in methanol) was added and boiling was continued for 15 min. The optical density was read at 540 nm.
Total sulfated polysaccharides were determined by metachromatic assay using heparin as standard [30]. 0.005% toluidine blue solution and 0.2% NaCl were added to the test sample (10mg/ml) and was mixed well for 30 sec. Then n-hexane was added to the above mixture and the 5ml aqueous layer was separated. Equal volume of absolute ethanol was added and the optical density was read at 631nm.
Fucose content was determined using cysteine hydrochloride [31]. Concentrated sulfuric acid was added to the test sample (10mg/ml) for 3 min. 3% cysteine hydrochloride was added and the difference in the optical density at 396nm and 427nm was calculated.
The monosaccharide xylose was estimated using orcinol method [32]. 0.1 ml of sample (10mg/ ml) was heated in a boiling water bath for 30 min. Optical density was read at 670nm
In vitro antioxidant activity of isolated polysaccharides.
The antioxidant activity of all the three sulfated polysaccharides at different concentration (0.5mg/ml-2mg/ml) was determined by standard protocols. The antioxidant assays include DPPH (1-1-diphenyl 2-picryl hydrazyl) radical scavenging activity [33], hydroxyl radical scavenging activity [34], hydrogen peroxide Scavenging activity [35], Total Antioxidant Activity [36] and Reducing power [37].
Statistics
All the results were expressed as mean ± standard deviation. One way ANOVA was calculated by using an online software statistic calculator. The P values <0.05 were considered to be significant [38], [39].
Results
The sulfated polysaccharides were obtained from Sargassum swartzii, Ulva fasciata and Chaetomorpha antennina by ethanol precipitation and the total yield was found to be 11%, 1.5% and 1.3% respectively. The chemical compositions all the three algae are given in Table1. S.swartzii showed a higher yield when compared to other two algae. It also showed an elevated amount of total carbohydrate, sulfate, uronic acid and fucose content when compared to other algae.
| Composition (%) | Sargassum swartzii | Ulva fasciata | Chaetomorpha antennina |
|---|---|---|---|
| Carbohydrates | 12.9 ± 1.59 | 12.3 ± 1.02 | 12.7 ± 1.08 |
| Sulfate | 10.6 ± 0.57 | 2.3 ± 0.13 | 1.5 ± 0.11 |
| Uronic acid | 7.7 ± 1.11 | 4 ± 0.54 | 4.5 ± 0.32 |
| Fucose | 2.9 ± 0.22 | 2.2 ± 0.05 | 3 ± 0.16 |
| Sulfated polysaccharide |
1.2 ± 0.19 | 0.15 ± 0.12 | 2.2 ± 0.91 |
| Xylose | 0.16 ± 0.07 | 0.26 ± 0.04 | 0.02 ± 0.01 |
Table 1: Yield and chemical composition of sulfated polysaccharides
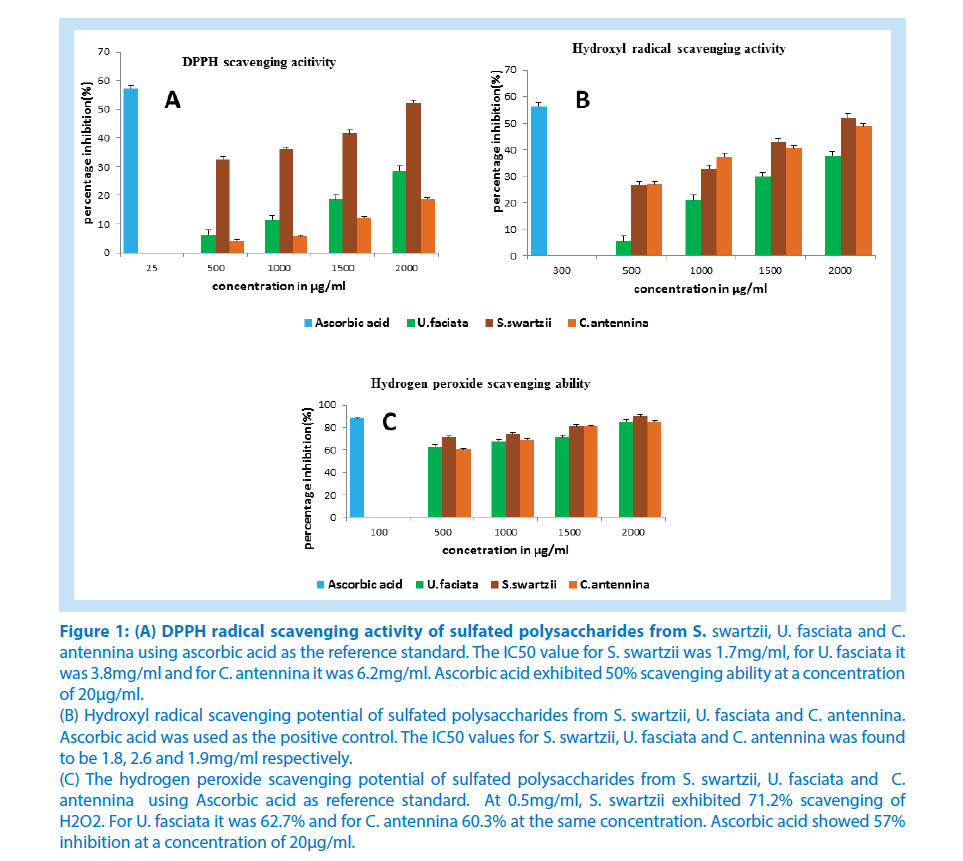
The antioxidant potential of crude sulfated polysaccharide from all the three algae was determined using various methods and compared with standard antioxidants. The DPPH radical scavenging activity is depicted in Figure 1A. All the samples exhibited a dose dependent increase in the DPPH scavenging activity. On comparing the scavenging potential of three algae, S. swartzii showed a higher DPPH scavenging effect. The half-maximal inhibitory concentration (IC50) value for S. swartzii was 1.7mg/ml, for U. fasciata it was 3.8mg/ml and for C. antennina it was 6.2mg/ml. Ascorbic acid exhibited 50% scavenging ability at a concentration of 20μg/ml.
Figure 1: (A) DPPH radical scavenging activity of sulfated polysaccharides from S. swartzii, U. fasciata and C. antennina using ascorbic acid as the reference standard. The IC50 value for S. swartzii was 1.7mg/ml, for U. fasciata it was 3.8mg/ml and for C. antennina it was 6.2mg/ml. Ascorbic acid exhibited 50% scavenging ability at a concentration of 20μg/ml. (B) Hydroxyl radical scavenging potential of sulfated polysaccharides from S. swartzii, U. fasciata and C. antennina. Ascorbic acid was used as the positive control. The IC50 values for S. swartzii, U. fasciata and C. antennina was found to be 1.8, 2.6 and 1.9mg/ml respectively. (C) The hydrogen peroxide scavenging potential of sulfated polysaccharides from S. swartzii, U. fasciata and C. antennina using Ascorbic acid as reference standard. At 0.5mg/ml, S. swartzii exhibited 71.2% scavenging of H2O2. For U. fasciata it was 62.7% and for C. antennina 60.3% at the same concentration. Ascorbic acid showed 57% inhibition at a concentration of 20μg/ml.
Figure 1B shows the hydroxyl radical scavenging activity of all the samples. Here also S. swartzii showed a better activity when compared to others. The half-maximal inhibitory concentration (IC50) values for S. swartzii, U. fasciata and C. antennina was found to be 1.8, 2.6 and 1.9mg/ ml respectively where as ascorbic acid control showed IC50 at 282μg/ml. The scavenging ability of all samples was in a dose-dependent manner.
All the samples were potent dose-dependent inhibitors of hydrogen peroxide and exhibited a better effect below 0.5mg/ml (Figure 1C). At 0.5mg/ml, S. swartzii exhibited 71.2% scavenging of H2O2, for U. fasciata it was 62.7% andfor C. antennina was 60.3%. Ascorbic acid showed 57% inhibition at a concentration of 20μg/ml.
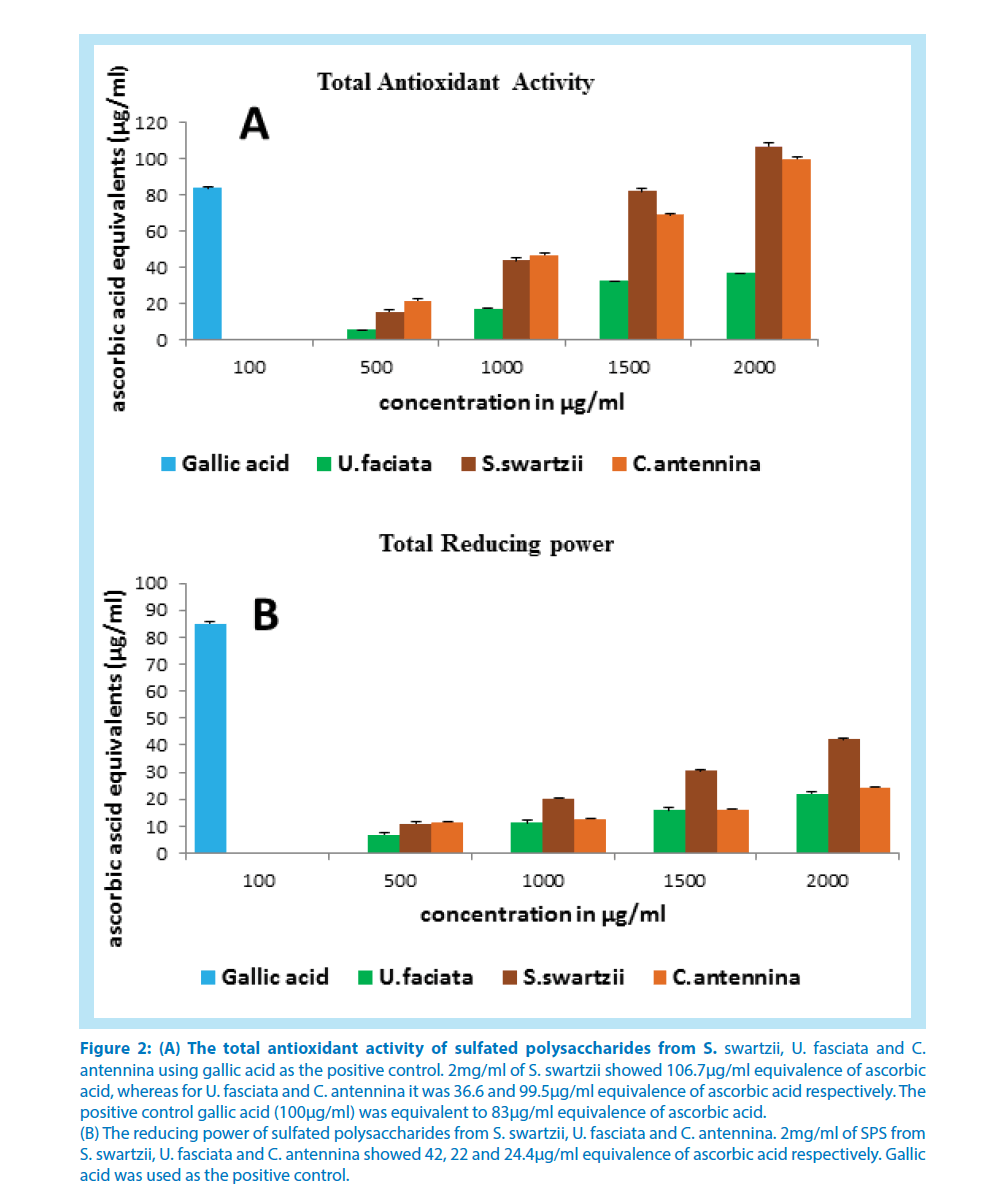
The total antioxidant activity of sulfated polysaccharides from all the three algae is displayed in Figure 2A. Here also S. swartzii showed a good activity at higher concentration than at lower dose. 2mg/ml of S. swartzii showed 106.7μg/ml equivalence of ascorbic acid, whereas for U. fasciata and C. antennina it was 36.6and 99.5μg/ml equivalence of ascorbic acid respectively. The positive control gallic acid (100μg/ml) was equivalent to 83μg/ml equivalence of ascorbic acid.
Figure 2: (A) The total antioxidant activity of sulfated polysaccharides from S. swartzii, U. fasciata and C.
antennina using gallic acid as the positive control. 2mg/ml of S. swartzii showed 106.7μg/ml equivalence of ascorbic
acid, whereas for U. fasciata and C. antennina it was 36.6 and 99.5μg/ml equivalence of ascorbic acid respectively. The
positive control gallic acid (100μg/ml) was equivalent to 83μg/ml equivalence of ascorbic acid.
(B) The reducing power of sulfated polysaccharides from S. swartzii, U. fasciata and C. antennina. 2mg/ml of SPS from
S. swartzii, U. fasciata and C. antennina showed 42, 22 and 24.4μg/ml equivalence of ascorbic acid respectively. Gallic
acid was used as the positive control.
The reducing power of sulfated polysaccharide from S. swartzii was found to be more efficient when compared to other two algae (Figure 2B). 2mg/ml of SPS from S. swartzii, U. fasciata and C. antennina showed 42, 22 and 24.4μg/ ml equivalence of ascorbic acid respectively. 100μg/ml of gallic acid, the positive control, was equivalent to 85μg/ml equivalence of ascorbic acid.
Discussion
The study is aimed at the comparison of sulfated polysaccharides from three marine algae, Sargassum swartzii, Ulva fasciata and Chaetomorpha antennina, with respect to their chemical composition and antioxidant activity.
Acidic extraction method was applied for all the three algae. The yield and properties of isolated polysaccharides were highly influenced by the extraction procedure adopted for the same [40]. It is well known that the acidic extraction yields more sulfated polysaccharide than water extraction in Sargassum sps. [41]. Also in another method, which includes the precipitation of sulfated polysaccharide after removing alginate, the yield was found to be very less (4.3%) [42]. The brown algae Sargassum swartzii gave higher yield of sulfated polysaccharide when compared to other two green seaweeds. This higher yield is comparable to the yield obtained from Sargassum polycystum, 11.32±0.12% (w/w) [41]. When the acidic extraction method was used for both the green algae in our study, the yield was very less. Extraction using hot water at 75–850C, was found to be effective for polysaccharide isolation in Ulva species [43]. Thus, we could see that the current method is suitable for the isolation of sulfated polysaccharides from brown algae.
Sulfated polysaccharides are complex group of molecules and marine algae is its one of the major non-animal source [44]. They are composed of mainly sulfate and monosaccharides repeats and display a wide structural diversity among different groups of marine algae [45]. Fucose, mannose, galactose, glucose, xylose and uronic acid are the main monosaccharides found in marine sulfated polysaccharides [18]. Earlier studies confirmed that the seaweeds contain large amount of polysaccharides [46]. All the three algal polysaccharides evaluated herewere found to have almost similar and considerable percentage of total carbohydrate content. A sulfated polysaccharide isolated by Kokilam G et al. from Sargassum tenerrimum was found to posses 8.21% of total carbohydrate [47]. Also in another report, the biochemical analysis of Chaetomorpha antennina [48] exhibited 19.68% and 18.4% of carbohydrate content respectively. Total carbohydrate content of the current three algae also correlates with these results.
The brown algae Sargassum swartzii possess very high amount of sulfate content (10.6%) when compared to other two green algae. Evaluation of sulfate content of another brown algae Sargassum polycystum also reported comparable fraction of sulfate [41]. In another study, it was reported that brown algae possess more sulfate content than green algae [49]. Fucose is the most important monosaccharide in brown algae and our samples contained significant amount of fucose. But there is also presence of fucose in green algal polysaccharide. The fucose can also be present in the polysaccharides of green algae in rare cases [50]. Uronic acid content was found to be high in Sargassum than the other two green algae and similar trend is already reported [45] Brown algae are known to contain high amount of uronic acid in their polysaccharide structure [21].
The antioxidant capacity of sulfated polysaccharides from seaweeds is well-studied [51]. They are known to posses various antioxidant activities such as scavenging of free radicals like superoxide, hydroxyl and DPPH, lipid peroxide inhibition and ferric reducing antioxidant power [52]. In the current study the antioxidant property of all the three sulfated polysaccharides were evaluated and all of them exhibited antioxidant effects in a concentration dependent manner.
DPPH radical scavenging assay is a simple method for the determination of antioxidant capacity of a compound. DPPH (2,2-diphenyl- 1-picryl-hydrazyl-hydrate) is a stable free radical that gives purple color in ethanol solution and on reduction in the presence hydrogen donating antioxidants, turns the solution colorless [53]. Thus, the characteristic absorption shown by DPPH reduce in accordance with increased concentration of the antioxidant compound, which indicates the DPPH scavenging potential of the compound [54]. In the present examination, sulfated polysaccharides from all the three algae exhibited a dose dependent increase in the scavenging of DPPH radical and Sargassum was found to be more efficient. However, the effective concentration was found to be 2mg or above in all the three cases, which is much higher when compared to ascorbic acid.
There are reports that substantiate this high concentration of sulfated polysaccharide from seaweeds for DPPH scavenging [54,55].
The hydroxyl radical scavenging effect of the samples is determined by using Fenton reaction. Here, hydroxyl radicals are generated using Ferric-ascorbate–EDTA–H2O2 system, which will later react with deoxyribose to produce thiobarbituric acid reactive substances (TBARS). When heated with Thiobarbituric acid (TBA), TBARS forms a pink chromogen and the intensity of the color will get reduced depending up on the antioxidant ability of the test compound [34]. Evaluation of the current samples results in the effective scavenging of hydroxyl radical in a dose dependent manner. At a concentration of 2mg/ ml sulfated polysaccharides from Sargassum swartzii was found to be more effective in scavenging hydroxyl radical. In many literatures, it was reported that sulfated polysaccharides exhibit moderate or no defense against hydroxyl radical [56].
Hydrogen peroxide is a weak oxidizing agent that can cross membranes very rapidly and can oxidize and inactivate many essential enzymes. Thus, the cells have to eliminate H2O2 immediately for their biological existence [57].The H2O2 scavenging ability of an antioxidant compound can be evaluated by means of decrease in the optical density. Here sulfated polysaccharides from all the three algae showed a better ability to scavenge H2O2 in a dose dependent manner. All the three samples showed a better activity at a concentration below 0.5mg/ml. This is much better than other reports on the H2O2 scavenging ability of sulfated polysaccharides [58]. The sulfated polysaccharide isolated from the red algae Pterocladia capillacea showed 45.76% inhibition of hydrogen peroxide at a concentration of 1mg/ml [59].
The total antioxidant activity of the samples is evaluated as their ability to reduce molybdenum VI to molybdenum V and form the green colored phosphomolybdenum complex. There will be an increase in the intensity of the green color as the concentration of the sample increases [36]. Even though sulfated polysaccharides from Sargassum swartzii showed more activity, it is much lower when compared to that of standard gallic acid. Reducing power of a compound is another potent indicator of its antioxidant activity. It is the ability of the compound to reduce ferric ion to ferrous form, which is associated with a color change from yellow to Pearl’s Prussian Blue [60]. In the present study, all the samples exhibited a dose dependent increase in the reducing power. But, when compared to standard gallic acid it is too low. Thus total antioxidant and reducing power contributes much less to the antioxidant capacity of the sulfated polysaccharides in our study.
In all the antioxidant assays, sulfated polysaccharides from the brown algae Sargassum swartzii showed better activity when compared to other two algae. This can be explained easily by the percentage of sulfate in the extracted polysaccharides. It has already proven that, sulfate content is the main factor that contributes to the biological activity of the sulfated polysaccharides [51]. This may vary with degree of sulfation and position of sulfate groups [61]. Since the polysaccharide isolated from Sargassum swartzii contains higher amount of sulfate, it can scavenge free radicals more effectively. The possible mechanism behind the ROS scavenging potentials of sulfated polysaccharide was suggested to be H-atom transfer and electron transfer between polysaccharides and free radicals [62].
Conclusion
In conclusion, the sulfated polysaccharides isolated from all the three marine algae contained significant amount of carbohydrate. In brown algae Sargassum swartzii, sulfate content was found to be higher than the other two. All samples exhibited potent antioxidant capacity and the higher activity of Sargassum swartzii can be due to the presence high sulfate. Thus, these SPS can be effectively used for scavenging ROS in vitro and thus can reduce the risk of many diseases. Potential application of marine derived, novel antioxidant, sulfated polysaccharides in the food industry reveals a wide scope of these edible seaweeds.
Acknowledgements
We gratefully acknowledge UGC for financial assistance in the form of Basic Scientific Research- Research Fellowship in Science for Meritorious Students (BSR-RFSMS).
References
- Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects. Med. 32(4-6), 234-246 (2011).
- Thankam Finosh G, Jayabalan M. Reactive oxygen species—Control and management using amphiphilic biosynthetic hydrogels for cardiac applications.Adv.Biosci.Biotechnol. 4(12), 1134-1146 (2013).
- Villanueva C, Kross RD. Antioxidant-Induced Stress. Int. J. Mol.Sci. 13(2), 2091-2109 (2012).
- Thankam FG, Muthu J. Infiltration and sustenance of viability of cells by amphiphilic biosynthetic biodegradable hydrogels. J. Mater. Sci. Mater. Med. 25(8), 1953-1965(2014).
- Salim S (2014) Oxidative Stress and Psychological Disorders. CurrNeuropharmacol 12:140-147.
- Domej W, Oetll K, Renner W (2014) Oxidative stress and free radicals in COPD &ndash; implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis 1207. doi: 10.2147/COPD.S51226
- Samak G, Shenoy RP, Manjunatha SM, Vinayak KS (2009) Superoxide and hydroxyl radical scavenging actions of botanical extracts of Wagateaspicata. Food Chem 115:631–634. doi: 10.1016/j.foodchem.2008.12.078
- Ndhlala AR, Moyo M, Van Staden J (2010) Natural Antioxidants: Fascinating or Mythical Biomolecules? Molecules 15:6905–6930. doi: 10.3390/molecules15106905
- Anusha Bhaskar N (2011) Phytochemical screening and in vitro antioxidant activities of the ethanolic extract of Hibiscus rosasinensis L. V.G3 Annals of Biological Research, 2 (5) :653-661
- Padayatty SJ, Katz A, Wang Y, et al (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am CollNutr 22:18–35.
- Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z FürLebensm-Unters -Forsch 196:329–338.
- Raghavender, U. S., Bhaswati, B., Indranil, S., Rajagopal, A., Shamala, N., Balaram, P., Entrapement of a Water Wire in a Hydrophobic Peptide Channel with an Aromatic Lining. J. Phys. Chem, B, 2011, 115(29), 9236-9243.
- Rajagoapl, A., Charles, B. C., Alexey, Y. K., Joshua, D. S., Frederick J. K., Andrew, Z., and Jai, S. R., Enhancing the Magnitude of Antibody Responses through Biomaterial Stereochemistry. ACS Biomater. Sci. Eng., 2015, 1(7), 601-609.
- Balboa EM, Conde E, Moure A, et al (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785. doi: 10.1016/j.foodchem.2012.11.026
- Plaza M, Cifuentes A, Ibanez E (2008) In the search of new functional food ingredients from algae. Trends Food SciTechnol 19:31–39.doi: 10.1016/j.tifs.2007.07.012
- Lee J-C, Hou M-F, Huang H-W, et al (2013) Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int 13:55. doi: 10.1186/1475-2867-13-55
- Costa LS, Fidelis GP, Cordeiro SL, et al (2010) Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother64:21–28. doi: 10.1016/j.biopha.2009.03.005
- Jiao G, Yu G, Zhang J, Ewart H (2011) Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar Drugs 9:196–223. doi: 10.3390/md9020196
- Patel S (2012) Therapeutic importance of sulfated polysaccharides from seaweeds: updating the recent findings. 3 Biotech 2:171–185.doi: 10.1007/s13205-012-0061-9
- Wijesekara I, Pangestuti R, Kim S-K (2011) Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. CarbohydrPolym 84:14–21. doi: 10.1016/j.carbpol.2010.10.062
- Suresh V, Senthilkumar N, Thangam R, et al (2013) Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassumplagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochem 48:364–373. doi: 10.1016/j.procbio.2012.12.014
- Khanavi M, Toulabi PB, Abai MR, et al (2011) Larvicidal activity of marine algae, Sargassumswartzii and Chondriadasyphylla, against malaria vector Anopheles stephensi. J Vector Borne Dis 48:241–244.
- Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC (2012) Influence of surfactant on the preparation of silver nanoparticles by polyol method. Adv Nat SciNanosciNanotechnol
- Priyadharshini S, Bragadeeswaran S, Prabhu K, Ran SS (2011) Antimicrobial and hemolytic activity of seaweed extracts Ulvafasciata (Delile 1813) from Mandapam, Southeast coast of India. Asian Pac J Trop Biomed 1:S38–S39. doi: 10.1016/S2221-1691(11)60118-4
- Abirami. R. G and Kowsalya (2012). Anticancer activity of methanolic and aqueous extract of ulva fasciata in albino mice Int J Pharm Pharm Sci, Vol 4, Issue 2, 681-684
- Thanigaivel S, Vijayakumar S, Mukherjee A, et al (2014) Antioxidant and antibacterial activity of Chaetomorphaantennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 433:467–475. doi: 10.1016/j.aquaculture.2014.07.003
- P. Thinh, R. Menshova, S. Ermakova, S. Anastyuk, B. Ly, T. Zvyagintseva, Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei, (2013) Mar. Drugs. 11 1456–1476. doi:10.3390/md11051456.
- B.W.S. Souza, M.A. Cerqueira, A.I. Bourbon, A.C. Pinheiro, J.T. Martins, J.A. Teixeira, et al., (2012). Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae, Food Hydrocoll. 27 287–292. doi:10.1016/j.foodhyd.2011.10.005.
- V. Kumar, S. Nagar, Y.C. Tripathi, (2014) Do assorted approaches aid in estimation of uronic acids? Case studies on Tinospora sinensis polysaccharides, Int. J. Biol. Macromol. 70 360–363. doi:10.1016/j.ijbiomac.2014.07.010.
- D. Liao, X. Wang, P.H. Lin, Q. Yao, C. Chen, (2009) Covalent linkage of heparin provides a stable anti-coagulation surface of decellularized porcine arteries, J. Cell. Mol. Med. 13 2736–2743. doi:10.1111/j.1582-4934.2008.00589.x.
- Sawke N, Sawke G (2010) Serum fucose level in malignant diseases. Indian J Cancer 47:452. doi: 10.4103/0019-509X.73549
- P.F. Blackmore, J.F. Williams, (1974) Estimation of pentose 5-phosphate in the presence of heptulose and hexose phosphates by the orcinol method,Int. J. Biochem. 5 343–348. doi:10.1016/0020-711X(74)90128-1.
- B. Manochai, Y. Paisooksantivatana, H. Choi, J.H. Hong, (2010)Variation in DPPH scavenging activity and major volatile oil components of cassumunar ginger, Zingiber montanum (Koenig), in response to water deficit and light intensity, Sci. Hortic. 126 462–466. doi:10.1016/j.scienta.2010.07.011.
- Corpuz, M. J. A. T, Osi, M. O, Santiago, L. A(2013), Free radical scavenging activity of Sargassum siliquosum J. G. Agardh, Int. Food Res. J. 20 291–297.
- Ramani R, Sudini S, Boddupalli BM, Anisetti RN (2012) Antioxidant, free radical scavenging and invitro cytotoxic studies of ethanolic extract of Leucasindicavarlavandulifolia and Leucasindicavarnagalapuramiana. Asian Pac J Trop Biomed 2:S1637–S1642. doi: 10.1016/S2221-1691(12)60468-7
- Do QD, Angkawijaya AE, Tran-Nguyen PL, et al (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophilaaromatica. J Food Drug Anal 22:296–302. doi: 10.1016/j.jfda.2013.11.001
- Zhao Y, Du S, Wang H, Cai M (2014) In vitro antioxidant activity of extracts from common legumes. Food Chem 152:462–466. doi: 10.1016/j.foodchem.2013.12.006
- Finosh GT, Jayabalan M (2015) Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics for cardiac tissue engineering. RSC Adv 5:38183–38201. doi: 10.1039/C5RA04448K
- Thankam FG, Muthu J (2015) Alginate–polyester comacromer based hydrogels as physiochemically and biologically favorable entities for cardiac tissue engineering. J Colloid Interface Sci 457:52–61. doi: 10.1016/j.jcis.2015.06.034
- Alves A, Sousa RA, Reis RL (2013) Processing of degradable ulvan 3D porous structures for biomedical applications. J Biomed Mater Res A 101A:998–1006. doi: 10.1002/jbm.a.34403
- AttachaiKantachumpoo AC (2010) Components and Antimicrobial Activity of Polysaccharides Extracted from Thai Brown Seaweeds. Kasetsart J - Nat Sci 44:220 – 233.
- García-Ríos V, Ríos-Leal E, Robledo D, Freile-Pelegrin Y (2012) Polysaccharides composition from tropical brown seaweeds: Polysaccharides from seaweeds. Phycol Res 60:305–315. doi: 10.1111/j.1440-1835.2012.00661.x
- Alves A, Caridade SG, Mano JF, et al (2010) Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr Res 345:2194–2200. doi: 10.1016/j.carres.2010.07.039
- Costa LS, Fidelis GP, Telles CBS, et al (2011) Antioxidant and Antiproliferative Activities of Heterofucans from the Seaweed Sargassumfilipendula. Mar Drugs 9:952–966. doi: 10.3390/md9060952
- Silva TH, Alves A, Popa EG, et al (2012) Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2:278–289. doi: 10.4161/biom.22947
- Vera J, Castro J, Gonzalez A, Moenne A (2011) Seaweed Polysaccharides and Derived Oligosaccharides Stimulate Defense Responses and Protection Against Pathogens in Plants. Mar Drugs 9:2514–2525. doi: 10.3390/md9122514
- Vijayabaskar P, Vaseela N (2012) In vitro antioxidant properties of sulfated polysaccharide from brown marine algae Sargassumtenerrimum. Asian Pac J Trop Dis 2:S890–S896. doi: 10.1016/S2222-1808(12)60287-4
- Manju Chandraprabha. M. (2012) Biochemical and Nanotechnological Studies in Selected Seaweeds of Chennai Coast. J Appl Pharm Sci. doi: 10.7324/JAPS.2012.21118
- Cavas L, Pohnert G (2010) The Potential of Caulerpa spp. for Biotechnological and Pharmacological Applications. In: Seckbach J, Einav R, Israel A (eds) Seaweeds and their Role in Globally Changing Environments. Springer Netherlands, Dordrecht, pp 385–397
- Mamedov T, Yusibov V (2011) Green algae Chlamydomonasreinhardtii possess endogenous sialylated N-glycans. FEBS Open Bio 1:15–22. doi: 10.1016/j.fob.2011.10.003
- Barahona T, Chandía NP, Encinas MV, et al (2011) Antioxidant capacity of sulfated polysaccharides from seaweeds.A kinetic approach. Food Hydrocoll 25:529–535. doi: 10.1016/j.foodhyd.2010.08.004
- Ngo D-H, Wijesekara I, Vo T-S, et al (2011) Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. Food Res Int 44:523–529. doi: 10.1016/j.foodres.2010.12.030
- Garcia EJ, Oldoni TLC, Alencar SM de, et al (2012) Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J 23:22–27.
- Dore CMPG, das C Faustino Alves MG, Will LSEP, et al (2013) A sulfated polysaccharide, fucans, isolated from brown algae Sargassumvulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. CarbohydrPolym 91:467–475. doi: 10.1016/j.carbpol.2012.07.075
- Shao P, Chen X, Sun P (2014) Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassumhorneri. CarbohydrPolym 105:260–269. doi: 10.1016/j.carbpol.2014.01.073
- Magalhaes KD, Costa LS, Fidelis GP, et al (2011) Anticoagulant, Antioxidant and Antitumor Activities of Heterofucans from the Seaweed Dictyopterisdelicatula.Int J MolSci 12:3352–3365. doi: 10.3390/ijms12053352
- Gülden M, Jess A, Kammann J, et al (2010) Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free RadicBiol Med 49:1298–1305. doi: 10.1016/j.freeradbiomed.2010.07.015
- Abdelmalek BE, Sila A, Krichen F, et al (2015) Sulfated polysaccharides from Loligo vulgaris skin: potential biological activities and partial purification. Int J BiolMacromol 72:1143–1151. doi: 10.1016/j.ijbiomac.2014.09.041
- Fleita D, El-Sayed M, Rifaat D (2015) Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae; Pterocladiacapillacea. LWT - Food SciTechnol 63:1236–1244. doi: 10.1016/j.lwt.2015.04.024
- Liu J, Jia L, Kan J, Jin C-H (2013) In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricusbisporus). Food ChemToxicolInt J Publ Br IndBiol Res Assoc 51:310–316. doi: 10.1016/j.fct.2012.10.014
- Hu T, Liu D, Chen Y, et al (2010) Antioxidant activity of sulfated polysaccharide fractions extracted from Undariapinnitafida in vitro.Int J BiolMacromol 46:193–198. doi: 10.1016/j.ijbiomac.2009.12.004
- Yan J-K, Wang W-Q, Ma H-L, Wu J-Y (2012) Sulfation and Enhanced Antioxidant Capacity of an Exopolysaccharide Produced by the Medicinal Fungus Cordycepssinensis. Molecules 18:167–177. doi: 10.3390/molecules18010167