Research Article - Interventional Cardiology (2019) Volume 11, Issue 4
Incidence, angiographic features, clinical phenotype and therapeutic challenges of myocardial infarction due to spontaneous Coronary artery dissection in central Greece
- Corresponding Author:
- John Papanikolaou
Department of Cardiology
General Hospital of Trikala
Trikala, Thessaly, Greece
E-mail: y_papanikolaou@hotmail.com
Received Date: July 21, 2019 Accepted Date: July 26, 2019 Published Date: August 02, 2019
Abstract
Background: Spontaneous coronary artery dissection (SCAD) is a rare cause of myocardial infarction (MI), typically affecting subjects with low index of suspicion for coronary artery disease. Data regarding SCADrelated MI in central Greece is extremely sparse. Methods: In this three-year study (January 2016-December 2018), all patients who admitted/transferred due to ST-elevation MI (STEMI) or non-STEMI (NSTEMI) were prospectively evaluated. Baseline, predisposing conditions, angiographic and revascularization data, in-hospital and long-term events were meticulously recorded. Results: Among 474 MI patients enrolled, 439 (92.6%) underwent coronary angiography. SCAD accounted for 1.82% (8/439) of MIs overall [2.5% (6/235) of STEMIs and 1% (2/204) of NSTEMIs; P=0.219]. SCAD affected predominately women (7/8; 87.5%). The incidence of SCAD was significantly increased in women with STEMI (7% vs. 0%, P=0.001) but not NSTEMI (1.3% vs. 0.79%, P=0.7194), compared to men. Among women with SCAD, 57.1%(4/7) were post-menopausal, none was pregnant, and 100% (7/7) revealed a left anterior descending artery (LAD) dissection. The prevalence of SCAD in women aged<55 compared to women ≥ 55 years-old was significantly increased in MI overall, STEMI and NSTEMI subsets (13.95% vs. 0.85%; 18.5% vs. 1.7%; 6.2% vs. 0%, P=0.0003;0.0049;0.0494, respectively). Type 2 lesion (diffuse smooth stenosis) was the most common angiographic feature (6/8;75%), while type 1 (multiple radiolucent lumen) was less found (2/8;25%). Established risk factors or precipitating stressors reported in other SCAD populations either failed to be related or showed weak relationship with SCAD in our specific region. Medical therapy was prioritized against early coronary revascularization with favorable outcomes. Conclusion: Our study provides an incidence of 1.82% for SCAD-induced MI in central Greece. SCAD mainly affects women, who are typically aged<55, non-pregnant (rather postmenopausal), and it predominately occurs as type 2 LAD disease; established precipitating SCAD stressors/disorders show weak pathogenic role in our specific population.
Keywords
Spontaneous coronary artery dissection; Myocardial infarction incidence; Medical therapy; Revascularization; Central Greece
Abbreviations
SCAD: Spontaneous coronary Artery Dissection; CAD: Coronary Artery Disease; ACS: Acute Coronary Syndrome; SCD: Sudden Cardiac Death; MI: Myocardial Infarction; STEMI: ST-segment Elevation Myocardial Infarction; NSTEMI: Non-ST Elevation Myocardial Infarction; CT: Computed Tomography; LAD: Left Anterior Descending Artery; TTC: Takotsubo Cardiomyopathy; MACE: Major Cardiovascular Events
Introduction
Spontaneous coronary artery dissection (SCAD) is a rare non-traumatic, noniatrogenic and non-atherosclerotic separation of coronary arterial wall [1-3]. The development of a false lumen within coronary arterial wall may exert external compression to the true lumen, impairing thus or even obstructing normal blood flow [1-3].
Consequently, similarly to atherosclerotic coronary artery disease (CAD), coronary dissection may be clinically associated with the whole spectrum of acute coronary syndrome (ACS), from myocardial ischemia and/or infarction (MI) to lethal ventricular arrhythmias and sudden cardiac death (SCD) [1-3]. It is of note, however, that SCAD typically affects subjects with low index of suspicion for CAD, especially younger women; this renders diagnosis extremely challenging [1-3].
Data regarding SCAD-induced MI in central Greece is extremely sparse in the literature. In this study, we aimed at identifying the prevalence of SCAD in central Greece, and also reviewing potential clinical features, predisposing factors and therapeutic implications of the entity in our geographic region.
Methods
In this three-year study (January 2016 – December 2018), we prospectively evaluated all patients who admitted/transferred in our Cardiology Department due to ST-elevation MI (STEMI) and non-STEMI (NSTEMI). Baseline, predisposing and precipitating conditions, coronary angiography and revascularization data, in-hospital and long-term events were recorded. SCAD was defined as the detection of non-traumatic, non-iatrogenic and non-atherosclerotic separation of coronary arterial wall in coronary angiography. Renal artery evaluation for fibromuscular dysplasia was performed either during coronary angiography or via CT angiography. All of our SCAD-induced MI patients underwent a final follow-up on January 2019. Informed consent was obtained from the patients or their relatives. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Statistical Analysis
Categorical variables were expressed as numbers (n) and percentages (%), and were compared by chisquare or Fisher’s exact test, as indicated. Post-hoc sample size calculations [with a probability of a type-I error of 5% (alpha=0.05)] were performed in order to calculate the statistical power of differences in the incidence of SCAD observed within several subgroups examined. The statistical package SPSS 17.0 (Chicago, IL, USA) was used. The statistical tests were two-sided. A P-value<0.05 was considered statistically significant.
Results
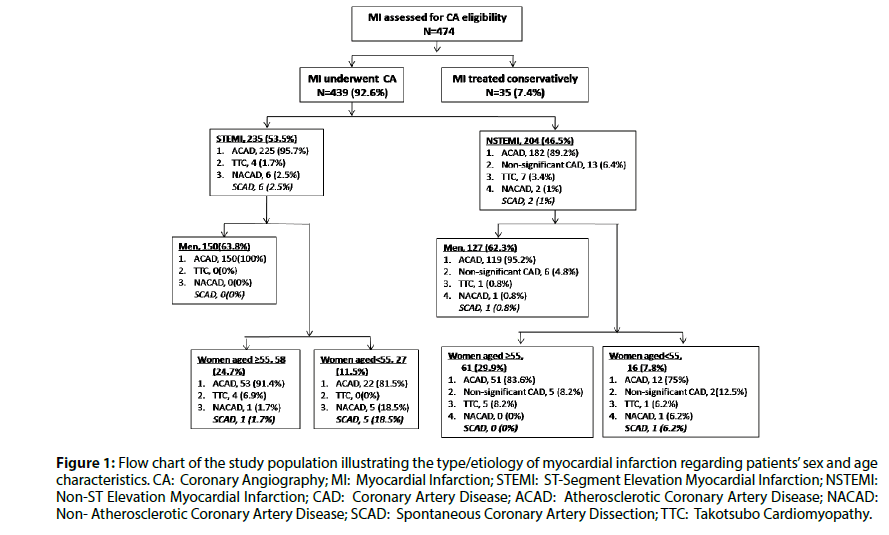
Figure 1 illustrates the patients’ flow chart and the distribution of SCAD regarding the type of MI, sex and age characteristics. In our cohort, the incidence of SCAD as the underlying cause of MI was 1.82% (2.5% of STEMIs and 1% of NSTEMIs; P=0.219, Fisher’s exact test). Table 1 demonstrates baseline clinical characteristics, while Table 2 illustrates the angiographic features and therapeutic strategies followed in our eight patients with SCAD-associated MI. They were predominately women (7/8;87.5%), aged under 55 years-old (7/8;87.5%) and principally post-menopausal (4/7;57.1%).
Figure 1: Flow chart of the study population illustrating the type/etiology of myocardial infarction regarding patients’ sex and age characteristics. CA: Coronary Angiography; MI: Myocardial Infarction; STEMI: ST-Segment Elevation Myocardial Infarction; NSTEMI: Non-ST Elevation Myocardial Infarction; CAD: Coronary Artery Disease; ACAD: Atherosclerotic Coronary Artery Disease; NACAD: Non- Atherosclerotic Coronary Artery Disease; SCAD: Spontaneous Coronary Artery Dissection; TTC: Takotsubo Cardiomyopathy.
| Case number | Year | Age (years) | Sex | ECG on presentation | Clinical presentation | CAD/SCAD risk factors | Follow-up CAA |
|---|---|---|---|---|---|---|---|
| 1 | 2016/Feb | 53 | F | STEMI (ST-segment elevation in V2-V5); T-wave inversion in V2-V6 | Intermittent epigastralgia after meals; duration of 2-3 days | Post-menopausal situation (menopause at 44) | 2 months Angiographic improvement |
| 2 | 2017/Sep | 31 | F | Normal on day-1 ST-segment elevation in V1-V5 on day-6 |
Atypical chest pain Pain relapse on day-6 |
Premature menopause (22) Smoking 15py Family history (father; SCD at 52) |
2 months Vascular patency (Figure 2). |
| 3 | 2017/Nov | 48 | F | Anterior wall STEMI (ST-segment elevation in I, aVL, V2-V6) | Severe typical chest pain Preceding emotional stress |
Precipitating emotional stress Menopause at 42 Hypothyroidism Family history (brother; MI at 45 |
8 months Distal LAD patency restoration (Figure 2) |
| 4 | 2018/Jan | 51 | F | Anteroseptal STEMI (ST-segment elevation in V1-V4) | Out-of-hospital cardiac arrest (VF); Intubation-sedation, mechanical ventilation and vasopressor support. |
Family history (brother; SCD at 44) | 1month Angiographic improvement (Figure 2). |
| 5 | 2018/Jul | 67 | F | Non-specific ST-T disorders in precordial leads | Chest pain; no relapses after initial medical treatment | Arterial hypertension Hypothyroidism |
- |
| 6 | 2018/Nov | 62 | M | ST depression in lateral leads | Nocturnal chest pain; no relapses after initial medical treatment | Arterial Hypertension | - |
| 7 | 2018/Dec | 51 | F | Anterior wall STEMI (ST-segment elevation in V2-V6) | Chest pain; no relapses after initial medical treatment | Post-menopausal situation (menopause at 42) | - |
| 8 | 2018/Dec | 51 | F | Anterior wall STEMI (ST-segment elevation in V1-V6) | Chest pain; no relapses after initial medical treatment | Smoking 25py Crohn’s disease |
- |
Abbreviations: CAD: Coronary Artery Disease; SCAD: Spontaneous Coronary Artery Dissection; CAA: Coronary Artery Angiography; STEMI: ST-Segment Elevation Myocardial Infarction; py: Pack-Years; LAD: Left Nterior Decending Artery; VF: Ventricular Fibrillation; SCD: Sudden Cardiac Death
Table 1: Clinical characteristics of our eight patients with SCAD-induced myocardial infarction.
| Case number | Vessel(s) involved | Type of SCAD | Vessel tortuosity/ Intravascular thrombus | Medical therapy | Interventional therapy | Surgical therapy |
|---|---|---|---|---|---|---|
| 1 | Mid-LAD | 2 | +/- | Nitrates iv; ASA+Clopidogrel; LMWH; Statin; β-blocker; ACE-i | - | - |
| 2 |
|
1 | +/+ | Nitrates iv; GP IIb-IIIa inhibitor; ASA+Clopidogrel; LMWH; Statin; β-blocker; ACE i |
PCI on day-6 (stents in LAD and D1) | - |
| 3 | Mid/distal LAD (occlusion distal to D3) | 2 | +/- | Nitrates iv; ASA+Clopidogrel; LMWH; Statin;β-blocker |
- | - |
| 4 |
|
2 | +/- | Sedation iv; Noradrenaline iv; Amiodarone iv; ASA+Clopidogrel; Statin | - | Referred for surgical consultation; no CABG was performed (see text) |
| 5 | Distal LAD | 2 | +/- | ASA+Clopidogrel; LMWH; Statin; β-blocker; ACE-i | - | - |
| 6 | Mid-RCA | 1 | -/- | ASA+Clopidogrel; LMWH; Statin; β-blocker; ACE-i | - | - |
| 7 | Mid-LAD | 2 | +/- | ASA+Clopidogrel; Statin; β-blocker; ACE-i | - | - |
| 8 | Mid-LAD | 2 | +/- | ASA+Clopidogrel; Statin; β-blocker; ACE-i | - | - |
Abbreviations: SCAD: Spontaneous Coronary Artery Dissection; LAD: Left Anterior Descending Artery; D1, D3;: Diagonal Branches (first and third, respectively); ASA: Aspirin; LMWH: Low Molecular Weight Heparin; ACE-i: Angiotensin Converting Enzyme Inhibitor; GP: Glycoprotein; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Grafting
Table 2: Angiographic characteristics and therapeutic interventions in our eight patients with SCAD-induced myocardial infarction.
The prevalence of SCAD was significantly increased in women with STEMI [7% vs. 0%, P=0.001 (Fisher’s exact test), post-hoc power of 84.6% with alpha=0.05) but not NSTEMI [1.3% vs. 0.79%, P=0.7194 (Fisher’s exact test)], compared to men. In addition, the prevalence of SCAD in women aged<55 suffering an MI compared to women ≥ 55 years-old was significantly increased in MI overall [13.95% vs. 0.85%, P=0.0003 (Fisher’s exact test), post-hoc power of 86.8% with alpha=0.05), as well as for STEMI (18.5% vs. 1.7%, P=0.0049 (Fisher’s exact test), post-hoc power of 74.7% with alpha=0.05) and NSTEMI [6.2% vs.0%, P=0.0494 (Fisher’s exact test), post-hoc power of 50.1% with alpha=0.05) subsets.
All women with SCAD (7/7;100%) suffered a left anterior descending artery (LAD) dissection, while the only male patient demonstrated a right coronary artery SCAD. LAD-related SCAD was associated with left ventricular systolic dysfunction, partially reversed on follow-up examination(s) (Table 1).
Type 2 lesion (diffuse smooth stenosis) was the most common angiographic SCAD feature in our series, while type 1 (multiple radiolucent lumen) was less found [(75%(6/8) and 25%(2/8), respectively] (Table 2; Figure 2).
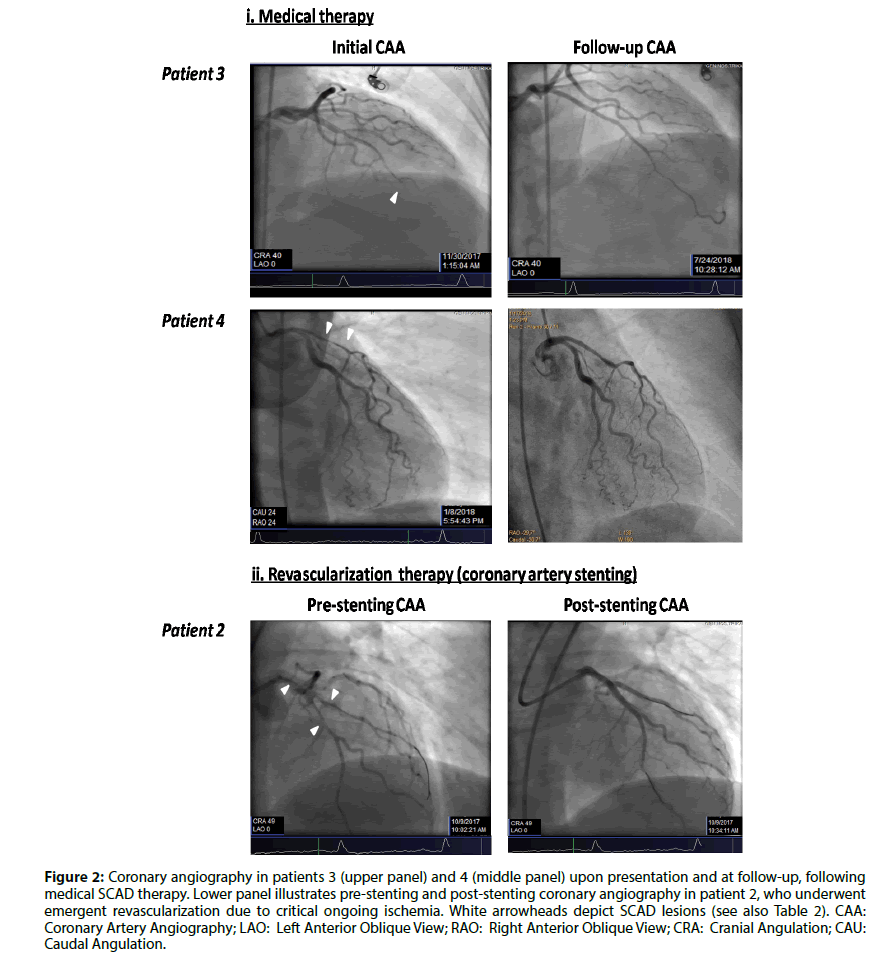
Figure 2: Coronary angiography in patients 3 (upper panel) and 4 (middle panel) upon presentation and at follow-up, following medical SCAD therapy. Lower panel illustrates pre-stenting and post-stenting coronary angiography in patient 2, who underwent emergent revascularization due to critical ongoing ischemia. White arrowheads depict SCAD lesions (see also Table 2). CAA: Coronary Artery Angiography; LAO: Left Anterior Oblique View; RAO: Right Anterior Oblique View; CRA: Cranial Angulation; CAU: Caudal Angulation.
Except for patient-3, our SCAD patients stated no emotional stress prior to the induction of cardiac symptoms. Instead, severe anxiety was reported to precede Takotsubo Cardiomyopathy (TTC) in our cohort [1 male; 10 female (90% of them aged ≥ 55 years-old)] (Figure 1).
None of our patients demonstrated evidence of underlying fibromuscular dysplasia, while only one subject (patient-8) had a history remarkable of Crohn’s disease (Table 1).
Discussion
The present study provides a SCAD incidence of 1.82% among patients presenting with MI in our specific geographic region (central Greece). Despite non-availability of high-resolution intracoronary techniques (intravascular ultrasound, optical coherence tomography) [1,2], our results are in line to previously reported data, where SCAD accounts for 0.1-4% of ACS [3-5].
Our data suggests that, among patients suffering an MI in our district, SCAD predominately occurs in young<55 years-old and primarily post-menopausal women. Previous studies [6-9] have also highlighted the predominance of female sex (82%-98%) in SCAD; interestingly, a significant percentage of those women in these studies (up to 60%) were post-menopausal as well. In addition, while pregnancy has also been associated with (up to 2-30% of) SCAD through a constellation of proposed mechanisms [6-11], such a relationship was not supported by our limited data. Finally, several parameters that had been associated with SCAD occurrence in previous reports were also examined in our study; however, the possible pathogenic role of such factors, including arteriopathies (fibromuscular dysplasia, connective tissue disorders) or precipitating stressors (intense emotional stress, physical activities, hormone therapy, sympathomimetic drugs and intense Valsalva-like activities) was not confirmed in our series [7-9]. Interestingly, however, our data provides evidence that the combination of severe anxiety and age ≥ 55, however, is likely to clinically discriminate TTC rather than SCAD in our specific population (Results section; Table 1).
Clinical manifestations in SCAD may vary from mild chest pain to fulminant cardiogenic shock [1,3,7,12,13]. The incidence of STEMI and NSTEMI ranges significantly among various populations (26.1-49% and 8-70%, respectively) [6,7,9-14]. In our cohort, the percentage of STEMI and NSTEMI in SCAD-induced MI was 75%(6/8) and 25%(2/8), respectively (Table 1). Left ventricular dysfunction in LAD-SCADs reversed significantly over time, as previously reported [15]. Ventricular arrhythmia occurs in 3.6-14% of patients [3]; in our series, only patient-4 experienced ventricular fibrillation. None of our patients reported post-exertion symptomatology, a feature that is more frequent in men [6]. It is of note that cigarette smoking or other classical CAD risk factors are not likely to be causally related with SCAD in our series (Table 1).
It has previously been reported [7] that type 2 lesion (diffuse smooth stenosis) represents the most common angiographic SCAD feature (67%), while type 1 (multiple radiolucent lumen) and type 3 (mimic atherosclerosis) lesions are less found (29.1% and 3.9%, respectively). Our limited data (Table 2; Figure 2) are in line with this report [(type 2: 75% (6/8); type 1: 25% (2/8)]. In angiographic series, LAD involvement has been reported in 38-77% of cases [1,16], while multivessel disease accounts for 9-23% of cases [1,6,9]. Interestingly, LAD was involved in the majority of our SCAD patients, and exclusively in our female subset (Table 2). It is also of note that increased coronary tortuosity, an angiographic risk factor of SCAD [3], was a common angiographic feature in our female subgroup (Table 2).
Our three-year study showed an uneventful shortterm clinical course of our patients, on the basis of clinical, echocardiographic and angiographic (Table 1), follow-up criteria. Notably, both short-term and longterm SCAD outcome is generally good; in-hospital, one-year and 10-year mortality is relatively low (1- 10%; 1%-4%; 7.7%, respectively) [5-7,11]. Although survivors are at risk for recurrent dissection, MI, need for revascularization and major cardiovascular events (MACEs) [5-7,11], the natural history of SCAD is usually spontaneous healing in a time-period up to 40 months [7,11,17]. Female gender, in contrast to our results, has been associated with a poorer prognosis [1,3,7,8,18].
The management of SCAD-related MI is extremely challenging. This is because recommendations still remain empiric and largely based on expert opinion. However, consensus has been reached, broadly, that medical management (watchful waiting strategy) should be the initial approach in patients with single-vessel non-left main/proximal LAD artery SCAD, while coronary intervention (stenting) or coronary artery bypass should be confined in patients with ongoing/refractory ischaemia, haemodynamic instability or when SCAD affects major arteries with sizable myocardial jeopardy and adequate anatomy [1,7,14]. Notably, stenting has been associated with technical failure, short-term and long-term-MACEs (1,3,6,7). Instead, lower stenting rates were recently associated with improved survival in women presenting with SCAD-induced NSTEMI [19].
According to the existing algorithms [1,7], medical management in our patients was based on dual antiplatelet therapy (aspirin life-long and clopidogrel up to 1-year, respectively) and beta-blockage (Table 2). Antiplatelets reduce false lumen thrombotic burden and consequently true lumen compression, and counteract the prothrombotic effect of the intimal tear within SCAD [3]. On the other hand, beta-blockers reduce heart rate, cardiac contractility, cardiac workload and coronary arterial wall stress [3], while they have recently been associated with reduced SCAD recurrence rates [20].
None of our six SCAD-associated STEMI patients received thrombolysis. Early coronary angiography has probably prevented them from catastrophic complications, since thrombolysis has been associated with angiographic and clinical deterioration of SCAD in up to 60% of cases [3,7]. This further highlights the importance of early angiography in STEMI, especially in young women without conventional CAD risk factors. For the same reasons, the role of anticoagulation (low molecular-weight heparin, LMWH) has also been challenged in SCAD; yet, in our series, LMWH was used extensively, without adverse events (Table 2).
Patient-2 received a 24-hour regimen of tirofiban (glycoprotein IIb-IIIa inhibitor) due to increased thrombotic burden on initial angiography with temporary clinical recession (Table 2); however, on day-6 of hospitalization, the patient suffered an acute anterior STEMI and underwent urgent coronary stenting to prevent catastrophic retrograde propagation of the dissection into left main (Table 1; Figure 2). One could argue that tirofiban precipitated dissection worsening [3]; however, there was no angiographic deterioration on follow-up angiography, stent implantation was successful and the patient’s course was uneventful afterwards. Nevertheless, the role of glycoprotein IIb-IIIa inhibitors in SCAD is still to be elucidated in the future.
Patient-4 survived an out-of-hospital cardiac arrest due to an anterior STEMI (Figure 2); while on cardiogenic shock she referred for coronary artery bypass, however her clinical condition improved rapidly and was also treated conservatively. In this respect, we feel that the decision for revascularization of severe SCAD should be considered with skepticism in the event of clinical improvement under medical treatment.
Certainly, a sample size of only eight patients with MI secondary to SCAD could be considered as exceptionally small; we acknowledge this as a limitation of our study. The small sample size of our SCAD patients did not allow the performance of multivariate regression analysis in order to examine the possible effect of several clinical determinants on the occurrence of SCAD. Accordingly, no inference on the possible pathogenic role of established risk factors or precipitating stressors reported in other SCAD populations could be drawn from our limited data.
Conclusion
The incidence of SCAD as underlying etiology of MI in central Greece approximates data previously reported worldwide. However, in our specific population, SCAD is likely to manifest a unique clinical phenotype, predominately affecting women aged<55 years-old, non-pregnant, postmenopausal, with no precipitating stressors or underlying connective tissue disorders. Type 2 (diffuse smooth stenosis) LAD disease dominates SCAD-related MI, especially in the women subset. Finally, our data indicates that initial conservative management may be prioritized, as it is associated with favorable outcomes; this may also concern patients who are initially considered as eligible for revascularization due to severe clinical/angiographic SCAD, yet they are rapid responders to medical therapy.
Author’s Contributions
All authors accept full responsibility for the design and conduct of the study, and had full access to the data and controlled the decision to publish them. All co-authors agree with this and have participated in the study to a sufficient extent to be named as authors. All authors read and approved the final manuscript.
Acknowledgement
Triantafyllia Koukoubani provided significant language help and writing assistance in the article.
References
- Hayes SN, Kim ESH, Saw J, et al. American heart association council on peripheral vascular disease, council on clinical cardiology council on cardiovascular and stroke nursing, council on genomic and precision medicine; and stroke council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American heart association. Circulation. 137(19): 523-557 (2018).
- Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 5(3): 263-270 (2016).
- Yip A, Saw J. Spontaneous coronary artery dissection-A review. Cardiovasc Diagn Ther. 5(1): 37-48.
- Buccheri D, Piraino D, Latini RA, et al. Spontaneous coronary artery dissections: a call for action for an underestimated entity. Int J Cardiol. 214: 333-335 (2016).
- Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 35: 250-254 (2009).
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical Features, Management and Prognosis of Spontaneous Coronary Artery Dissection. Circulation. 126: 579-588 (2012).
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 7: 645-655 (2014).
- Vijayaraghavan R, Verma S, Gupta N, et al. Pregnancy-related spontaneous coronary artery dissection. Cardiovasc Res. 33: 527-532 (1997).
- Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC CardiovascInterv. 6: 44-652 (2013).
- Chou AY, Sedlak T, Aymong E, et al. Spontaneous Coronary Artery Dissection Misdiagnosed as Takotsubo Cardiomyopathy: A Case Series. Can J Cardiol. 31(8): 1073.e5-8 (2015).
- Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv 74: 710-717 (2009).
- DeMaio SJ, Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 64: 471-474 (1989).
- Tokura M, Taguchi I, Kageyama M, et al. Clinical features of spontaneous coronary artery dissection. J Cardiol. 63:119-122 (2014).
- Bergen E, Huffer L, Peele M. Survival after spontaneous coronary artery dissection presenting with ventricular fibrillation arrest. J Invasive Cardiol. 17: 4-6 (2005).
- Hering D, Piper C, Hohmann C, et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol. 87: 961-970 (1998).
- Vrints CJ. Spontaneous coronary artery dissection. Heart. 96: 801-808 (2010).
- Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 59: 1073-1079 (2012).
- Thompson EA, Ferraris S, Gress T, et al. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J Invasive Cardiol 17: 59-61 (2005).
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv. 11(1): 80-90 (2018).
- Saw J, Humphries K, Aymong E, et al. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 70(9): 1148-1158 (2017).