Research Article - Imaging in Medicine (2013) Volume 5, Issue 6
Incidence of gallium-67 citrate uptake in brown adipose tissue in neonates
Rajan Rakheja1*, William Makis2, Raymond Lambert3and Sophie Turpin31Department of Nuclear Medicine, Royal University Hospital, University of Saskatchewan, Saskatoon, SK, Canada
2Department of Nuclear Medicine, Cross Cancer Institute, Department of Diagnostic Imaging, Edmonton, AB, Canada
3Department of Nuclear Medicine, McGill University, Hospital Ste-Justine, Montreal, QC, Canada
Abstract
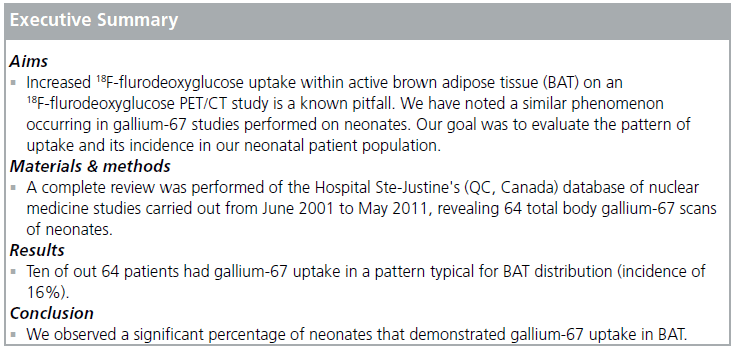
Increased 18F-flurodeoxyglucose uptake within active brown adipose tissue (BAT) on an 18F-flurodeoxyglucose PET/CT study is a known pitfall that has been well described in the literature. We have noted a similar phenomenon occurring in gallium-67 studies performed on neonates, which introduces an undesirable element of complexity when evaluating for the presence of infection or malignancy. Since the incidence of gallium-67 uptake in BAT in neonates has not been previously described in the literature, the goal of this study was to evaluate the pattern of uptake and its incidence in our neonatal patient population. Materials & methods: A complete review was performed of the Hospital Ste-Justine's (QC, Canada) database of nuclear medicine studies carried out from June 2001 to May 2011, revealing 64 total body gallium-67 scans of neonates (carried out within the first 28 days after birth). These studies were reviewed for BAT gallium-67 uptake. Results: Ten of out 64 patients had gallium-67 uptake in a pattern typical for BAT distribution (incidence of 16%). Conclusion: We observed a significant percentage of neonates that demonstrated gallium-67 uptake in BAT. As total body gallium scans are not routinely performed in the neonatal age group at most institutions, we feel that nuclear medicine specialists should be aware of gallium-67 uptake in BAT tissue, as a potential pitfall that may be confused with pathologic uptake.
Keywords
brown adipose tissue; gallium-67; neonate
Gallium-67 citrate has been used since the early 1970s and was the radiotracer of choice for infection imaging at most nuclear medicine centers [1]. A total body gallium scan can be a useful diagnostic imaging modality used in the evaluation of a broad range of pediatric infections, in searching for occult inflammatory disease, and in detecting and staging malignant disease.
Bone and gallium scans have been shown to have high sensitivity for distinguishing cellulitis from osteomyelitis, for precise localization of a focus of infection and for separating active from chronic osteomyelitis [2,3]. A gallium-67 scan is extremely reliable in confirming or excluding the site of purulent material as a cause of sepsis [4]. Given its high sensitivity, gallium-67 scintigraphy can be used in the evaluation of children with fever of unknown origin (FUO) when there is a high suspicion for localized infection, as it may be helpful even if other diagnostic imaging studies are negative [5]. Gallium-67 can also be used for the diagnosis, staging and monitoring of response to therapy of lymphoma and rhabdomyosarcoma [6].
While gallium-67 scintigraphy is very sensitive, gallium uptake is nonspecific and many normal variants have been described [4,7]. 18F-fluorodeoxyglucose (FDG) uptake is frequently observed in brown adipose tissue (BAT) with varying degrees of metabolic activity; Cohade et al. reported an incidence of 4% with the 'USA-Fat' pattern in 347 patients [8]. However, gallium uptake in BAT has not been previously described in the literature. Our experience suggests that a significant percentage of neonatal gallium studies demonstrate gallium uptake within BAT tissue. We aimed to clarify the incidence of this physiologic phenomenon, and to describe the pattern of this uptake as a potential pitfall in the evaluation of neonatal gallium studies.
Materials & methods
All of the neonatal gallium studies were ordered for localization of known infection or fever of unknown origin (FUO), after other tests could not localize a source of fever. The dose of gallium-67 was determined by Webster's rule (adult dose × [age + 1]/[age + 7]) with a minimum dose of 1 mCi and was administered intravenously. Images were acquired with a wide field of view gamma camera with a medium energy collimator approximately 48–72 h after injection. Images were acquired for 5 min or 500,000 counts. Additional delayed views and/or SPECT were obtained when warranted. Bowel preparation was not utilized. Sedation was very rarely used at our institution.
Results
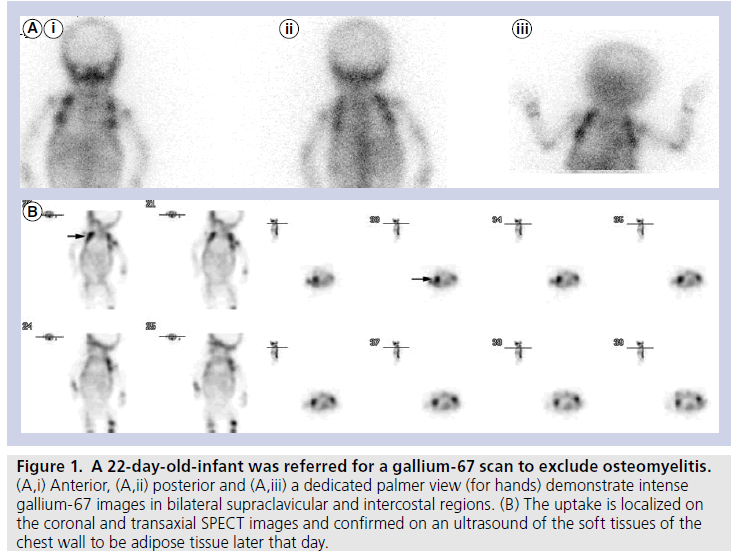
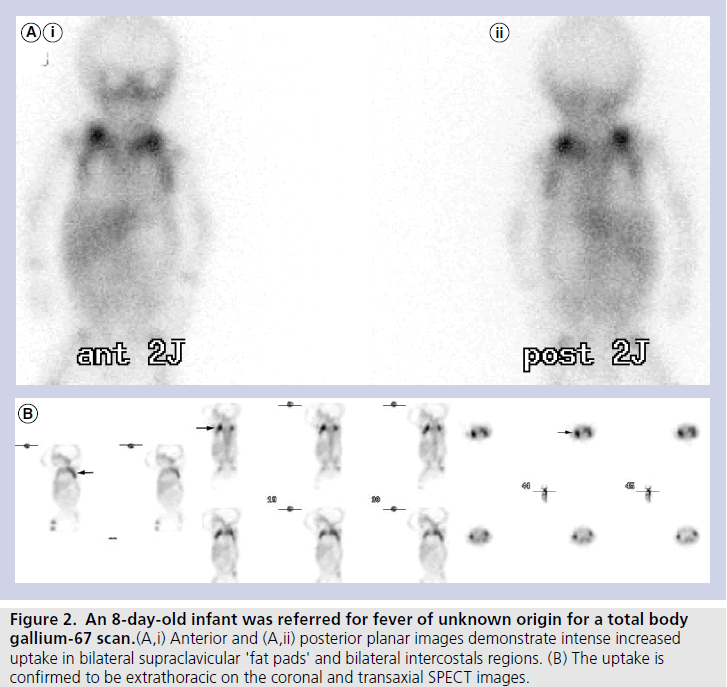
The mean age of our cohort was 20.5 days. Ten out of 64 patients demonstrated gallium uptake in a pattern typical for BAT (incidence of 16%). Out of the ten cases that showed gallium uptake in BAT, four of the cases had been referred for FUO, and six had been referred to exclude osteomyelitis, while none were known (or suspected) to have lymphoma or any other type of malignancy. Seven patients demonstrated gallium uptake in BAT located in bilateral supraclavicular and intercostals regions (lateral thoracic wall), and three showed uptake only in supraclavicular regions (Figures 1A, 1B, 2A & 2B).
Discussion & conclusion
Gallium-67 citrate is produced by a cyclotron via charged particle bombardment of enriched Zn-68. Gallium-67 is then complexed with citric acid to form gallium-67 citrate. The half-life of gallium-67 is 78 h. Within 24 h after injection, approximately 10–25% of the administered dose is excreted via the kidneys. Beyond 24 h, the principal excretory pathway is the large bowel and thus, large bowel uptake is very variable [9]. At 48–72 h after injection, approximately 75% of the administered dose remains within the body and is equally distributed among soft tissues, liver and bone/bone marrow [10].
There are numerous mechanisms of gallium uptake in infectious and inflammatory foci [11,12]. The normal biodistribution of gallium is extremely variable. Activity in the nasal regions, oropharynx and lacrimal glands can be quite prominent, even in the absence of any pathology [13]. Breast uptake is commonly mild and bilateral, but can be intense in pregnancy, during lactation, caused by numerous drugs, renal failure and hypothalamic lesions [14,15]. In addition, Handmaker et al. describe a number of normal variations including increased gallium uptake in growth plates, thymus and spleen (in the presence of sepsis). These children were aged from 3 months to 20 years and their analysis did not include any neonates, nor did they describe gallium uptake in BAT [4].
Physiologic uptake of gallium in BAT has not been described previously in the literature, despite the fact that BAT plays a significant role in thermogenesis, principally in this age group. Brown and white adipose tissues differ in their composition. BAT contains multilocular intracellular lipid droplets, whereas white fat contains a single-lipid droplet in each adipocyte. Consequently, their functions are unique. White adipose tissue primarily stores energy in the form of fat. BAT is a thermogenic organ oriented toward heat production that helps maintain normal body temperature in newborn infants and regresses with increasing age [16]. BAT is more active in young subjects; Yeung et al. reported a 15% incidence in pediatric patients versus 1.9% in adult patients [17]. Interestingly, this figure closely resembles our finding of a 16% incidence of gallium uptake in BAT in neonatal patients.
Figure 1. A 22-day-old-infant was referred for a gallium-67 scan to exclude osteomyelitis. (A,i) Anterior, (A,ii) posterior and (A,iii) a dedicated palmer view (for hands) demonstrate intense gallium-67 images in bilateral supraclavicular and intercostal regions. (B) The uptake is localized on the coronal and transaxial SPECT images and confirmed on an ultrasound of the soft tissues of the chest wall to be adipose tissue later that day.
Figure 2. An 8-day-old infant was referred for fever of unknown origin for a total body gallium-67 scan.(A,i) Anterior and (A,ii) posterior planar images demonstrate intense increased uptake in bilateral supraclavicular 'fat pads' and bilateral intercostals regions. (B) The uptake is confirmed to be extrathoracic on the coronal and transaxial SPECT images.
Fatty acids are the main metabolic substrate of BAT [18]. Cold exposure will activate BAT via adrenergic stimulation [18]. In fact, Cohade et al. showed that BAT was more frequent during the colder period of the year (13.7%; January to March) than during the warmer period (4.1%; April to December) [19]. The cellular density of BAT and rich neurovascular supply accounts for the preferential uptake of 18F-FDG in BAT over white adipose tissue. Moreover, BAT is richer in mitochondria than white fat, as demonstrated by the greater abundance of cytochrome c, a mitochondrial marker [20]. Virtanen et al. assessed tissue-biopsy specimens of BAT and showed that BAT overexpresses uncoupling protein UCP1 by a factor of 1000 in comparison with white fat [20]. The function of UPC1 is adaptive thermogenesis, which generates heat instead of ATP. We can only postulate whether these same characteristics that result in increased 18F-FDG uptake in BAT, may also be responsible for increased gallium uptake.
BAT uptake is often bilateral and symmetric and located in distinct locations. In comparison, 18F-FDG uptake has been observed most frequently in the neck (2.3%), paravertebral area (1.4%), perinephric space (0.8%) and mediastinum (0.9%) [17]. In the ten patients in our cohort who demonstrated gallium uptake in BAT, all ten were in the supraclavicular/infraclavicular regions and/or intercostal spaces and were bilateral and symmetric. Given the lower resolution of gallium compared to 18F-FDG PET/CT and the small size of these infants, it was difficult to demonstrate gallium uptake in other areas known for the presence of BAT, such as the paravertebral area or perinephric space (even with SPECT).
BAT is intermediate in attenuation between fat and muscle on CT and demonstrates enhancement with some washout on delayed imaging. BAT has been described as minimally hyperintense to muscle on MRI T1-weighted images and heterogeneously hyperintense to muscle on T2-weighted images, with maintenance of hyperintensity on chemical fat-suppressed T2-weighted images and some loss of signal on fat-suppressed T1-weighted images [21].
Besides 18F-FDG and gallium-67, several other radiopharmaceuticals have been described to accumulate in BAT: F18-6-fluorodopamine [22], I-123 metaiodobenzylguanidine [23], Tc-99m tetrofosmin [24] and Tc-99m methoxyisobutylisonitrile [25,26]. F18-6-fluorodopamine and metaiodobenzylguanidine are thought to be concentrated in the sympathetic nerve terminals in BAT. It is postulated that uptake of tetrofosmin and methoxyisobutylisonitrile is dependent on the rich mitochondria supply in BAT.
The mechanisms of gallium uptake in BAT have not been previously described. We believe it is likely multifactorial, with one etiology being hypervascularization of BAT due to thermogenesis in neonates. We also hypothesize that this cohort of neonates likely had adrenergic system activation (they were often very ill infants), and thus may have been more likely to demonstrate BAT gallium uptake compared to healthy neonates (BAT activation is thought to be stimulated by its adrenergic innervations) [27].
It is important to note that gallium-67 imaging in neonates has been largely replaced by 18FFDG imaging. One of the major problems with gallium-67 scanning is the associated radiation dose; for a 1 mCi dose, a neonate would receive an effective dose of 4.2 rem [28]. For FUO, FDG PET/CT is playing an increasing role, although it is still not widely used. FDG PET has been shown to be more sensitive and specific than gallium- 67 in the workup of FUO [29] and can be performed within a few hours at a significantly lower radiation dose. Nonetheless, 18F-FDG imaging is still not widely available and, at times, PET radiopharmaceuticals may not be available on site for various reasons.
In a literature review, we could only find one other reference of BAT gallium uptake, which was a case report of a 10-year-old boy with hip pain who was referred for gallium scintigraphy for possible osteomyelitis [30]. The literature review did not show any reported cases of gallium uptake in neonates or adults. Given the high incidence of gallium BAT uptake in our cohort (16%) and the relative rarity of total body gallium scanning in neonates at most institutions, we felt nuclear medicine specialists should be aware of this physiologic uptake as a potential pitfall, to avoid unnecessary treatment and/ or biopsy. Given the bilateral and symmetrical intensity of gallium uptake in the supraclavicular/ infraclavicular regions and/or intercostal spaces in our cohort, there is a very limited differential diagnosis that could account for this pattern of uptake. It is possible that bilateral supraclavicular lymph nodes could show intense gallium uptake in lymphoma and less likely, in sarcoidosis. However, there would be other diagnostic findings to exclude physiologic BAT gallium uptake.
A noted limitation of our analysis is the lack of objectivity in confirming BAT gallium avidity, as it was not practical to confirm every case with SPECT/CT. Similarly, this study was only intended to assess brown tissue avidity in neonates, while a broader analysis would be of clinical interest.
It is also important to note that out of the ten patients who demonstrated BAT gallium uptake, eight had studies performed during the winter months of Montreal (QC, Canada; October to March). This is consistent with the reported increased incidence of 18F-FDG uptake in BAT during winter months, as previously reported by Cohade et al. [19]. If infection or inflammation in the thorax region is suspected clinically and a gallium scan is requested in a neonate during winter, it may be warranted to use some of the simple and noninvasive methods of reducing BAT activity that have been described for 18FFDG, such as wearing warm clothes, maintaining a warm ambient environment and providing blankets (we use many of these measures at our institution to keep infants warm) [31].
Disclosure
Work was performed at Hopital Ste-Justine, Universite de Montreal and McGill University (Montreal, QC, Canada).
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Treves ST. Pediatric Nuclear Medicine (2nd Edition). Springer-Verlag, NY, USA, 546 ( 1995).
- Lisbona R, Rosenthall L. Observations on the sequential use of 99mTc-phosphate complex and 67Ga imaging in osteomyelitis, cellulitis, and septic arthritis. Radiology 123, 123– 129 ( 1977).
- Turpin S, Lambert R. Role of scintigraphy in musculoskeletal and spinal infections. Radiol. Clin. North Am. 39( 2), 169– 189( 2001).
- Handmaker H, RE O'Mara. Gallium imaging in pediatrics. J. Nucl. Med. 18, 1057– 1063 ( 1977).
- Buonomo C, Treves ST. Gallium scanning in children with fever of unknown origin. Pediatr. Radiol. 23, 307– 310 ( 1993).
- Bekerman C, Port RB, Pang E Scintigraphic evaluation of childhood malignäncies by 67Ga-citrate. Radiology 127, 719– 725 ( 1978).
- Desai AG, Intenzo C, Park C Druginduced gallium uptake in the breasts. Clin. Nucl. Med. 12, 703– 704 ( 1987).
- Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat ("USA-Fat"): description on 18F-FDG PET/ CT. J. Nucl. Med. 44, 170– 176 ( 2003).
- Love C, Palestro C. Altered biodistribution and incidental findings on gallium and labeled leukocyte/bone marrow scans. Semin. Nucl. Med. 40, 271– 282 ( 2010).
- Palestro CJ. The current role of gallium imaging in infection. Semin. Nucl. Med. 24, 128– 141 ( 1994).
- Weiner RE. The mechanism of 67Ga localization in malignant disease. Nucl. Med. Biol. 23, 745– 751 ( 1996).
- Ando A, Nitta K, Ando I Mechanism of gallium 67 accumulation in inflammatory tissue. Eur. J. Nucl. Med. 17, 21– 27 ( 1990).
- Nelson B, Hayes RL, Edwards CL Distribution of gallium in human tissues after intravenous administration. J. Nucl. Med. 13, 92– 100 ( 1972).
- Vazquez R, Oates E, Sarno RC Gallium-67 breast uptake in a patient with hypothalamic granuloma (sarcoid). J. Nucl. Med. 29, 118– 121 ( 1988).
- Desai AG, Intenzo C, Park C Druginduced gallium uptake in the breasts. Clin.Nucl. Med. 12, 703– 704 ( 1987).
- Lean ME. Brown adipose tissue in humans. Proc. Nutr. Soc. 48, 243– 256 ( 1989).
- Yeung HW, Grewal RK, Gonen M Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J. Nucl. Med. 44, 1789– 1796 ( 2003).
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444– E452 ( 2007).
- Cohade C, Mourtzikos KA, Wahl RL. "USA-Fat": prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J. Nucl. Med. 44, 1267– 1270( 2003).
- Virtanen KA, Lidell ME, Orava J Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518– 1525 ( 2009).
- Dundamadappa SK, Shankar S, Danrad R Imaging of brown fat associated with adrenal pheochromocytoma. Acta Radiol. 48( 4), 468– 472 ( 2007).
- Hadi M, Chen CC, Whatley M Brown fat imaging with (18)F-6- fluorodopamine PET/CT, (18)F-FDG PET/CT, and (123) I-MIBG SPECT: a study of patients being evaluated for pheochromocytoma. J. Nucl. Med. 48, 1077– 1083 ( 2007).
- Okuyama C, Sakane N, Yoshida T (123) I-or (125)I-metaiodobenzylguanidine visualization of brown adipose tissue. J. Nucl. Med. 43, 1234– 1240 ( 2002).
- Fukuchi K, Ono Y, Nakahata Y Visualization of interscapular brown adipose tissue using (99m)Tctetrofosmin in pediatric patients. J. Nucl. Med. 44, 1582– 1585 ( 2003).
- Belhocine T, Shastry A, Driedger A Detection of 99mTc-sestamibi uptake in brown adipose tissue with SPECT-CT. Eur. J. Nucl. Med. Mol. Imaging 34, 149 ( 2007).
- Goetze S, Lavely WC, Ziessman HA Visualization of brown adipose tissue with 99mTcmethoxyisobutylisonitrile on SPECT/CT. J. Nucl. Med. 49, 752– 756 ( 2008).
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277– 359 ( 2004).
- Stabin MG, Gelfand G. Dosimetry of pediatric nuclear medicine procedures. Quartely J. Nucl. Med. 42, 93– 112 ( 1998).
- Meller J, Sahlmann C-O, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J. Nucl. Med. 48, 35– 45 ( 2007).
- Huany YC, Wang PW, Tang SW Identifying Ga-67 uptake in brown adipose tissue with SPECT/CT. Clin. Nucl. Med. 34, 12 ( 2009).
- Cohade C. Altered biodistribution on FDG-PET with emphasis on brown fat and insulin effect. Semin. Nucl. Med.40, 283–293 (2010).