Research Article - Neuropsychiatry (2016) Volume 6, Issue 6
Increased levels of Serum Nitrogen oxides are indicators of Post-treatment response in Mood disorder patients with Acute episodes
- Corresponding Author:
- Meng-Chang Tsai, MD
Department of Psychiatry
Chang Gung Memorial Hospital Kaohsiung Medical Center
123, Ta-Pei Rd, Niao-Sung, Kaohsiung 833, Taiwan, R.O.C
Tel: 886-7-7317123-6313
Fax: 886-7-7326817
Abstract
Background:
Nitrogen Oxides (NOx) has an important role in mood disorders. This study aims to investigate whether serum NOx levels are associated with bipolar disorder, manic episodes (BD-ME) and major depressive disorder, depressive episodes (MDD-DE), and to evaluate the impact of treatments on NOx levels and symptoms severity in BD-ME and MDD-DE patients.
Methods:
We enrolled 20 patients with BD-ME, 18 with MDD-DE, and 80 healthy subjects. Serum NOx levels were measured with assay kits. Symptom severity was evaluated using the Young Mania Rating Scale (YMRS) for BD-ME and the Hamilton Depression Rating Scale (HAM-D) for MDDDE. We collected and analyzed patients’ NOx, YMRS, and HAM-D data while pre-treatment (admission) and post-treatment (discharge).
Results:
Serum NOx levels of the healthy controls were higher than patients with BD-ME and lower than patients with MDD-DE, but the difference was not statistically significant. Serum NOx levels were both increased in BD-ME (p=0.0047) and in MDD-DE (p=0.0398) patients after treatment. The changes of NOx levels were not associated with the changes of YMRS scores in BD-ME patients after treatment. However, the changes of NOx levels were negatively correlated with the changes of HAM-D scores in MDD patients after treatment (p=0.0473).
Conclusions:
Our results suggest that increased levels of serum NOx are indicators in patients with BDME and MDD-DE after treatment. In addition, the changes of serum NOx levels might be measuring biomarkers for assessment of treatment response in MDD-DE patients.
Keywords
Nitrogen oxides, BD, Bipolar disorder, Mania, MDD, Depressive disorder, Depression
Introduction
Mood disorder, a category of severe psychiatric illness affecting many in the general population, mainly includes bipolar disorder (BD) and major depressive disorder (MDD). A number of assessment tools are used to diagnose or evaluate the severity of bipolar disorder with manic episode (BD-ME), bipolar disorder with depressive episode (BD-DE), and major depressive disorder with depressive episode (MDD-DE) [1-3]. However, these psychiatric rating scales should be used by psychiatrists or well-trained clinical professionals. Clinicians lack biological indicators which might stand for diagnosis and prognosis after treatment [4].
Nitrogen Oxides (NOx) has been considered as a potential biomarker for diagnosis assisting. It is an important neurotransmitter involved in the pathophysiology of many neuropsychiatric disorders, such as BD, MDD, schizophrenia, and drug addiction [5-8]. Several studies have used NOx levels in the blood as biomarkers to detect BD-ME [9,10], BD with a euthymic state SS[11], BD-DE [12], and MDD-DE [13- 15]. However, the results have contradictory outcome. Furthermore, few studies have investigated the changes of NOx levels after treatment in BD-ME and MDD-DE patients, and showed unsatisfactory results [16-18].
The aim of our study is to verify the hypothesis, the serum NOx levels as diagnosis tool in BD-ME and MDD-DE patients compared with healthy controls. Moreover, we investigate the serum NOx levels and evaluate the severity of BD-ME by The Young Mania Rating Scale (YMRS)1 and MDD-DE by tshe 17-item Hamilton Depression Rating Scale (HAM-D) [19] in pre-treatment and post-treatment state. Then we assess the relationship between the changes of serum NOx levels and symptom severity scores after treatment in BD-ME and MDD-DE patients.
Materials and Methods
▪ Patients
All BD-ME and MDD-DE patients in the psychiatric inpatient ward of Kaohsiung Chang Gung Memorial Hospital were enrolled. They met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for BD-ME and MDD-DE, and were diagnosed by psychiatrists. Background data including gender, age, weight, height, and body mass index (BMI) were recorded. Serum NOx levels at pre-treatment (admission) and posttreatment (discharge), and during hospitalization were collected. The severity of manic symptoms in BD-ME and depressive symptoms in MDDDE were evaluated using the YMRS1 and the 17- item HAM-D [2,19], respectively. Severity was assessed at pre-treatment and post-treatment. All patients were aged from 18 to 65, and underwent blood pressure, routine blood tests, electrocardiogram (EKG), and chest X-ray at admission. Those with currently unstable physical problems, a history of heavy smoking, or a history of substance abuse were excluded. Twenty-three BD-ME patients and 21 MDDDE patients initially were enrolled. They were treated with antipsychotic agent, mood stabilizer, and antidepressant according to their symptoms. The dosage of medications was titrated to treatment dose within 2 weeks, and then maintained to date of discharge. Three BD-ME patients and 3 MDD-DE patients dropped out of the study; the remaining 20 BD-ME patients and 18 MDD-DE patients were included.
The healthy controls were recruited at Kaohsiung Chang Gung Memorial Hospital. Similar background data were collected. Those with a history of mental disorders or severe medical disease were excluded. All the controls were free of medication. Eighty individuals finally were included in this study.
The study was carried out at Kaohsiung Chang Gung Memorial Hospital from November 2012 to October 2014, and was approved by the Institutional Review Board of the hospital and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects signed informed consent forms after receiving a full explanation of the study.
▪ Laboratory data
Venous blood samples were collected in the morning after overnight fasting (at least 8 hours). The serum samples were separated by centrifugation at 3000 g for 10 min, and then stored at −80°C for further analysis soon after blood pumping. A nitrate/nitrite colorimetric assay kit was used to assay serum NOx (Cayman Chemical Company, Ann Arbor, USA). All analyses were performed at the same laboratory and according to the manufacturer’s protocol. The website of the protocol was https://www. caymanchem.com/product/780001.
▪ Statistical analysis
Continuous variables were expressed as mean ± standard deviation. The Kolmogorov-Smirnov test was used for normality. The independent samples t-test was used to compare patient and control data that were normally distributed, and the Mann-Whitney U test was used for data that were not normally distributed. Analysis of covariance (ANCOVA) was used to compare the serum NOx levels at pre-treatment between patients and controls while controlling for the gender, age, and BMI. Paired samples t-test was used to analyze the changes of serum NOx levels, YMRS scores and HAM-D scores in the patients. Pearson’s correlation coefficient was used to evaluate the relationship between serum NOx levels and YRMS scores or HAM-D scores. A p value of less than 0.05 was used to indicate statistical significance. All data were analyzed using MedCalc Statistical Software version 16.2.0 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016).
Results
Table 1 shows the characteristics of patients and controls. A total of 118 subjects met the recruitment criteria, including 20 BD-ME patients (12 males and 8 females), 18 MDD-DE patients (3 males and 15 females), and 80 controls (30 males and 50 females). There was a significant age difference between the BD-ME patients vs controls (p=0.0025) and the MDD-DE patients vs controls (p<0.0001). There were no significant differences in the other background data (weight, height, and BMI) between BD-ME patients vs controls and MDD-DE patients vs controls (p>0.05). The mean hospitalization durations of the patients in the BD-ME and MDD-DE groups were 25.8±5.0 days and 24.8±6.0 days, respectively. Serum NOx levels were reduced in BD-ME patients vs control (25.8±18.6 vs. 21.9±12.8) and increased in MDD-DE patients vs. controls (34.6±21.0 vs. 25.8±18.6), but without significant differences (p=0.7110 and p=0.0766, respectively). ANCOVA showed that the age had no significant effect on serum NOx levels between BD-ME patients vs controls (F=1.441, p=0.233) and MDD-DE patients vs controls (F=2.058, p=0.155) after controlling the gender, age, and BMI (Data not shown). Table 2 shows the medications and maintenance dose received by the BD-ME and MDD-DE patients.
| BD-ME (n=20) | MDD-DE (n=18) | Controls (n=80) | |
|---|---|---|---|
| Gender (Male/Female) | 12/8 | 3/15 | 30/50 |
| Age (years) | 40.8 ± 12.3a | 50.9 ± 6.7b | 31.7 ± 5.5 |
| Weight (kg) | 61.5 ± 8.7 | 63.3 ± 16.8 | 59.9 ± 11.8 |
| Height (cm) | 165.1 ± 7.1 | 160.6 ± 6.7 | 163.9 ± 8.4 |
| BMI (kg/m2) | 22.6 ± 3.3 | 24.6 ± 6.5 | 22.2 ± 3.0 |
| NOxc (µmol/L) | 21.9 ± 12.8 | 34.6 ± 21.0 | 25.8 ± 18.6 |
| Hospitalization (days) | 25.8 ± 5.0 | 24.8 ± 6.0 | NA |
Data values are given as mean ± standard deviation ap<0.01, BD-ME vs Controls; bp<0.0001, MDD-DE vs Controls; cMean serum nitrogen oxides level in pre-treatment or healthy controls Abbreviation: BD-ME = bipolar disorder with manic episode; MDD-DE = major depressive disorder with depressive episode; BMI = body mass index; NOx = nitrogen oxides; NA = not applicable
Table 1: Characteristics of BD-ME patients, MDD-DE patients, and healthy controls in the study.
| BD-ME (n=20) | MDD-DE (n=18) | ||
|---|---|---|---|
| Patient | Medications | Patient | Medications |
| 1 | Haloperidol 5 mg + Lithium 900 mg | 1 | Fluoxetine 20 mg |
| 2 | Olanzapine 20 mg + Lithium 900 mg | 2 | Agomelatine 50 mg + Quetiapine 25 mg |
| 3 | Risperidone 3 mg + Lithium 1200 mg | 3 | Duloxetine 60 mg + Quetiapine 150 mg |
| 4 | Risperidone 4 mg + Valproate 1000 mg | 4 | Bupropion 150 mg + Fluoxetine 40 mg |
| 5 | Haloperidol 5 mg + Valproate 1200 mg | 5 | Fluoxetine 80 mg + Quetiapine 500 mg |
| 6 | Risperidone 3 mg + Lithium 900 mg | 6 | Fluoxetine 60 mg + Aripiprazole 2.5 mg |
| 7 | Valproate 1000 mg | 7 | Paroxetine 40 mg + Aripiprazole 5 mg |
| 8 | Risperidone 3 mg + Lithium 1200 mg | 8 | Agomelatine 25 mg |
| 9 | Olanzapine 20 mg + Valproate 1000 mg | 9 | Escitalopram 20 mg |
| 10 | Risperidone 3 mg + Lithium 1200 mg | 10 | Duloxetine 30 mg + Quetiapine 100 mg |
| 11 | Aripiprazole 15 mg + Valproate 400 mg | 11 | Escitalopram 10 mg + Trazodone 50 mg |
| 12 | Clozapine 50 mg + Valproate 1000 mg | 12 | Paroxetine 40 mg |
| 13 | Paliperidone 3 mg + Valproate 1200 mg | 13 | Venlafaxine 225 mg |
| 14 | Risperidone 6 mg + Valproate 1200 mg | 14 | Agomelatine 50 mg |
| 15 | Aripiprazole 15 mg + Lithium 600 mg | 15 | Fluoxetine 40 mg |
| 16 | Risperidone 3 mg | 16 | Escitalopram 15 mg + Amitriptyline 50 mg |
| 17 | Valproate 1000 mg | 17 | Mirtazapine 60 mg + Quetiapine 50 mg |
| 18 | Risperidone 4 mg + Lithium 600 mg | 18 | Bupropion 300 mg |
| 19 | Risperidone long-acting injection 25 mg + Lithium 600 mg |
||
| 20 | Risperidone long-acting injection 37.5 mg + Lithium 600 mg | ||
Table 2: Medications used by the BD-ME patients and MDD-DE patients in the study.
| BD-ME (n=20) | MDD-DE (n=18) | |||
|---|---|---|---|---|
| NOx (µmol/L) | YMRS | NOx (µmol/L) | HAM-D | |
| Pre-treatment | 21.9 ± 12.8 | 35.3 ± 7.3 | 34.6 ± 21.0 | 27.4 ± 5.9 |
| Post-treatment | 38.8 ± 24.1 | 3.2 ± 2.9 | 51.8 ± 36.4 | 8.3 ± 5.7 |
| pvalue | 0.0047** | <0.0001** | 0.0398* | <0.0001** |
Data values are given as mean ± standard deviation
* p<0.05; ** p<0.01
Abbreviation: NOx = nitrogen oxides; YMRS = Young Mania Rating Scale; HAM-D = Hamilton Depression Rating Scale; BD-ME = bipolar disorder with manic episode; MDD-DE = major depressive disorder with depressive episode
Table 3: Mean changes in NOx levels, and YMRS and HAM-D scores of BD-ME and MDD-DE patients after treatment.
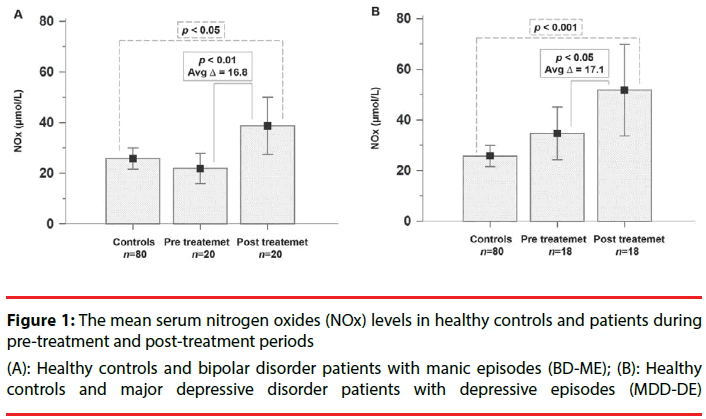
The BD-ME patients showed significantly increased levels of serum NOx at post-treatment compared with pre-treatment and the healthy controls (p=0.0047 and p=0.0224). The BDME patients also had significant improvement in YMRS scores between pre-treatment and posttreatment (p<0.0001). The MDD-DE patients presented significantly increased levels of serum NOx at post-treatment compared with pretreatment and the healthy controls (p=0.0398 and p=0.0009). The MDD-DE patients also had significantly improved HAM-D scores from pretreatment to post-treatment (p<0.0001) (Table 3) (Figure 1A &1B).
(A): Healthy controls and bipolar disorder patients with manic episodes (BD-ME); (B): Healthy controls and major depressive disorder patients with depressive episodes (MDD-DE)
Figure 1: The mean serum nitrogen oxides (NOx) levels in healthy controls and patients during pre-treatment and post-treatment periods
Serum NOx levels and YMRS scores of pretreatment BD-ME patients showed no significant correlation (r=0.1391, p=0.5588). Also, no significant correlation was noted between serum NOx levels and HAM-D scores in pre-treatment MDD-DE patients (r=-0.0045, p=0.9858). (Data not shown)
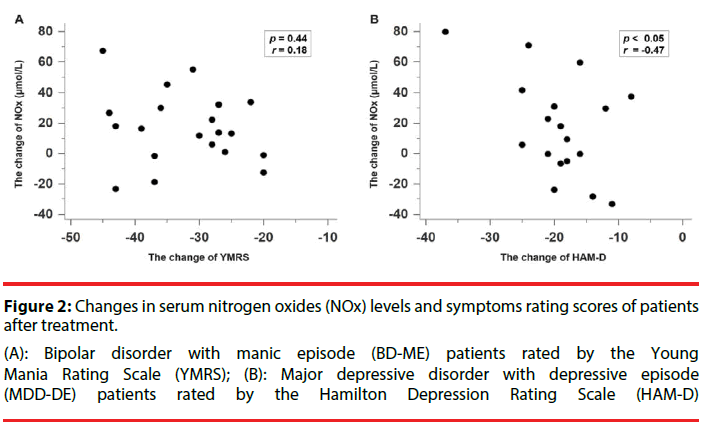
The changes of serum NOx levels and YMRS scores in BD-ME patients after treatment showed no significant correlation (r=-0.1831, p=0.4396) (Figure 2A). However, a significant correlation was noted between changes of serum NOx levels and HAM-D scores in MDD-DE patients after treatment (r=-0.4732, p=0.0473) (Figure 2B).
(A): Bipolar disorder with manic episode (BD-ME) patients rated by the Young Mania Rating Scale (YMRS); (B): Major depressive disorder with depressive episode (MDD-DE) patients rated by the Hamilton Depression Rating Scale (HAM-D)
Figure 2: Changes in serum nitrogen oxides (NOx) levels and symptoms rating scores of patients after treatment.
Discussion
Our study found that serum NOx levels were non-significantly reduced and increased in BDME and MDD-DE patients, respectively, while treatment significantly increased these values in both groups. We also found that the changes of NOx levels in MDD-DE patients after initial treatment had a negative correlation with the changes of HAM-D scores.
▪ Blood NOx levels in BD, MDD, and healthy subjects
Few clinical trials in past decades have investigated blood NOx levels in BD-ME patients. Savas et al. found that the mean plasma NOx level in BD-ME patients (n=44) was significantly higher than that in controls [9]. Case-control studies by Yanik et al. (n=43 in the BD-ME group and n=31 in the control group) and Gergerlioglu et al. (n=29 in the BD-ME group and n=30 in the control group) had similar results [16,20].
However, a study by Ozcan et al. found decreased plasma NOx levels in the pre-treatment bipolar patient group compared to the control group [10]. Differing from previous findings, our study showed no significant differences between BD-ME patients and controls. The reasons for the discrepancy might be differences in race, in sample sizes, and in severity of BD-ME.
The role of blood NOx levels in MDD-DE has been debated since the 2000s. Some studies proposed that blood NOx levels had a positive association [13,17,21-27], others suggested no significant association [18], and still others reported a negative association with MDD-DE [14,15,28,29]. Our study found higher mean serum NOx levels in MDD-DE patients than in healthy controls, but without a significant difference. These discrepancies might be the result of differences in case heterogeneity. For instance,patients in some studies had comorbid physical disease (colorectal carcinoma or leukemia) [26,27] or other psychiatric disorders [14,18,28]; some had been diagnosed with a first episode of MDD [13,21,29] or had recurrent depressive episodes of MDD25, or had attempted suicide [22,23]; one study enrolled only female groups [15], and another only Han Chinese subjects [17]. Our study population was purely mood disorder without major physical problem, but we didn’t distinguish first or recurrent episode, gender, and severity of MDD. All these factors might affect blood NOx levels [18,22,25,29,30].
Taken together, these studies and our research had inconsistent results of blood NOx levels in BD-ME and MDD-DE patients compared with healthy controls. Gene polymorphism may structurally interpret them. Nitric oxide (NO) is synthesized from L-arginine and oxygen (O2) with the help of nitric oxide synthases (NOSs) [7]. The neuronal isoform of NOS (encoded as NOS1) is the main source of NO in humans, and a certain single nucleotide polymorphisms (SNP) in the NOS1 might pathologically disturb NO production by its activity [31]. NOS1 has a complex haplotype structure and diverse characteristics, which might have led to the results of different NOx levels in BD-ME and MDD-DE patients [11,12,18,27,29].
▪ The changes of blood NOx levels in BD and MDD patients
Gergerlioglu et al. evaluated the changes of serum NOx levels in acute-phase BD-ME patients during treatment. They found mildly higher NOx levels in BD-ME patients on the 30th day (57.05±15.47 μmol/L) than on the 1st day (54.87±6.03 μmol/L), but without significance16. Ozcan et al. also reported higher NOx levels in the erythrocytes of patients with affective disorders at post-treatment (298.0±28.9 nanomol/g) than at pre-treatment (267.6±19.1 nanomol/g) [10]. Our results showed that the serum NOx levels in BD-ME patients were significantly increased after treatment, and supported previous findings.
Besides, two studies from Turkey investigated the relationship between NOx levels and Bech-Rafaelson Mania Scale scores [32] in pretreatment BD-ME patients, and reported no significant correlations [9,16]. Our data also supported these results, finding no significant correlation between serum NOx levels and YMRS scores in pre-treatment BD-ME patients. Furthermore, we found that the changes of serum NOx levels were not correlated to the improvement of YMRS scores in BD-ME patients after treatment. Based on the above results, increased levels of serum NOx might not relate with initial status or symptoms improvement in BD-ME patients, which could be explained by mood stabilizer using. Previous studies indicated that lithium and valproic acid would induce NOx synthase and elevated NOx levels after these medications use, and supported the results [33,34]. Almost all patients in our study took mood stabilizer during hospitalization, and then they were found increased the serum NOx levels after treatment, which were not correlated to the change of YMRS scores.
Few studies have compared the changes of NOx levels pre-treatment and post-treatment in MDD-DE patients [17,18]. Herken et al. (n=32) detected serum NOx levels at pre-treatment and post-treatment, in a study period of 8 weeks, and found significantly decreased NOx levels [18]. Lu et al. revealed that NOx levels remained elevated, without a significant difference (n=7, p ≥ 0.128), even after 2 months of fluoxetine treatment [17]. Baranyi et al. indicated increased NO production might be an indicator of remission in major depression patients [35]. Our data revealed significantly increased serum NOx levels in MDD-DE patients after initial treatment. Two differences in study methodologies might account for above results. One, different antidepressants could produce the same anti-depressant effects but they might decrease or elevate NOx levels [36]. In our study, we used 8 kinds of antidepressant in [18] MDDDE patients with obvious heterogeneity, so it might indicate the increasing trend of NOx levels after “general” antidepressant treatment. Second, the length of treatment time was different. We collected NOx data at around 4 weeks compared to the 8 weeks of previous studies. The difference in time of NOx collection might have affected the results.
Hence, we used another new perspective for the trend of NOx levels after treatment. We investigated the relationship between the changes of NOx levels and the degree of HAM-D improvement after treatment, which was more valuable than duration after treatment, and our data found a significant negative association between them. The meaning of our result suggested the changes of NOx levels might be an objective marker for assessment improvement in MDD-DE patients after initial treatment.
One NOx protective hypothesis explained increased NOx level after treatment. NOx caused a reaction with superoxide (O2-) to form highly oxidant and neurotoxic peroxynitrite (ONOO-) [37]. ONOO- can attack neural cells and has a calamitous role in neuro-degeneration and mood switching [7,38]. In contrast, NOx had a benefit in functional recovery and synaptic plasticity from cerebral ischemia in some studies [39-41]. The patients showed increasing NO level after treatment, which regulated endothelial function via arginase activity, L-arginine/ asymmetric dimethylarginine (ADMA) ratio, and global arginine bioavailability [35]. Taken together, NOx was not toxic, and played a neuroprotective role via endothelial regulation system.
Limitations
Several limitations should be highlighted. First, measuring NOx levels in blood samples from different locations, such as brain tissue and peripheral blood (RBC, plasma, or serum), may result in some discrepancies. We collected NOx data of all subjects from peripheral venous serum. Second, we prescribed heterogeneous antipsychotic agents, mood stabilizers, and antidepressants for each patient. Not prescribing the same medication may have influenced the results. Serum NOx may be increased after antipsychotic treatment [42], increased while mood stabilizers using [33,34], and increased or unaffected after using antidepressant [43,44]. Third, age differences may influence oxidative stress biomarkers and result in discrepancies [45]. There was a significant difference in mean age of the BD-ME and MDD-DE patients and healthy controls in our study, but multiple regressions showed that it was not a significant factor affecting NOx levels. Fourth, sample sizes were too small to group individuals by gender, BMI, and medications, which may yield differences in NOx levels and hinder adequate statistical analysis [46]. Future studies should include follow-up NOx data of patient different states, and use large sample sizes to distinguish different kinds of medications, gender, age, and BMI, to determine the association between mood disorder and serum NOx levels.
Conclusions
In this study, we found that the levels of serum NOx in both BD-ME and MDD-DE patients at post-treatment were significantly increased compared to pre-treatment. Increased levels of serum NOx were indicators of post-treatment in mood disorder patients with acute episodes. In addition, the changes of serum NOx levels in MDD-DE patients after treatment had a negative correlation to the changes of HAM-D scores. The changes of serum NOx levels might be the measuring biomarker of symptoms improvement in MDD-DE patients.
Declaration of interests
There is no conflict of interest regarding publication of this paper. We obtained no financial support from any drug company.
Acknowledgments
The work was supported by grants from Chang Gung Memorial Hospital in Taiwan (Research number: CMRPG8B0511, CMRPG8C0681) provided to Tsai MC.
References
- Young RC, Biggs JT, Ziegler VE, et al.A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry133(1), 429-435 (1978).
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry23(), 56-62 (1960).
- Beck AT, Ward CH, Mendelson M, et al.An inventory for measuring depression. Arch. Gen. Psychiatry 4(1), 561-571 (1961).
- Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry. Res 218(1-2), 61-68 (2014).
- Andreazza AC, Kauer-Sant'anna M, Frey BN, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J. Affect. Disord111(2-3), 135-144 (2008).
- Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric. Oxide 24(3), 125-131 (2011).
- Kudlow P, Cha DS, Carvalho AF, et al. Nitric Oxide and Major Depressive Disorder: Pathophysiology and Treatment Implications. Curr. Mol. Med(2016).
- Siwek M, Sowa-Kucma M, Dudek D, et al. Oxidative stress markers in affective disorders. Pharmacol. Rep 65(6), 1558-1571 (2013).
- Savas HA, Herken H, Yurekli M, et al. Possible role of nitric oxide and adrenomedullin in bipolar affective disorder. Neuropsychobiology 45(2), 57-61 (2002).
- Ozcan ME, Gulec M, Ozerol E, et al. Antioxidant enzyme activities and oxidative stress in affective disorders. Int. Clin. Psychopharmacol19(2), 89-95 (2004).
- Savas HA, Gergerlioglu HS, Armutcu F, et al. Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes. World. J. Biol. Psychiatry7(1), 51-55 (2006).
- Selek S, Savas HA, Gergerlioglu HS, et al. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J. Affect. Disord 107(1-3), 89-94 (2008).
- Suzuki E, Yagi G, Nakaki T, et al. Elevated plasma nitrate levels in depressive states. J. Affect. Disord63(1-3), 221-224 (2001).
- Selley ML. Increased (E)-4-hydroxy-2-nonenal and asymmetric dimethylarginine concentrations and decreased nitric oxide concentrations in the plasma of patients with major depression. J. Affect. Disord80(2-3), 249-256 (2004).
- Arslan A, Uzun M. Does the lower nitric oxide level cause cardiovascular changes in major depressed women? Eur. Rev. Med. Pharmacol. Sci12(5), 309-313 (2008).
- Gergerlioglu HS, Savas HA, Bulbul F, et al.Changes in nitric oxide level and superoxide dismutase activity during antimanic treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry31(3), 697-702 (2007).
- Lu YR, Fu XY, Shi LG, et al.Decreased plasma neuroactive amino acids and increased nitric oxide levels in melancholic major depressive disorder. BMC. Psychiatry14:123 (2014).
- Herken H, Gurel A, Selek S, et al.Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch. Med. Res 38(2), 247-252 (2007).
- Akdemir A, Turkcapar MH, Orsel SD, et al.Reliability and validity of the Turkish version of the Hamilton Depression Rating Scale. Compr. Psychiatry42(2), 161-165 (2001).
- Yanik M, Vural H, Tutkun H, et al.The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder. Eur. Arch. Psychiatry. Clin. Neurosci254(1), 43-47 (2004).
- Akpinar A, Yaman GB, Demirdas A, et al.Possible role of adrenomedullin and nitric oxide in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry46(), 120-125 (2013).
- Kim YK, Paik JW, Lee SW, et al.Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog. Neuropsychopharmacol. Biol. Psychiatry30(6), 1091-1096 (2006).
- Lee BH, Lee SW, Yoon D, et al.Increased plasma nitric oxide metabolites in suicide attempters. Neuropsychobiology 53(3), 127-132 (2006).
- Moreno J, Gaspar E, Lopez-Bello G, et al.Increase in nitric oxide levels and mitochondrial membrane potential in platelets of untreated patients with major depression. Psychiatry. Res209(3), 447-452 (2013).
- Talarowska M, Galecki P, Maes M, et al.Nitric oxide plasma concentration associated with cognitive impairment in patients with recurrent depressive disorder. Neurosci. Lett510(2), 127-131 (2012).
- Wei YC, Zhou FL, He DL, et al.The level of oxidative stress and the expression of genes involved in DNA-damage signaling pathways in depressive patients with colorectal carcinoma. J. Psychosom. Res66(3), 259-266 (2009).
- Zhou F, Zhang W, Wei Y, et al.The changes of oxidative stress and human 8-hydroxyguanine glycosylase1 gene expression in depressive patients with acute leukemia. Leuk. Res31(3), 387-393 (2007).
- Chrapko WE, Jurasz P, Radomski MW, et al.Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol. Psychiatry56(2), 129-134 (2004).
- Garcia RG, Zarruk JG, Barrera C, et al.Plasma nitrate levels and flow-mediated vasodilation in untreated major depression. Psychosom. Med73(4), 344-349 (2011).
- Moylan S, Berk M, Dean OM, et al.Oxidative & nitrosative stress in depression: why so much stress? Neurosci. Biobehav. Rev45(), 46-62 (2014).
- Freudenberg F, Alttoa A, Reif A. Neuronal nitric oxide synthase (NOS1) and its adaptor, NOS1AP, as a genetic risk factors for psychiatric disorders. Genes. Brain. Behav14(1), 46-63 (2015).
- Karadag F, Oral T, Yalcin FA, et al.Reliability and validity of Turkish translation of Young Mania Rating Scale. Turk. Psikiyatri. Derg13(2), 107-114 (2002).
- de Sousa RT, Zanetti MV, Busatto GF, et al.Lithium increases nitric oxide levels in subjects with bipolar disorder during depressive episodes. J. Psychiatr. Res55(), 96-100 (2014).
- Chiu CT, Wang Z, Hunsberger JG, et al.Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol. Rev65(1), 105-142 (2013).
- Baranyi A, Amouzadeh-Ghadikolai O, Rothenhausler HB, et al.Nitric Oxide-Related Biological Pathways in Patients with Major Depression. PLoS. One10(11), e0143397 (2015).
- Schiavone S, Trabace L. Pharmacological targeting of redox regulation systems as new therapeutic approach for psychiatric disorders: A literature overview. Pharmacol. Res107(), 195-204 (2016).
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 87(1), 315-424 (2007).
- Uttara B, Singh AV, Zamboni P, et al. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol7(1), 65-74 (2009).
- Mori K, Togashi H, Ueno KI, et al. Aminoguanidine prevented the impairment of learning behavior and hippocampal long-term potentiation following transient cerebral ischemia. Behav. Brain. Res 120(2), 159-168 (2001).
- Zhu DY, Liu SH, Sun HS, et al. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J. Neurosci23(1), 223-229 (2003).
- Fujikawa DG. The role of excitotoxic programmed necrosis in acute brain injury. Comput. Struct. Biotechnol. J 13(1), 212-221 (2015).
- Maia-de-Oliveira JP, Trzesniak C, Oliveira IR, et al.Nitric oxide plasma/serum levels in patients with schizophrenia: a systematic review and meta-analysis. Rev. Bras. Psiquiatr34(Suppl 2), S149-S155 (2012).
- Krass M, Wegener G, Vasar E, et al.The antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis. Behav. Brain. Res218(1), 57-63 (2011).
- Ha E, Jung KH, Choe BK, et al.Fluoxetine increases the nitric oxide production via nuclear factor kappa B-mediated pathway in BV2 murine microglial cells. Neurosci. Lett397(3), 185-189 (2006).
- Faizal P, Satheeshan B, Milindkumar, et al.Antioxidant status and oxidative stress in the circulation of younger and elderly human subjects. Indian. J. Clin. Biochem28(4), 426-428 (2013).
- Maiti AK. Genetic determinants of oxidative stress-mediated sensitization of drug-resistant cancer cells. Int. J. Cancer130(1), 1-9 (2012).