Review Article - Interventional Cardiology (2014) Volume 6, Issue 5
Infectious complications of percutaneous cardiac procedures
- Corresponding Author:
- Jinnette Dawn Abbott
Cardiovascular Institute, The Warren Alpert School of Medicine at Brown University
814 APC, 593 Eddy Street, Providence, RI 02903, USA
Tel: +1 401 444 5328
Fax: +1 401 444 4652
E-mail: jabbott@lifespan.org
Abstract
Percutaneous coronary intervention (PCI) and interventional structural heart disease procedures have steadily increased worldwide over the last two decades. Patient infection is a potential complication of percutaneous cardiac procedures, but the incidence is very low due to the use of sterile techniques and percutaneous arterial puncture. Infection can be categorized as occurring at the access site, which includes vascular closure devices, or locally within the implanted device or a cardiac structure. Although rare, endovascular and cardiac infection can be challenging to diagnose and may have catastrophic consequences. In most cases, treatment requires prolonged parenteral antibiotics and surgical intervention. A comprehensive review of all reports of infection related to percutaneous cardiac procedures since 1994 is covered as are the current infection control guidelines.
Keywords
complications, infection, percutaneous coronary intervention, Staphylococcus aureus, vascular closure device
Infections are an inherent risk of any invasive procedure or indwelling temporary or permanent device. Fortunately, for percutaneous procedures performed with sterile technique the incidence is rare. Although infection rates may be underestimated by reporting bias or missed diagnosis, a large retrospective study involving over 22,000 cardiac procedures found a bloodstream infection rate of 0.11% [1]. Several potential sources may introduce bacteria to the access site, the blood stream or a remote location. Localized infection can occur at any site of vascular injury such as the access site, or in an implanted device. The factors influencing the pathogenesis of procedure related infections include the virulence of the pathogen, host response, and the properties of the device. In this review, infections related to arterial access, as well as implanted cardiac devices including stents, valves and septal closure devices are covered in detail. In addition, indications for antibiotic prophylaxis and standards for infection control are discussed.
Cardiac catheterization
Diagnostic coronary angiography and percutaneous coronary intervention (PCI) are the most common procedures performed in cardiac catheterization laboratory. In 2010, over 1,000,000 diagnostic cardiac catheterizations and 950,000 PCI procedures were performed in the USA [2]. In the current era, over 95% of PCI is performed with stents, with a predominance of drug-eluting stents (DES) over bare-metal stents (BMS) [3]. A small prospective study of 147 complex PCI patients demonstrated a rate of positive blood cultures of 18% immediately following the procedure and 12% at 12 h. However, in this series, no patients had evidence of clinical infection. This study demonstrates that transient bacteremia is common and generally clears without sequela [4]. A larger retrospective series found PCI related bacteremia in 0.64% of patients [5].
Access site infections
The Swedish physician Sven Seldinger is largely credited for introducing a guidewire technique to improve the safety of vascular access. With rare exception, percutaneous venous or arterial cannulation for cardiac catheterization is achieved utilizing a modification of Seldinger’s original technique. This procedure effectively places a plastic or composite sheath into a superficial blood vessel allowing catheters to be delivered to cardiac structures [6]. The majority of sheaths placed in the catheterization laboratory are removed at the completion of a procedure or shortly thereafter, which minimizes risk of infection. In the critical care setting where catheters remain inserted for longer periods, the risk of catheter related bloodstream infections is around 2% [7].
Manual compression has long been the method for achieving hemostasis after cardiac catheterization, and arterial infection after cardiac catheterization utilizing this method remains a rare event. Septic endarteritis is an infectious complication of femoral artery cannulation. On literature review, septic endarteritis after femoral artery catheterization and PCI with hemostasis by manual compression has been reported in 20 cases. All of these case reports were associated with certain risk factors such as repeat ipsilateral puncture and prolonged sheath times. Hematoma at the arterial access site can also serve as a nidus for infection. The majority of these infections were caused by staphylococcal infection and required arterial debridement, reconstruction and long-term intravenous antibiotics [8]. Without timely and appropriate treatment, septic arterial aneurysms can rupture or seed additional sites. Septic endarteritis following manual compression after diagnostic catheterization has not been reported. It appears that PCI, perhaps in the context of increased procedural time, provides the increased risk for septic endarteritis. In addition, the use of procedural anticoagulation such as heparin may increase the risk of local infection and bacteremia [8].
Practices that may reduce the risk of septic complications of catheterization include avoidance of access through vascular grafts or surgical sites such as prosthetic hips. Sheaths should not be left in place longer than required. For patients that require staged procedures, the sheath should be removed and alternative access used for the subsequent procedure. There is no data supporting the use of prophylactic antibiotics for catheterization access sites managed with manual pressure.
Vascular closure device infection
Percutaneous vascular closure devices (VCD) have been increasingly used in order to shorten time to ambulation and reduce access site complications in the setting of femoral artery cannulation. Recent data have reported device use in approximately 37% of PCIs and there are significant reductions in vascular complications and the need for blood transfusions [9]. The purported benefits of VCD must be considered against the infectious risks of a foreign body implant. These devices can be broadly separated into suture closure and vascular plugs with collagen or polyethylene glycol. There have been reported cases of septic endarteritis with the use of the Perclose device (Abbott Laboratories, Abbott Park, IL, USA) in diagnostic catheterization. In this setting, it appears that the VCD itself portends the increased infection risk regardless of whether or not coronary intervention was performed.
The Manufacturer and User Facility Device Experience (MAUDE) database is a voluntary reporting system maintained by the US FDA. There appear to be numerous infectious complications entered into this database, which are not reported in the medical literature. All of these cases tend to be similar with delayed presentation of infection (range of 4–28 days), extensive necrosis of the femoral artery requiring reconstruction, and infection with Staphylococcus aureus. All of these complications appear to be treated with long-term intravenous antibiotics [10].
The Mayo Clinic performed a retrospective analysis of VCD-related infections between1 January 2000 and 31 December 2003. A total of 46 cases in the medical literature and six cases from their own institution of VCD-related infections were reported. Diabetes mellitus and obesity were the most common comorbidities and Staphylococcus aureus was responsible for most (75%) of the infections. Interestingly, Mycotic pseudoaneurysms (22 cases) were the most common complications and most of these patients underwent surgical debridement and reconstructive procedures [11].
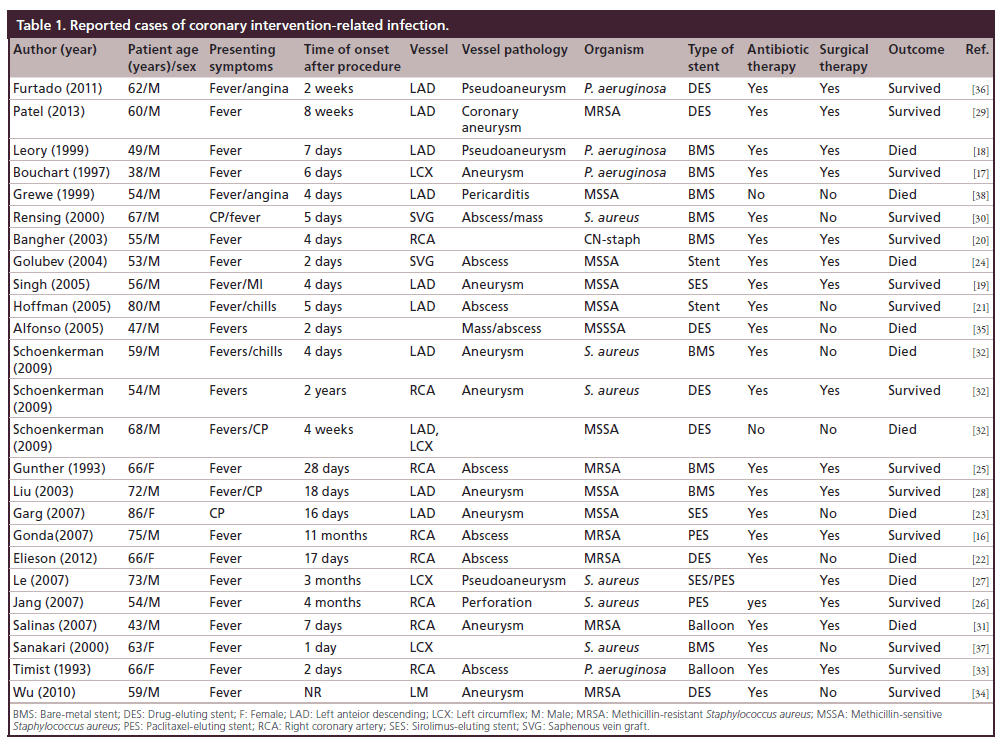
Coronary artery stent infections
Stent infection is equally as rare as access site infection but carries more significant morbidity and mortality [12–14]. With the increasing use of DES there is a theoretical increased risk of infection over BMS, in part due to their immunomodulatory properties and delayed endothelialization compared to BMS [15]. Review of the current literature yielded 25 reported cases of coronary stent infection (Table 1) [16–38]. The plurality of infections (48%) was in patients receiving DES followed by BMS (32%). Two patients (8%) developed a coronary infection in the setting of balloon angioplasty. The remainder of patients the stent type was unknown. The most common organism implicated was Staphylococcus aureus (80%) followed by Pseudomonas aeruginosa (20%).
Risk factors
The etiology of periprocedural infection is unknown; however, there are several risk factors that predispose patients to coronary stent infection. The major risk factors for developing infectious complications post procedure are congestive heart failure, age >60 years old, and procedure related risk factors such difficult vascular access, repeated catheter insertion and duration of sheath retention [17].
Clinical presentation & diagnosis
The diagnosis of coronary artery stent infection presents several challenges. In our review, the most common symptoms are fever and chills and chest pain. However, the nonspecific nature of these symptoms often lead to delay in diagnosis and treatment. Furthermore, the time from stent implant to presentation is also variable, ranging from 2 days to 4 months. A high degree of clinical suspicion, therefore, is required. In the absence of a clear etiology of symptoms stent infection should be considered.
One criteria proposed to aid in the diagnosis of stent infection is the presence of three or more of the following risk factors:
• Placement of a coronary stent within the previous 4 weeks;
• Multiple repeat procedures performed through the same arterial sheath;
• The presence of bacteremia, significant fever, or leukocytosis with no other cause;
• Acute coronary syndrome;
• Positive cardiac imaging [39].
Coronary stent infection manifests in many forms, including coronary artery abscess, aneurysm, and pericarditis. The most common modality used for diagnosis was coronary angiography, but there has been reported use of transthoracic echocardiography, transesophageal echocardiography [17–19], computed tomography coronary angiogram [18,39], cardiovascular magnetic resonance imaging [16], and more recently, PET imaging has been used to diagnosed endovascular stent infection [40].
As evident by this review there is a trend towards higher infection rates with DES. Theoretically, the higher infection rates with DES might be due to impairment of local host defense mechanism and endothelialization of the stent struts by the immunomodulatory drugs released from the stents, which serves as a nidus for infection.
Treatment
The ideal antibiotic duration or mode of treatment for coronary stent infection is unknown. A review by Elieson and colleagues suggested that early-onset infection, defined as <10 days after stent implantation, may be treated with prolonged broad spectrum antibiotics alone. Late-onset infection, defined as ≥10 days after implantation, or major complication, usually necessitate combined surgical and medical therapy [22].
Our contention, based on this case review is that stent infection should be treated similar to infected vascular and valvular prosthesis, where a combined antibiotic and surgical approach is preferred. The goal is total removal of infected material, and subsequent installation of an extra-anatomical or bypass graft. In this review, more than half of the patient survived (60%), and the majority required a combination of both prolonged broad spectrum antibiotic therapy and surgery.
Noncoronary device infections
Permanent implantation of non-coronary devices are being increasingly performed in the cardiac catheterization laboratory including percutaneous heart valves, septal closure devices and endovascular stent grafts. These procedures have a theoretically higher risk of infection compared to intracoronary stents due to larger sheaths, multiple access sites and in some circumstances need for arterial cutdown. In addition, these devices employ prosthetic graft material in addition to a metal alloy.
Transcatheter aortic valve replacement
Infectious complications have been reported with transcatheter aortic valve replacement (TAVR). A recent literature search utilizing MEDLINE-EBSCO and PubMed databases identified ten cases of infective endocarditis (IE) occurring after the TAVR procedure. Most of these IE cases occurred months after the procedure with a mean time to onset of 186 days. The infected valve was the CoreValve (Medtronic CV, Luxembourg) in six of the cases and the Edwards SAPIEN valve (Edwards Lifesciences, CA, USA) in the other four cases. Some of the predisposing factors that these investigators noted included recent PCI, recurrent recent urinary tract infections, probable pneumonia, recent dental procedure, repeated attempts at CoreValve implantation, and mechanical ventilation [41].
The initial clinical symptoms of IE in these TAVR cases included but were not limited to fever, dyspnea, malaise, and elevated inflammatory markers. The echocardiographic findings included vegetation in four of the cases, severe mitral regurgitation with rupture of the anterior leaflet in two cases, left ventricular outflow tract to left atrium fistula in two cases, one case of an echo-free space in the wall of the ascending aorta where the stents of the CoreValve were observed, and one case there were no echocardiographic findings. In contrast to access site infections, multiple different pathogens were identified including Staphylococcus species (three cases), Streptococcus species (two cases), and atypical bacteria such as Enteroccocus faecium, Corynebacterium, and Moraxella nonliquefaciens, as well as fungi such as Histoplasma capsulatum and Candida albicans. Seven patients were treated with antibiotics alone, three required surgery, and in follow-up analysis four patients died [41].
The estimated incidence of IE following TAVR ranges between 0 and 2.3%, which is comparable to surgical prosthetic valve endocarditis incidence of 0.1– 4% per year. Despite adequate and aggressive antibiosis and surgical revision, mortality remains high, ranging from 20 to 80% of affected patients [40]. The mechanisms of pathogen exposure include an intraoperative contamination event or a postoperative event from a remote infectious source. Transfemoral TAVR may be achieved via surgical cut-down to the common femoral artery, followed by primary closure at procedure completion or a by a fully percutaneous approach utilizing a VCD. A 2013 study of percutaneous access and closure compared to cut-down revealed that wound infections requiring prolonged antibiotics use or surgical debridement occurred significantly more frequently in the surgical group (0.7 vs 6.7%; p = 0.007) [42]. If a cut-down is utilized, infection control precautions need to be as strict as if the procedure were performed in a surgical suite. Due to the high morbidity associated with IE related to TAVR, clinicians should implement antibiotic prophylaxis directed against skin flora prior to the TAVR procedure. In addition, subacute bacterial endocarditis prophylaxis guidelines for prosthetic cardiac valves should be followed, particularly in the context of an increased risk of IE as a result of procedural aortic insufficiency [43].
Endovascular closure of septal defects
Congenital heart defects including atrial septal defect (ASD) and patent foremen ovale, have indications for closure and are often treated via an endovascular approach; however, there is a paucity of literature that details infectious complications of these procedures. An Italian study analyzed 417 patients that underwent transcatheter occlusion of secundum type ASD between December 1996 and January 2001. Complications were categorized as major and minor. The devices analyzed were the CardioSEAL/STARFlex (NMT Medical, MA, USA) in 159 patients and the Amplatzer Septal Occluder (St Jude Medical, MN, USA) in 258 patients. With respect to infectious complications, there were only two reported cases of IE post transcatheter ASD closure. This study highlights that infectious complications, more specifically IE is an exceedingly rare complication in the transcatheter septal closure era [44]. Prophylaxis against subacute bacterial endocarditis is recommended for 6 months after catheter based closure of congenital heart defects, during the period of device endothelialization [43].
Infection control in the cardiac catheterization laboratory
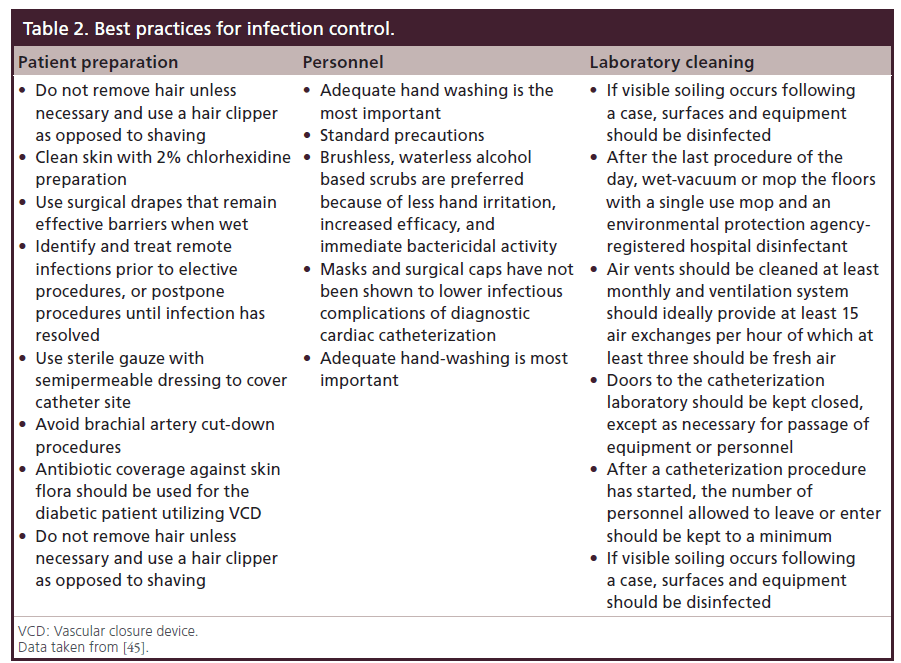
The standardization of central access procedures has resulted in the widespread use of sterile insertion measures. Many hospitals utilize checklists and involve nursing or other team members during procedures to ensure that not only is the correct procedure being performed, but that an experienced operator is following sterile technique and maximizing barrier precautions. Clinical practice guidelines for infection control in the cardiac catheterization laboratory have been proposed. The Society for Cardiovascular Angiography and Interventions (SCAI) most recently updated guidelines in 2006 [45]. Table 2 outlines the best practices for prevention of infections including patient preparation, access site management, personnel and laboratory cleaning recommendations.
Table 2: Best practices for infection control.
Conclusion & future perspective
Infectious complications of percutaneous cardiac procedures are rare but must be considered in any patient presenting with fevers, chills or bacteremia in the initial 4 weeks following a procedure. There are several clinical and procedural related risk factors for infection including presence of congestive heart failure, age over 60 years, difficult vascular access, sheath duration or repeated catheter insertion. Treatment generally includes prolonged antibiotics and surgical intervention to remove any infected prosthesis. SCAI has proposed guidelines for the prevention of infection and careful implementation of their recommendations may improve patient outcomes. In an era of patient centered medicine and accountability in healthcare, prevention of procedural-related infections is of paramount importance.
Percutaneous procedures performed in the cardiac catheterization laboratory or in a hybrid operating room are increasing in scope and complexity. Concurrent with this growth is the need for adequate training of staff and strict adherence to sterile procedures. Driven in part by financial penalty for nosocomial infections, the infection control field is in constant evolution. A variety of device related factors have been developed and tested with varying results. A recent large meta-analysis revealed a marked reduction in catheter related blood stream infection with the use of antibiotic impregnated or heparin coated catheters [46]. Although conceptually in its infancy, a recent study suggested the incorporation of a novel group of Factor XIIIa (plasma transglutaminase) inhibitors into a lubricious silicone elastomer in order to generate an optimized drug delivery system.
In this model, a secondary sustained drug-release profile occurs following an initial burst release for catheters and other medical devices, with the hope that this would reduce the incidence of associated staphylococcal infection [47]. Studies in vivo are yet to be performed. Further research aimed at understanding pathogenesis of central venous catheter-related infections is ongoing.
Executive summary
Infectious complications of percutaneous cardiac procedures are rare
• Procedure-related infections should be considered any in patient presenting with fevers, chills or bactremia in the first 4 weeks postprocedure.
Clinical risk factors of postprocedure infections
• Congestive heart failure.
• Age >60 years old.
Procedure-related risk factors
• Difficult vascular access.
• Repeated catheter insertion.
• Time of sheath retention in the vessel.
Treatment of procedure-related infection
• Prolonged antibiotic use.
• Surgical intervention in some case to remove infected prosthesis.
Infectious control standard
• Adherence to infectious control guidelines proposed by the Society for Cardiovascular Angiography and Interventions can minimize postprocedure infectious complication.
• Use of prophylaxis antibiotics in diabetic patient receiving vascular closure devices.
Future prospective
• Increasing complexity of cardiac catheterization procedure and development of hybrid or requires more stringent adherence to sterile technique.
• Improvement in technology will allow for more sterile delivery of catheterization equipment.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- Muñoz P, Blanco JR, Rodríguez-Creixéms M et al.Bloodstream infections after invasive nonsurgical cardiologicprocedures. Arch. Intern. Med. 161(17), 2110–2115 (2001).
- Go AS, Mozaffarian D, Roger VL et al. American HeartAssociation Statistics Committee and Stroke StatisticsSubcommittee. Heart disease and stroke statistics – 2014update: a report from the American Heart Association.Circulation 129(3), e28–e292 (2014).
- Williams DO, Abbott JD, Kip KE; DEScover Investigators.Outcomes of 6906 patients undergoing percutaneous coronaryintervention in the era of drug-eluting stents: report of theDEScover Registry. Circulation 1114(20), 2154–2162 (2006).
- Ramsdale DR, Aziz S, Newall N, Palmer N, Jackson M. Bacteremia following complex percutaneous coronaryintervention. J. Invasive Cardiol. 16(11), 632–634 (2004).
- Samore M, Wessolossky M et al. Frequency, risk factors, andoutcome for bacteremia after percutaneous transluminalcoronary angioplasty. Am. J. Cardiol. 79(7), 873–877 (1997).
- Seldinger SI. Catheter replacement of the needle inpercutaneous arteriography. Acta Radiol. 39, 368–376 (1953).
- Deshpande KS, Hatem C, Ulrich HL et al. The incidenceof infectious complications of central venous catheters at thesubclavian, internal jugular, and femoral sites in an intensivecare unit population. Crit. Care Med. 33(1), 13–20 (2005).
- Shea KW, Schwartz RK, Gambino AT, Marzo KP, CunhaBA. Bacteremia associated with percutaneous transluminalcoronary angioplasty. Cathet. Cardiovasc. Diagn. 36(1), 5–9(1995).
- Gurm HS, Hosman C, Share D, Moscucci M, Hansen BB;for the Blue Cross Blue Shield of Michigan CardiovascularConsortium. Comparative safety of vascular closure devicesand manual closure among patients having percutaneouscoronary intervention. Ann. Intern. Med. 159(10), 660–666(2013).
- Johanning JM, Franklin DP, Elmore JR, Han DC. Femoralartery infections associated with percutaneous arterial closuredevices. J. Vasc. Surg. 34(6), 983–985 (2001).
- Sohail MR, Khan AH, Holmes DR Jr, Wilson WR,Steckelberg JM, Baddour LM. Infectious complicationsof percutaneous vascular closure devices. Mayo Clin. Proc.80(8), 1011–1015 (2005).
- Gray NA, Baddour LM. Nonvalvular intravascular devicerelatedinfections. Curr. Infect. Dis. Rep. 4, 293–298(2002).
- Baddour LM, Bettmann MA, Bolger AF et al. Nonvalvularcardiovascular device-related infections. Circulation 108,2015–2031 (2003).
- Lim CP, Ho KL, Tan TT, Wong AS, Tan JW, Chua YL,Su JW. Infected coronary artery pseudoaneurysm afterrepeated percutaneous coronary intervention. Ann. Thorac.Surg. 91(2), e17–e19 (2011).
- Gautam SR, Mishra D, Goyal BK. Mechanical interventionand coronary artery aneurysm. J. Invasive Cardiol. 22(12),e213–e215 (2010).
- Gonda E, Edmundson A, Mann T. Late coronary stentinfection: a unique complication after drug-eluting stentimplantation. J. Invasive Cardiol. 19(10), e307–e308(2007).
- Bouchart F, Dubar A, Bessou JP, Redonnet M, BerlandJ, Mouton-Schleifer D, Haas-Hubscher C, Soyer R.Pseudomonas aeruginosa coronary stent infection. Ann.Thorac. Surg. 64(6), 1810–1813 (1997).
- Leroy O, Martin E, Prat A, Decoulx E, Georges H, GuilleyJ, Beuscart C, Beaucaire G. Fatal infection of coronary stentimplantation. Cathet. Cardiovasc. Diagn. 39(2), 168–170(1996).
- Singh H, Singh C, Aggarwal N, Dugal JS, Kumar A,Luthra M. Mycotic aneurysm of left anterior descendingartery after sirolimus-eluting stent implantation: a casereport. Catheter Cardiovasc. Interv. 65(2), 282–285 (2005).
- Bangher M, Liva P, Baccaro J. [Coronary stent infection:case report and definition]. Rev. Esp. Cardiol. 56(3),325–326 (2003).
- Hoffman M, Baruch R, Kaplan E, Mittelman M, AviramG, Siegman-Igra Y. Coronary stent bacterial infection withmultiple organ septic emboli. Eur. J. Intern. Med. 16(2),123–125 (2005).
- Elieson M, Mixon T, Carpenter J. Coronary StentInfections: a case report and literature review. Tex. HeartInstit. J. 396, 884–889 (2012).
- Garg N, Garg R, Gordon C, Singh R, Singh A. Acutecoronary syndrome caused by coronary artery mycoticaneurysm due to late stent infection localized withradiolabeled autologous leukocyte imaging. Clin. Nucl.Med. 34(11), 753–755 (2009).
- Golubev N, Schwammenthal E, Di Segni E, Pudil R, Hay I,Feinberg MS. Echocardiographic imaging of coronary arteryabscess following stent implantation. Echocardiography 21(1),87–88 (2004).
- Günther HU, Strupp G, Volmar J, von Korn H, Bonzel T,Stegmann T. [Coronary stent implantation: infection andabscess with fatal outcome]. Z. Kardiol. 82(8), 521–525(1993).
- Jang JJ, Krishnaswami A, Fang J, Go M, Ben VC. Images incardiovascular medicine. Pseudoaneurysm and intracardiacfistula caused by an infected paclitaxel-eluting coronarystent. Circulation 116(14), e364–e365 (2007).
- Le MQ, Narins CR. Mycotic pseudoaneurysm of the leftcircumflex coronary artery: a fatal complication followingdrug-eluting stent implantation. Catheter Cardiovasc. Interv.1, 69(4), 508–512 (2007).
- Liu JC, Cziperle DJ, Kleinman B, Loeb H. Coronary abscess:a complication of stenting. Catheter Cardiovasc. Interv. 58(1),69–71 (2003).
- Patel AJ, Mehta RM, Gandhi DB, Bossone E, Mehta RH.Coronary aneurysm and purulent pericardial effusion: olddisease with an unusual cause. Ann. Thorac. Surg. 95(5),1791–1793 (2013).
- Rensing BJ, van Geuns RJ, Janssen M, Oudkerk M, de FeyterPJ. Stentocarditis. Circulation 101(18), e188–e190 (2000).
- Salinas G, Kumar D, Lick S, Vijayakumar V, Rahman M,Uretsky BF. Infective coronary aneurysms: a complication ofpercutaneous coronary intervention. Tex. Heart Inst. J. 34(1),91 (2007)
- Schoenkerman AB, Lundstrom RJ. Coronary stentinfections: a case series. Catheter Cardiovasc. Interv. 73(1),74–76 (2009).
- Timsit JF, Wolff MA, Bédos JP, Lucet JC, Décré D. Cardiacabscess following percutaneous transluminal coronaryangioplasty. Chest 103(2), 639–641 (1993).
- Wu BE, Chan WM, Yu CM. Left main stem rupture causedby methicillin resistant staphylococcus aureus infection ofleft main stent treated with covered stenting. Int. J. Cardiol.144, e39–e41 (2010).
- Alfonso F, Moreno R, Vergas J. Fatal infection afterrapamycin eluting coronary stent implantation. Heart 91(6),e5 (2005).
- Furtado AD, Bhat SP, Peer SM, Chikkatur R. Infectedpseudoaneurysm involving a drug-eluting stent. Interact.Cardiovasc. Thorac. Surg. 12(4), 636–637 (2011).
- Sankari A, Kumar AN, Kabins S, Chandna H, LiebD. Staphylococcal pericarditis following percutaneoustransluminal coronary angioplasty. Catheter Cardiovasc.Interv. 50(1), 71–73 (2000).
- Grewe PH, Machraoui A, Deneke T, Müller KM.Suppurative pancarditis: a lethal complication of coronarystent implantation. Heart 81(5), 559 (1999).
- Dieter RS. Coronary artery stent infection. Clin. Cardiol.23(11), 808–801 (2000).
- Lee SH, Song PS, Kim WS, Park KB, Choi SH. A case ofstent graft infection coupled with aorto-esophageal fistulafollowing thoracic endovascular aortic repair in a complexpatient. Korean Circ. J. 42(5), 366–368 (2012).
- Eisen A, Shapira Y, Sagie A, Kornowski R. Infectiveendocarditis in the transcatheter aortic valve replacement era:comprehensive review of a rare complication. Clin. Cardiol.35, 11, e1–e5 (2012).
- Nakamura M, Chakravarty T, Jilaihawi H, Doctor N,Dohad S, Fontana G, Cheng W, Makkar RR. Completepercutaneous approach for arterial access in transfemoraltranscatheter aortic valve replacement: a comparison withsurgical cut-down and closure. Cathet. Cardiovasc. Intervent.doi:10.1002/ccd.25130 (2014) (Epub ahead of print).
- Wilson W, Taubert KA, Gewitz M et al. Prevention ofinfective endocarditis: guidelines from the AmericanHeart Association: a guideline from the American HeartAssociation Rheumatic Fever, Endocarditis, and KawasakiDisease Committee, Council on Cardiovascular Disease inthe Young, and the Council on Clinical Cardiology, Councilon Cardiovascular Surgery and Anesthesia, and the Qualityof Care and Outcomes Research Interdisciplinary WorkingGroup. Circulation 116, 1736 (2007).
- Chessa M1, Carminati M, Butera G et al. Early and latecomplications associated with transcatheter occlusion ofsecundum atrial septal defect. J. Am. Coll. Cardiol. 39(6),1061–1065 (2002).
- Chambers CE, Eisenhauer MD, McNicol LB et al. InfectionControl Guidelines for the Cardiac CatheterizationLaboratory: Society Guidelines Revisited. CatheterCardiovasc. Interv. 67(1), 78–86 (2006).
- Gilbert RE, Harden M. Effectiveness of impregnated centralvenous catheters for catheter related blood stream infection:a systematic review. Curr. Opin. Infect. Dis. 21(3), 235–245(2008).
- Daneshpour N, Collighan R, Perrie Y, Lambert P, RathboneD, Lowry D, Griffin M. Indwelling catheters and medicalimplants with FXIIIa inhibitors: A novel approach to thetreatment of catheter and medical device-related infections.Eur. J. Pharm. Biopharm. 83(1), 106–113 (2013).
• Bloodstream infection in associated with cardiac procedures between 1991 and 1998.
• Executive summary of incidence and prevalence of cardiovascular disease and associated mortality.
• Health insurance registry of safety of vascular closure devices and their benefit in reducing the incidence of major access site bleeding and bloodstream bacteremia.
• AHA Guidelines recommendation for antibiotic prophylaxis for prevention of endocarditis.
• Society of cardiovascular angiography and intervention infectious control guidelines in cardiac catheterization laboratory.