Review Article - Interventional Cardiology (2023)
Inflammation in atherosclerosis: Role in pathogenesis and promising therapeutic strategies
- Corresponding Author:
- Alexander V. Blagov
Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia, E-mail: al.blagov2014@gmail.com - Alexander N. Orekhov
Laboratory of Cellular and Molecular Pathology of Cardiovascular System, Federal State Budgetary Scientific Institution “Petrovsky National Research Center of Surgery” (FSBSI “Petrovsky NRCS”), Abrikosovsky per., 2, Moscow, 119991, Russia, E-mail: a.h.opexob@gmail.com
Received date: 13-Feb-2023, Manuscript No. FMIC-23-89248; Editor assigned: 15-Feb-2023, PreQC No. FMIC-23-89248 (PQ); Reviewed date: 01-Mar-2023, QC No. FMIC-23-89248;Revised date: 08-Mar-2023, Manuscript No. FMIC-23-89248 (R);Published date: 18-Mar-2023, DOI: 10.37532/1755-5310.2023.15 (S15).379
Abstract
Studying the factors influencing the development of inflammation in atherosclerosis is an important step to better understand the pathogenesis of this disease. In this review, we consider the process of development of inflammation in atherosclerosis, the influence of various cell types on the development of inflammation in atherosclerosis, and the relationship between the inflammatory response and the progression of the disease. In addition, several strategies for anti-inflammatory therapy of atherosclerosis have been proposed, which may find their practical application in the near future.
Keywords
Atherosclerosis . Inflammation . T-cells
Introduction
Atherosclerosis is a chronic inflammatory disease of the arteries and is the main cause of about 50% of all deaths in a Europeanized society [1]. It is primarily a lipid-dependent process initiated by the accumulation of low-density lipoproteins and residual lipoprotein particles, as well as an active inflammatory process in the focal areas of the arteries, especially in areas of impaired non-laminar blood flow at the arterial bifurcation points, and is considered the main cause of Atherosclerotic Cardiovascular Disease (ASCVD), leading to heart attacks, stroke, and peripheral arterial disease [2].
Currently, atherosclerosis is a common disease in which fatty deposits, called atheromatous plaques, appear in the inner layers of the arteries. The formation of these plaques begins with the deposition of small cholesterol crystals in the intima and underlying smooth muscle. Then the plaques grow with the growth of fibrous tissue and smooth muscles surrounding them and protrude into the arteries and, as a result, reduce blood flow. The production of connective tissue by fibroblasts and the deposition of calcium in the lesion cause sclerosis, or hardening of the arteries. Finally, the uneven surface of the arteries leads to the formation of blood clots and thrombosis, which leads to a sudden obstruction of blood flow [3]. Atherosclerosis can manifest as Coronary Heart Disease (CHD), Cerebrovascular Disease (CVD), Transient Ischemic Attack (TIA), Peripheral Arterial Disease (PAD), abdominal aneurysms, and renal artery stenosis in men [4].
Atherosclerosis has a multifactorial etiology. The most common risk factors include hypercholesterolemia (LDL cholesterol), arterial hypertension, diabetes mellitus, cigarette smoking, age (men over 45 and women over 55), and male gender. In addition, sedentary lifestyle, obesity, diets high in saturated and Tran’s fatty acids and some genetic mutations contribute to the risk [5].
Since atherosclerosis is predominantly an asymptomatic disease, it is difficult to accurately determine the incidence. Atherosclerosis is considered the main cause of cardiovascular disease. Atherosclerotic cardiovascular diseases mainly affect the heart and brain: Coronary Heart Disease (CHD) and ischemic stroke. IHD and stroke are the first and fifth causes of death in the world, respectively [6].
Atherosclerosis mainly develops as a result of a continuous process of damage to the arterial wall due to the retention of lipids by entrapment of the intima by a matrix such as proteoglycan, which leads to modification, which in turn exacerbates chronic inflammation in vulnerable areas of the arteries and plays an important role in all phases. Progression of atherogenesis, this process begins with nascent fatty streaks in the intima of the arteries, which develop into fibrous plaques and develop into complex atherosclerotic lesions prone to rupture. In addition, stenosis due to inward expansion of atheroma can lead to occlusion of vessels, such as coronary vessels [7].
Changes in plasma homeostasis, such as hypercholesterolemia, are one of the major risk factors for atherosclerosis. First, it affects Endothelial Cells (ECs), which, when activated, express new adhesion molecules and chemotactic factors that provoke an inflammatory process. The latter involves the recruitment of circulating immune cells, which exacerbates and accelerates the development of atheroma. The initial optimistic view that the correction of dyslipidemia would eliminate atherosclerosis did not materialize, as extensive data have shown that inflammation itself can be the initiator or key factor in all stages of this disease; from the initial lesion to plaque rupture [8].
To date, the treatment of atherosclerosis is the treatment of risk factors such as elevated LDL cholesterol, Blood Pressure (BP), diabetes, and others. In addition, patients should exercise regularly and follow a healthy diet low in saturated fats and trans fats. Inhibitors of 3-hydroxy-3-methylglutarylcoenzyme A-reductase (statins) are the basis for lowering LDL cholesterol levels and reducing cardiovascular events and mortality. BP control requires 2 or more drug classes, including Angiotensin-Converting Enzyme (ACE) inhibitors, Angiotensin II Receptor Blockers (ARBs), diuretics, beta-blockers, Calcium Channel Blockers (CCBs), and vasodilators. For clinical ASCVD, revascularization procedures such as angioplasty, bypass, and others are justified. In addition, thrombolysis is also a therapeutic option for CVA, acute limb ischemia due to a thrombus/ embolus [9].
Literature Review
General model for the development of inflammation in atherosclerosis
Most cardiovascular complications result from plaque rupture, the probability of which is inversely proportional to the content of Smooth Muscle Cells (SMCs) in the lesion. Therefore, the prevailing view of SMCs in progressive atherosclerosis postulates that these cells play a predominantly beneficial role as they provide a stabilizing fibrous cap and generate extracellular matrix [10]. In the early stages of atherosclerosis, activated platelets secrete chemokines, such as CC-motif chemokine 5 (CCL5), which promote monocyte and neutrophil adhesion. Neutrophils themselves secrete chemotactic granule proteins (including cathelicidin, cathepsin G, and CCL2), thereby paving the way for arterial monocyte infiltration. The chemokine environment is complemented by chemokines secreted by activated smooth muscle cells such as CCL2 and CCL5 [11]. In progressive atherosclerotic lesions, medial SMCs migrate to the developing fibrous operculum, where they undergo clonal expansion. Somatic mutations cause clonal expansion in non-malignant tissues during aging, and clonal expansion in myeloid cells is associated with an increased risk of cardiovascular events [12].
Once in the fibrous capsule, SMCs are the main producers of the extracellular matrix. Although definitive evidence is lacking, data from mice lacking COL15A1, which encodes the collagen chain, especially in α-Smooth Muscle actin (SMC), and proteomics secretomas of lipid-laden SMCs strongly suggest that SMCs contribute to the formation of a fibrous cap. However, lipid uptake and changes in interactions between SMC and extracellular matrix alter the SMC phenotype by increasing the expression of markers commonly attributed to macrophages [13]. In advanced atherosclerotic plaques in mice, 30%-70% of cells with macrophage markers (also known as foam cells) are SMC; similar observations have been made in human atherosclerotic plaques [14]. The detrimental consequences of this phenotypic switch to macrophage-like SMKs are supported by a study in which the transcription factor Kruppel-Like Factor 4 (KLF4), a transcription factor previously reported to control the phenotypic switch of SMKs during development was deleted specifically in SMK. These mice show reduced SMC switching, a distinct increase in fibrous cap thickness, and an increased content of αSMA+ cells in the fibrous cap [15]. The increased lipid load of SMC induces apoptosis of SMC and, if not quickly resolved, necrosis. SMCs also undergo cell death after interaction with histone H4 present in Neutrophil Extracellular Traps (NET) [16]. NET release is licensed by cholesterol crystals, lipopolysaccharides, modified lipids, and chemokines, such as CCL7. NETs exhibit cytotoxicity via NET-resident histones, activate the NLRP3 inflammasome in macrophages, and induce coagulation via factor XII cleavage and Tissue Factor Pathway Inhibitor (TFPI), as well as direct platelet activation. NET-associated cytotoxicity is observed during plaque erosion, when NETs released at sites of impaired blood flow cause desquamation of endothelial cells [17]. Thereby contributing to the accelerated growth of plaques under these conditions [18]. Monocyte-derived macrophages engulf modified lipids and release inflammatory chemokines and cytokines in response. Activation of atheroma macrophages decisively determines the inflammatory environment in the plaque. Cholesterol crystals in plaque coactivate inflammasome containing the NACHT, LRR and PYD domains containing protein 3 (NLRP3), which has received considerable attention in the context of atherosclerosis [19]. Crystal priming of lipid-modified cholesterol such as oxidized Low-Density Lipoprotein (LDL) or impaired cholesterol efflux triggers the Nuclear Factor-κB (NF-κB) signaling pathway, promoting transcription of NLRP3 and pro-IL-1β. Assembly of the NLRP3 inflammasome induces the activation of caspase 1, which cleaves pro-IL-1β to mature IL-1β. Excessive lipid uptake causes macrophage proliferation or even cell death [20].
The role of non- immune cells in the development of inflammation in atherosclerosis
Endothelial cells: Endothelial Dysfunction (ED) is the primary and decisive stage in the development of atherosclerosis. Numerous cardiovascular risk factors such as obesity and diabetes mellitus potentially initiate Endothelial Cell (ECs) damage, causing ED. Under normal conditions, the endothelium regulates vascular inflammation by secreting Nitric Oxide (NO), while dysfunctional endothelium accelerates the formation of Reactive Oxygen Species (ROS) and increases vascular inflammation, which is harmful to the vascular system [21]. Endothelial injury disrupts the balance between vasoconstriction and vasodilation, which is characterized by an increase in Endothelial Contraction Factor (EDCF) and initiates a number of pathophysiological changes that promote or exacerbate atherosclerosis, including increased vascular permeability to lipoproteins and increased leukocyte adhesion, platelet aggregation, and cytokine production [22]. On the other hand, various inflammatory cytokines such as Tumor Necrosis Factor α (TNF-α), Interleukin 1 (IL-1) and IL-6 induce endothelium to express Vascular Cell Adhesion Molecule (VCAM), Intercellular Adhesion Molecule (ICAM), Monocyte Chemoattractant Protein 1 (MCP-1) and other chemokines, hence promoting monocyte adhesion and migration [23]. Once in the intima, monocytes acquire the characteristics of tissue macrophages. Monocytes upregulate Scavenger Receptor (SR) expression and then internalize modified lipoproteins. The above processes consistently lead to the formation of foam cells (PC), which can be considered as an early atherosclerotic lesion [22]. After atherosclerotic plaque rupture, the physiological balance between antithrombotic and thrombotic agents is disrupted due to EC dysfunction, leading to an increase in thrombotic agents (eg, von Willebrand Factor (vWF), TXA 2) and a decrease in antithrombotic agents, such as heparin. These effects facilitate the process of thrombosis, causing devastating consequences [24]. In conclusion, all of the aforementioned factors contribute to the development of atherosclerosis, indicating an indispensable role for endothelial cells in the progression of atherosclerosis.
Smooth muscle cells: Smooth Muscle Cells (SMCs) have a fundamental effect on the development of atherosclerosis due to their migration into the intima and proliferation [25]. Interestingly, migrating SMCs play a key role in the process of luminal stenosis after damage to the intima and internal elastic lamina. Once numerous SMCs migrate into the intima, their excessive proliferation and suppression of apoptosis promotes extracellular matrix synthesis and lipid deposition, which consequently promotes fibrosis and thickening of the arterial wall, as well as luminal stenosis. Human SMCs express numerous lipid uptake receptors, such as the Low-Density Lipoprotein receptor (LDL) and SR, promoting the formation of myogenic PCs [22].
Fibroblasts: Fibroblasts are the main cell population involved in extracellular matrix remodeling. Fibroblasts arise as a result of differentiation of other cell populations, including stem cells and resting resident tissue cells in fibrous plaques in atherosclerosis [26]. Myofibroblasts also produce profibrotic factors such as Transforming Growth Factor-β1 (TGF-β1), Angiotensin II (Ang II), and Interleukin-1 (IL-1), which allow them to assist in the activation and migration of resident immune cells. After wound healing, these cells undergo apoptosis or senescence; however, in the presence of unresolved inflammation and persistence of signals for myofibroblast activation, tissue homeostasis is disrupted, leading to excessive secretion of Extracellular Matrix (ECM), ECM disorganization, and thickening of the affected tissue [22].
Adipocytes: Adipocytes are the main type of cells that make up adipose tissue. It has been shown that Perivascular Adipose Tissue (PVAT) not only stores triacylglycerols/triglycerides and Free Fatty Acids (FFAs) involved in energy metabolism, but also secretes certain amounts of adipokines such as leptin, adiponectin, visfatin, resistin, TNF-α. IL-6, IL-8, MCP-1 and Plasminogen Activator Inhibitor 1 (PAI-1), which play an indispensable role in atherosclerosis by mediating SMC migration and proliferation, promoting hyperplasia and neointima formation, stimulating inflammatory reactions and oxidative stress, and regulating vascular tone [27]. For example, lipid mediators such as FFAs and adipokines have been shown to affect SMC function by inducing increased proliferation and inflammatory signaling, and it has been suggested that elevated levels of fatty acids and adipokines released in obesity may reveal the relationship between dysfunction SMC, vascular inflammation and atherosclerosis [28]. In addition, adipocytes play an indispensable role in the inflammatory response to atherosclerosis. The result of proteomic analysis showed that Empirical Adipose Tissue (EAT) exhibits increased oxidative stress compared to Subcutaneous Adipose Tissue (SAT) in patients with cardiovascular disease, suggesting its possible association with myocardial stress. Similarly, perivascular visceral fat leads to endothelial dysfunction and accelerates the development of atherosclerosis, as demonstrated by visceral adipose tissue transplantation in mice [29].
The role of immune cells in inflammation in atherosclerosis
Macrophages: It has been noted that in atherosclerosis, macrophages take an active part in the accumulation of lipoprotein deposits, which leads to the transformation of macrophages into foam cells filled with lipid droplets [30]. In turn, an increase in the concentration of foam cells leads to an even greater accumulation of lipids and, as a result, to the development of atherosclerotic plaques. At the same time, macrophages located in an atherosclerotic plaque are not capable of effective migration, which leads to chronic inflammation and subsequent pathological development of a plaque into a complicated atherosclerotic plaque [30].
Macrophages lacking the ability to migrate, however, actively produce pro-inflammatory cytokines, chemokines, and Reactive Oxygen Species (ROS). After death, the accumulation of macrophages form a necrotic core in the developing atherosclerotic plaques [31]. It is known that atherosclerotic plaques contain a large number of pro-inflammatory macrophages or M1-macrophages, while at the same time there are also anti-inflammatory macrophages (M2-macrophages) [32]. M2 type macrophages have been shown to reside in more stable plaque regions and have better resistance to transformation into foam cells [33]. Therefore, the ratio of pro-inflammatory and anti-inflammatory macrophages can reflect the progression and regression of atherosclerotic plaque formation. The polarization of macrophages in atherosclerosis is directly affected by various lipoproteins. Thus, it is known that LDL, including both native and modified forms, contributes to the pro-inflammatory polarization of macrophages [34]. In a study [35], it was found that the cultivation of macrophages in a medium supplemented with LDL led to an increase in the expression of pro- inflammatory molecules TNF-α and IL-6 and a decrease in the expression of anti-inflammatory molecules CD206 and CD200R. At the same time, HDL has the opposite effect. In an atherosclerotic mouse model, it was demonstrated that normalization of serum HDL levels led to a decrease in the proportion of pro- inflammatory macrophages and, in turn, an increase in the proportion of M2 type macrophages [36].
CD8+ T-lymphocytes: It is known that one of the populations of leukocytes present in atherosclerotic lesions are CD8+ T-lymphocytes, and in comparison with the population of circulating CD8+ T-lymphocytes, CD8+ T-cells of atherosclerotic plaques are predominantly activated, which implies the antigenic specificity of this subpopulation of cells [37]. At the same time, it remains unclear whether CD8+ T cells can be primed or activated locally in lesions. Research in vitro showed that oxLDL epitopes are able to activate CD8+ T-lymphocytes in the presence of dendritic cells [38]. CD8+ T-lymphocytes can have both atherogenic and atheroprotective effects. Thus, it was shown that CD8+ T-lymphocytes can control the maturation of monocytes and the accumulation of macrophages in the early stages of atherosclerosis [39]. In addition, CD8+ T-lymphocytes are able to exhibit cytotoxic activity against cells involved in the stabilization of atherosclerotic lesions, such as endothelial cells and vascular smooth muscle cells [40]. The production of Tumor Necrosis Factor (TNF) and interferon-γ by CD8+ T cells further enhances inflammation and causes further progression of atherosclerotic plaque formation [40]. At the same time, atherosclerotic lesions also contain a subpopulation of regulatory CD8+ T cells, which has an atheroprotective effect through cytotoxic activity directed at antigen-presenting cells, as well as through inhibition of the polarization of CD4+ T lymphocytes into proatherogenic subtypes [40].
CD4+ T-lymphocytes: CD4+ T lymphocytes are important regulators of the adaptive immune response and are present in large numbers in atherosclerotic plaques. Different subpopulations of CD4+ T-lymphocytes can affect the pathogenesis of atherosclerosis in different ways, which determines the multifaceted significance of CD4+ T-lymphocytes in atherosclerosis. It is known that peptide fragments of oxidized LDL molecules, apolipoprotein B-100, and heat shock proteins are processed and presented on the surface of antigen-presenting cells as part of the MHCII complex as antigens in atherosclerosis to CD4+ T-lymphocytes [41]. In experimental mouse models, the atherogenic role of the Th1 CD4+ T-lymphocyte subpopulation and the atheroprotective role of Th2 CD4+ T-lymphocytes were shown [42]. In advanced atherosclerotic lesions, T-helpers with the Th1 phenotype are present in significantly higher numbers compared to Th2, as demonstrated by increased levels of the cytokines IL2 and interferon-γ and decreased levels of IL4, IL5, and IL10 [42]. Dendritic cells and macrophages are able to activate Th1 through the production of IL22. In addition, an atheroprotective role has been shown for a population of regulatory T-helpers (Treg.) Thus, in the ApoE-/-mouse model, it was demonstrated that the introduction of Treg. led to a decrease in the secretion of IFN-γ, an increase in the production of IL-10 and a significant decrease in the size of the atherosclerotic lesion [43]. In the study in vitro Treg. Inhibited the formation of foam cells and shifted the polarization of maturing macrophages towards the M2 phenotype [44].
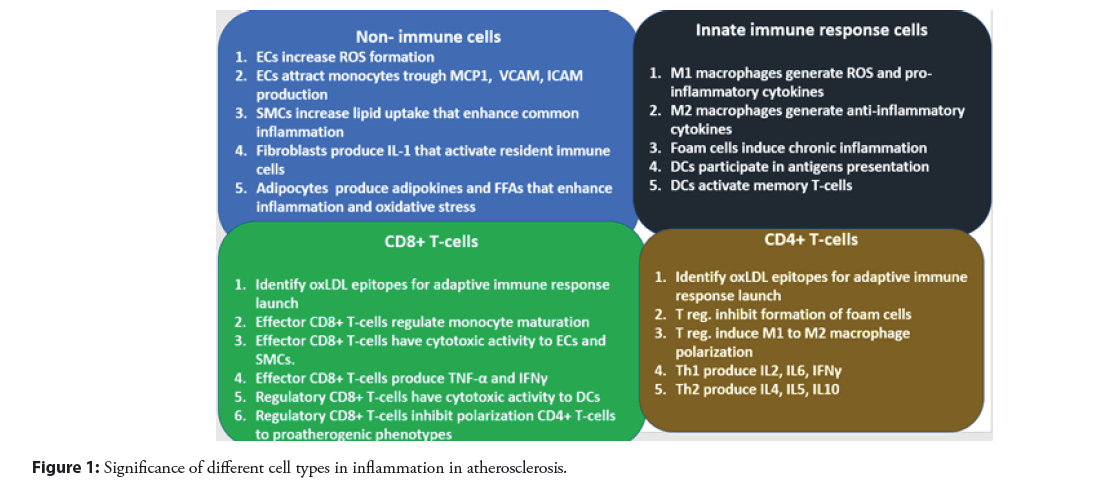
Dendritic cells: Dendritic cells (DCs) have the unique property of being conductors between innate and adaptive immune responses. They are the primary activators of both naive and memory T cells. The cholesterol-accumulating subpopulation of foam cells most likely originates from dendritic cells [45]. The mechanism of this transformation is not yet fully understood, but most likely involves the processes of macropinocytosis, receptor-mediated endocytosis, scavenger receptor-mediated uptake, and direct uptake from the bloodstream through dendritic processes and efferocytosis, apoptotic cells [45]. It has been shown that LDL itself can influence the function of dendritic cells in atherosclerosis. Both native LDL and modified LDL are able to initiate the maturation of dendritic cells, thus affecting the efficiency of antigen presentation to T cells [46]. Mature dendritic cells are able to present antigens to T-lymphocytes, which leads to increased inflammation in atherosclerosis. In mouse model studies, it has been demonstrated that atherosclerotic lesions are reduced in mice deficient in co-stimulatory molecules for CD80 and CD86 T cells, which are activated during dendritic cell maturation, and in mice deficient in CD74, a receptor that regulates antigen load on MHC-II [47]. All this indicates that the maturation of dendritic cells and antigen presentation with their participation leads to an atherogenic effect. The roles of the described cells in inflammation development in atherosclerosis is depicted on Figure 1.
Strategies for anti-inflammatory therapy of atherosclerosis
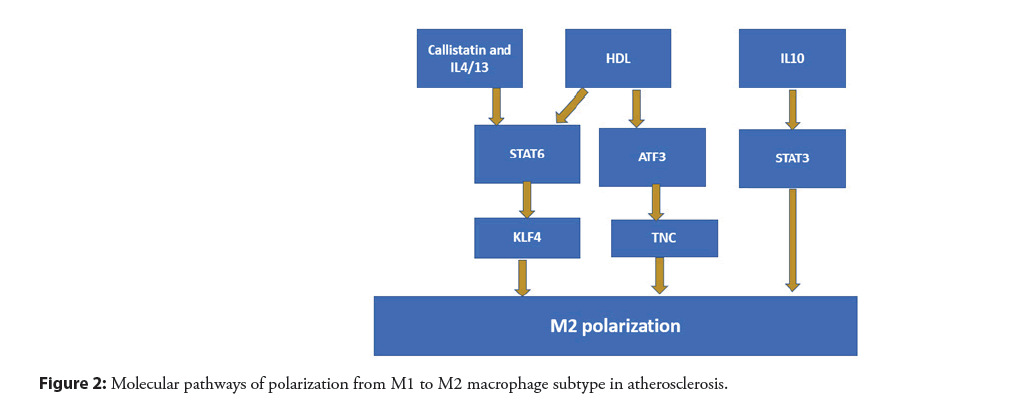
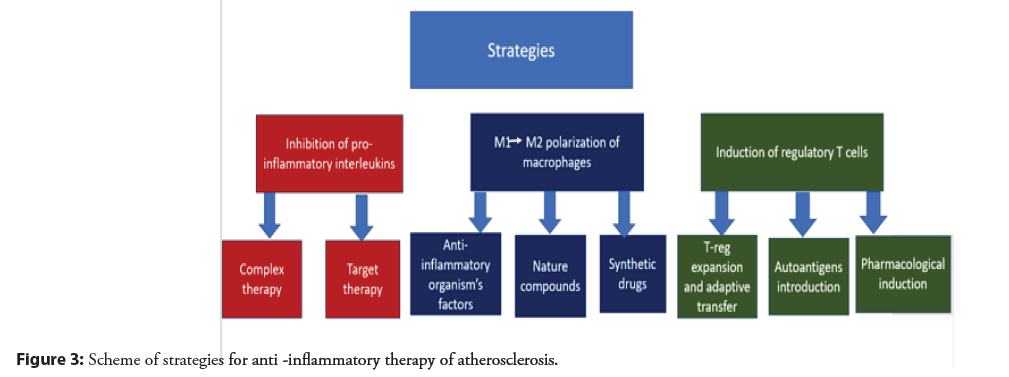
Change in the polarization of macrophages: Since it is known that M1 macrophages are the dominant subtype in progressive atherosclerotic plaques, and M2 macrophages are more represented in regressing plaques, and also that M1 and M2 macrophages can easily transform into each other under the influence of certain stimuli, then a promising therapeutic strategy for atherosclerosis looks like a change in the polarization of macrophages from M1 to M2 subtype. In vivo it has been shown that various anti-inflammatory factors such as: HDL, adiponectin, ApoE, callistatin, IL 19, IL-4, IL-13, etc. are able to induce the transformation of M1 into M2 macrophages [48]. At the same time, different factors can affect the polarization of macrophages through different molecular pathways. So HDL particles contribute to the transformation of M1 macrophages into M2 through the activation of ATF 3 and STAT 6 [49]. Callistatin, which is a blood plasma protein, activates macrophage polarization towards the M2 phenotype through activation of KLF 4 [21]. For anti-inflammatory cytokines: IL-19, IL-4 and IL-13, the ability to cause a decrease in M1 macrophages and increase the amount of M2 through the STAT, KLF 4 and PPARγ pathways has been shown [50]. For all types of this effect, a clinical manifestation is noted in the form of a decrease in plaque damage. The ability to induce polarization of macrophages towards the M2 phenotype has been shown for a number of natural therapeutic compounds, such as: Curcumin, natural polyphenols, ginsenoside Rb1 and Rg3 [48]. To modulate the macrophage phenotype in atherosclerosis, synthetic drugs that are currently used for other purposes can also be used: Metmorphine, sitagliptin, melatonin [48]. Molecular pathways of polarization from M1 to M2 macrophage subtype are shown in Figures 2 and 3.
Figure 1: Significance of different cell types in inflammation in atherosclerosis.
Induction of regulatory T cells: Since regulatory T-cells (T-reg.) are one of the main populations of immune cells with a complex atheroprotective effect, various ways to increase their activity and quantity in atherosclerosis are promising strategies for the treatment of this disease. One of these strategies is based on the use of T-reg expansion and adaptive transfer, which reduces the development of atherosclerotic plaques and reduces the inflammatory response, which has been shown in studies in mouse models of atherosclerosis [51]. In a certain context, atherosclerosis can be considered as an autoimmune disease caused by a decrease in immune tolerance to self-proteins such as: ox-LDL, Apolipoprotein B (ApoB) and Heat Shock Protein 60 (HSP60) [52]. The introduction of these autoantigens stimulates the production of antigen-specific Tregs in addition to tolerogenic dendritic cells, which may also be an effective therapeutic strategy for the treatment of atherosclerosis, which has also been shown in a number of studies on animal models [53]. In addition, the possibility of pharmacological induction of an increase in T-reg function has also been shown. As therapeutic compounds that have shown their effectiveness in increasing the amount and functional activity of T-reg. drugs such as rapamycin, fingolimod, amygdalin and vitamin D 3 are being considered [53]. Also, for a number of cholesterol-lowering drugs, an additional therapeutic effect was shown based on a positive modulation of the ratio of Treg/effector T cells [54].
Inhibition of pro- inflammatory interleukins: Cytokines released during inflammation by immune cells are one of the main humoral regulators of the immune response. Accordingly, for any disease associated with inflammation, including atherosclerosis, it makes sense to develop drugs that block the action of pro-inflammatory cytokines. The effect on pro-inflammatory cytokines is already a fairly well-known area of research, on the basis of which several dozen clinical studies have already been carried out. These clinical trials can be divided into two groups: broadly targeted therapies (e.g. LoDoCo clinical trials) and targeted therapies targeting specific inflammatory targets (e.g. CANTOS clinical trials) [55]. At the same time, compounds (mostly monoclonal antibodies) directed to the Interleukin (IL)-6/IL-1β receptor showed the best efficiency among targeted drugs, and low-dose colchicine turned out to be the most promising among drugs for complex effects [56]. However, these drugs still need to be assessed for their long-term safety in the treatment of atherosclerosis [56]. The anti- inflammatory therapeutic strategies described in this section are shown schematically in Figure 3.
Discussion
Despite the fact that there are more and more new data on the importance of inflammation in the pathogenesis of atherosclerosis, however, there is still a range of issues that require further resolution. In particular, it is important to study the correlation between the degree of inflammation, including the involvement of various types of immune cells in the development of the inflammatory response, and the clinical manifestations of atherosclerosis. How close the relationship between these processes can be traced? In this regard, the search for new inflammatory biomarkers of atherosclerosis and the development of diagnostic test systems based on them can become a potential practical application. An example of such an effective marker is uric acid. Thus, in the review (1c), based on the described studies, it was found that uric acid has a dose-dependent inflammatory effect at serum concentrations above 0.24 (mmol/l), which was noted for a number of metabolic diseases. The role of uric acid in the development of the disease pathogenesis has also been shown directly for atherosclerosis, which consisted in the development of endothelial dysfunction, increased vascular stiffness and enhanced production of inflammatory factors (2c). Lipoprotein A can also be a potential marker for the development of an inflammatory reaction in atherosclerosis, since its mechanism of action, based, in particular, on the induction of M1 macrophage differentiation and the formation of proatherogenic DAMPs, has long been known (3c). However, due to the presence of a large number of lipoprotein A isoforms, the measurement of its level in quantitative analysis is fraught with difficulties, which requires he development of non-standard diagnostic tests (3c,4c). In addition, deeper study requires understanding the role of some subpopulations of immune cells and the cytokines they produce, for example, Th 17 CD4+ T-lymphocytes and IL 17, which can have different effects on the progression of atherosclerosis depending on the conditions [57]. Of course, it is important to search for new therapeutic strategies aimed at reducing inflammation and reducing the progression of atherosclerotic lesions, however, for existing strategies, including those described in this review, more targeted preclinical, and subsequently, if successfully completed, clinical studies to determine the effectiveness of safety, pharmacokinetics, pharmacodynamics, methods and doses of administration of specific therapeutic compounds. Conducting a large number of such studies, in addition to the direct product in the form of the release of a new drug for the treatment of atherosclerosis, will also contribute to the accumulation of useful statistics on “vulnerabilities” in the pathogenesis of atherosclerosis, which will make it possible to identify certain most effective therapeutic targets for intervention.
Conclusion
Evidence of the significant role of inflammation in the development of atherosclerosis is the involvement in inflammatory pathogenesis of various populations of both immune (macrophages, T-helpers, T-killers, dendritic cells, neutrophils) and non-immune cells (adipocytes, fibroblasts, endothelial cells and smooth muscle cells). Moreover, immune cells can influence the progression of atherosclerosis by providing both an atherogenic effect (M1-macrophages, dendritic cells) and an atheroprotective effect (M2-macrophages, T-regulatory cells). Promising strategies in reducing inflammation in atherosclerosis, which have shown their effectiveness in a number of studies, are: reversal of macrophage polarization, induction of regulatory T cells, and inhibition of pro-inflammatory interleukins, and only for the latter strategies there is already a wealth of evidence from clinical studies.
Author Contributions
Writing-original draft preparation, AVB, MAP; writing-review and editing, VNS, IIE, IIN,NKS, ANO.
Funding
This work was supported by the Russian Science Foundation (Grant#23- 25-00196).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dichgans M, Pulit SL, Rosand J, et al. Stroke genetics: Discovery, biology, and clinical applications. Lancet Neurol. 18(6): 587-599 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Mohd NS, Khateeb AM, Chua YA, et al. Heterozygous familial hypercholesterolaemia in a pair of identical twins: A case report and updated review. BMC Pediatrics. 19(1) (2019).
[CrossRef] [Google Scholar] [PubMed]
- Reiss AB, Grossfeld D, Kasselman LJ, et al. Adenosine and the cardiovascular system. Am J Cardiovasc Drugs. 19(5): 449-464 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Doodnauth SA, Grinstein S, Maxson ME, et al. Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Philos Trans R Soc Lond B Biol Sci. 374(1765): 20180147 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 5(1) (2019).
[CrossRef] [Google Scholar] [PubMed]
- Ala Korpela M. The culprit is the carrier, not the loads: Cholesterol, triglycerides and apolipoprotein B in atherosclerosis and coronary heart disease. Int J Epidemiol. 48(5):1389-1392 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Rafieian-Kopaei M, Setorki M, Doudi M, et al. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 5(8): 927-946 (2014).
[Google Scholar] [PubMed]
- Gimbrone MA, García G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 118(4): 620-636 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Spannella F, Giulietti F, Pentima C, et al. Prevalence and control of dyslipidemia in patients referred for high blood pressure: The disregarded “double-trouble” lipid profile in overweight/obese. Adv Ther. 36(6): 1426-1437 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 13(6): 368-380 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Basatemur GL, Jørgensen HF, Clarke MCH, et al. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 16(12):727-744 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 377(2): 111-121 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Vengrenyuk Y, Nishi H, Long X, et al.Cholesterol loading reprograms the microrna-143/145– myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 35(3): 535-546 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Wang Y, Dubland JA, Allahverdian S, et al. Smooth muscle cells contribute the majority of foam cells in apoe (apolipoprotein e)-deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol. (2019).
[CrossRef] [Google Scholar] [PubMed]
- Shankman LS, Gomez D, Cherepanova OA, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 21(6): 628-637 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Silvestre-Roig C, Braster Q, Wichapong K, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 569(7755): 236-240. (2019).
- Paulin N, Viola JR, Maas SL, et al. Double-strand DNA sensing aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation. 138(3): 321-323 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Schumski A, Ortega-Gómez A, Wichapong K, et al. Endotoxinemia accelerates atherosclerosis via electrostatic charge-mediated monocyte adhesion. Circulation. 143(3):254-266 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Tang J, Lobatto ME, Hassing L, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv. 1(3): e1400223-e1400223. (2015).
[CrossRef] [Google Scholar] [PubMed]
- Van der Heijden T, Kritikou E, Venema W, et al. NLRP3 inflammasome inhibition by mcc950 reduces atherosclerotic lesion development in apolipoprotein e–deficient mice-brief reporthighlights. Arterioscler Thromb Vasc Biol, 37(8); 1457-1461 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Park KH, Park WJ. Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 30(9): 1213 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Wang D, Wang Z, Zhang L, et al. Roles of cells from the arterial vessel wall in atherosclerosis. Mediators Inflamm. 2017: 8135934. (2017).
[CrossRef] [Google Scholar] [PubMed]
- Brunetti ND, Salvemini G, Cuculo A, et al. Coronary artery ectasia is related to coronary slow flow and inflammatory activation. Atherosclerosis. 233(2): 636-640. (2014).
[CrossRef] [Google Scholar] [PubMed]
- Loon JE, Kavousi M, Leebeek FWG, et al. Von willebrand factor plasma levels, genetic variations and coronary heart disease in an older population. J Thromb Haemost. 10(7): 1262-1269. (2012).
[CrossRef] [Google Scholar] [PubMed]
- Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 34(10): 2191-2198. (2014).
[CrossRef] [Google Scholar] [PubMed]
- Xu F, Liu Y, Shi L, et al. NADPH oxidase p47phox siRNA attenuates adventitial fibroblasts proliferation and migration in apoE (-/-) mouse. Journal of Translational Medicine. 13(1): 38 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Qi XY, Qu SL, Xiong WH, et al. Perivascular Adipose Tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovascular Diabetology, 17(1) (2018).
[CrossRef] [Google Scholar] [PubMed]
- Chang L, Villacorta L, Li R, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor- deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 126(9): 1067-1078. (2012).
[CrossRef] [Google Scholar] [PubMed]
- Alexopoulos N, Katritsis D, Raggi P, et al. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 233(1): 104-112. (2014).
[CrossRef] [Google Scholar] [PubMed]
- Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 114(11):1757 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 50: S382 (2009).
[CrossRef] [Google Scholar] [PubMed]
- De Paoli F, Staels B., Chinetti-Gbaguidi G. Macrophage phenotypes and their modulation in atherosclerosis.Circ J Circ. 78(8): 1775-1781 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Chinetti G, Baron M, Bouhlel MA, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARα and LXR α pathways. Circ Res Inserm. 108(8): 985 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Bobryshev YV, Ivanova EA, Chistiakov DA, et al. Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis.Biomed Res Int Hindawi Limited. (2016).
[CrossRef] [Google Scholar] [PubMed]
- Al-Sharea A, Rong JX, Shamir R, et al. Native LDL promotes differentiation of human monocytes to macrophages with an inflammatory phenotype.Thromb haemost. 115(4): 762-772 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Feig JE, Rong JX, Shamir R, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci. 108 (17): 7166-7171 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Depuydt MAC, Prange KHM, Slenders L, et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics.Circ Res.127(11): 1437-1455 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Cimmino G, Cirillo P, Conte S, et al. Oxidized low-density lipoproteins induce tissue factor expression in T-lymphocytes via activation of lectin-like oxidized low-density lipoprotein receptor-1.Cardiovasc Res. 116(6): 1125-1135.
[CrossRef] [Google Scholar] [PubMed]
- Schäfer S, Zernecke A. CD8+ T cells in atherosclerosis.Cells. 10(1): 37 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Saigusa R, Winkels H, Ley K, et al. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17(7):87 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Kimura T, Tse K, Sette A, et al. Vaccination to modulate atherosclerosis. Informa Healthcare. 48(3): 152-160 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Baidya SG, Zeng QT. Helper T cells and atherosclerosis: The cytokine web.Postgrad Med J. 81(962): 746-752 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 12(2): 178-180 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Lin J. et al. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res. 51:(5) 1208 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Subramanian M, Tabas I. Dendritic cells in atherosclerosis.Semin Immunopathol. 36(1): 93 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Zaguri R. Verbovetski I, Atallah U, et al. Danger effect of Low-Density Lipoprotein (LDL) and oxidized LDL on human immature dendritic cells.Clin Exp Immunol. 149(3): 543 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Sun J. Deficiency of antigen presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 122(8): 808 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Lin P. Macrophage plasticity and atherosclerosis therapy. Front Mol biosci. 8 (679797) (2021).
[CrossRef] [Google Scholar] [PubMed]
- Sha H, Zhang D, Zhang Y, et al. ATF3 promotes migration and M1/M2 polarization of macrophages by activating tenascin‑C via Wnt/ β ‑catenin pathway. Mol Med Rep.16(3): 3641-3647 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Li B. Kallistatin inhibits atherosclerotic inflammation by regulating macrophage polarization. Hum Gene Ther.30(3): 339-351 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Ou H, Guo BB, Liu Q, et al. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol Sin.39(8): 1249 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Nilsson J, Lichtman A, Tedgui A, et al. Atheroprotective immunity and cardiovascular disease: Therapeutic opportunities and challenges. J Intern Med. 278(5): 507-519 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Bullenkamp J, Dinkle S, Kaski JC, et al. Targeting T cells to treat atherosclerosis: Odyssey from bench to bedside. Eur Hear J. 2(3): 194 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Maganto E. Dynamic changes in regulatory t cells are linked to levels of diet-induced hypercholesterolemia.Circulation. 124:185 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Deroissart J. Anti-inflammatory and immunomodulatory therapies in atherosclerosis. Handb Exp Pharmacol. 270: 359-404 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Ma J, Chen X. Anti-inflammatory therapy for coronary atherosclerotic heart disease: Unanswered questions behind existing successes. Front Cardiovasc Med. 7(415) (2021).
[CrossRef] [Google Scholar] [PubMed]
- Taleb S, Tedgui A, Mallat Z, et al. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arterioscler Thromb Vasc Biol. 35(2): 258-264 (2015).
[CrossRef] [Google Scholar] [PubMed]