Editorial - Pharmaceutical Bioprocessing (2018) Volume 6, Issue 1
Intensification of large-scale mammalian fed-batch processes
- *Corresponding Author:
- Martin Jordan
Merck Healthcare
Biotech Process Sciences
CH-1809 Fenil-sur-Corsier, Switzerland
E-mail: martin.jordan@merckgroup.com
Abstract
The term process intensification usually refers to a strategy aimed at transforming established processes into more efficient and economical processes. The overall productivity is increased by streamlining operations and applying more advanced technologies. While process intensification has been widely applied in chemical engineering as well as in the food and pharma industries, it has only recently become an area of interest in industrial bioprocessing. Here we wish to briefly describe and discuss the opportunities to intensify fed-batch processes for mammalian cells by means of high cell seeding.
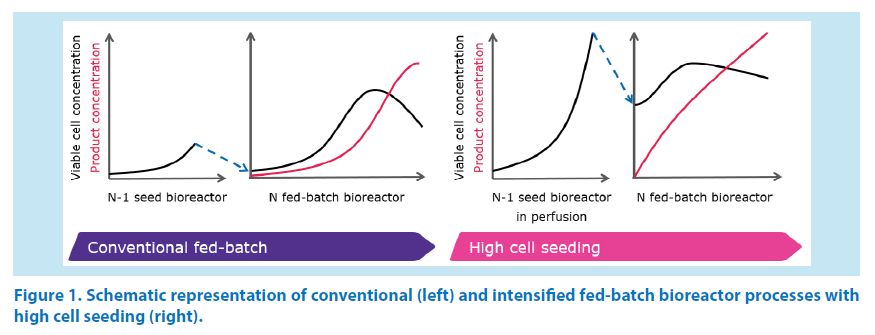
In general, this approach relies on a seed bioreactor (N-1) operated in perfusion mode in order to reach elevated cell densities within a few days (typically 30 to 50 mio cells/ mL). Subsequently, the fed-batch bioreactor (N) is started at a cell concentration which can range between 5 and 10 mio cells/mL or more, i.e. substantially higher than what is done with conventional fed-batch processes. The cells reach the peak concentration within 3 to 5 days and the production bioreactor is harvested after another few days. This results in shorter production cycles [1] and possibly in higher harvest product concentrations compared to conventional fed-batch processes (Figure 1).
High cell seeding processes are obviously not the only option when it comes to bioprocess intensification. Indeed, alternatives such as perfusion or concentrated fed-batch processes may be interesting modalities at scales up to 1 or 2 kL and for certain scenarios [2,3]. However, one of the key advantage of the above described intensified fed-batch process is the fact that it can be applied to existing large-scale facilities with bioreactors up to 20 kL. The implementation requires relatively minor modifications to the N-1 seed bioreactor with the addition of a cell retention system and adequate media hold tanks. Most importantly, no changes are in principle required for the actual fed-batch production bioreactor. As a consequence, existing facilities may be adjusted and the overall production capacity tremendously increased.
In addition and in contrary to perfusion, intensified fed-batch bioreactor processes may be considered as less disruptive and rely on process knowledge and control strategies developed for conventional fed-batch platforms. The definition of a batch is very clear and strategies used for in-process testing can be maintained. Adequate cell culture media and feed formulations can be derived from existing fed-batch processes and adjusted to the requirements of cultures starting with high cell concentrations. Yet another advantage which is common to fed-batch processes in general resides in the high level of flexibility of this approach. This is especially valuable in the context of multi-product manufacturing facilities and the production of diverse molecule formats to supply clinical trials. It is noteworthy to mention that the intensification of the fed-batch process does not impact the downstream processes. In short, the potential of fed-batch process intensification is becoming more and more evident. This could translate in a widespread industry adoption in the coming years.
Considering all the advantages of the high seeding strategy, one might wonder why such approaches were not adopted earlier in the industry. One of the reason is clearly related to the lack of adequate technologies for the perfusion in the N-1 step. In recent years however, suitable technologies have been made available and both TFF and ATF systems can now be reliably implemented in manufacturing environments. Another reason is the relatively slow adoption of new technologies in bioprocess development in general, simply because the development of a new proprietary cell culture platform takes considerable time and energy. Although no longer true, the relatively low pressure on cost of goods for biologics manufacturing may have been a contributing factor as well.
One of the earliest description of an intensified fed-batch bioreactor process with high cell concentration seeding was published in 2013 [4]. The authors describe a monoclonal antibody production process which was shortened from 14 to 8 days with a similar harvest product concentration and quality. Fed-batch bioreactor seeding concentrations of 5 and 8 mio cells/mL were tested, which represent a 25- and 40-fold increase over the control, respectively. Interestingly, the cell culture profiles resulting from the high seeding reached slightly higher peak cell concentrations and lower maximal lactate concentrations in the culture supernatant when compared to the control condition. This is linked to the shorter growth phase when using high cell seeding and maybe to the fact that the cells in the inoculum come with essentially fresh cell culture medium due to the perfusion applied in the N-1 seed bioreactor.
However, there are also potential risks when handling a high concentration cell culture inoculum between the N-1 and N steps.
Indeed, the transfer conditions and time need to be well characterized and tightly controlled in order to ensure consistent performance in the production bioreactor. A prolonged oxygen depletion or elevated shear stress during transfer may have a negative impact on the fed-batch culture. This is probably not a challenge at lab scale but it is certainly not trivial when transferring a large volume of highly concentrated cells to a 20 kL bioreactor. A study by Roche [5] describes the implementation of a high seeding fed-batch process in a large-scale facility, which indicates that this can be adequately controlled.
To conclude, the development of intensified fed-batch bioreactor processes with high cell seeding represents a promising approach and might open an opportunity for companies who intend to increase the utilization of their existing large-scale manufacturing capacity. This is most likely already being pursued by a number of biopharma companies and it might represent an intermediate step before moving towards even more agile and possibly continuous endto- end bioprocessing in the future.
Financial and competing interest disclosure
The authors are employed by Merck Healthcare in Switzerland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Yang WC, Lu J, Kwiatkowski C et al. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol. Progress. 30(3), 616–625 (2014).
- Croughan M, Konstantinov KB, Cooney CL. The future of industrial bioprocessing: batch or continuous? Biotechnol. Bioeng. 112(4), 648–651 (2015).
- Yang WC, Minkler DF, Kshirsagar R et al. Concentrated fed-batch cell culture increases manufacturing capacity without additional volumetric capacity. J. Biotechnol. 217, 1–11 (2016).
- Padawer I, Ling WLW, Bai Y. Case Study: An accelerated 8-day monoclonal antibody production process based on high seeding densities. Biotechnol. Progress. 29 (3), 829–832 (2013).
- Pohlscheidt M, Jacobs M, Wolf S et al. Optimizing capacity utilization by large scale 3000 L perfusion in seed train bioreactors. Biotechnol. Progress. 29 (1), 222–229 (2013).