Review Article - Imaging in Medicine (2013) Volume 5, Issue 6
Interventional biliary radiology: current state-of-the-art and future directions
Andrew Shawyer1, Mark D Goodwin*2 and Robert N Gibson31Radiology Department, Royal Bournemouth Hospital, Bournemouth, UK
2 Radiology Department, University of Melbourne, Austin Health, Heidelberg, Melbourne, Australia
3 Radiology Department, University of Melbourne, Royal Melbourne Hospital, Parkville, Melbourne, Australia
Abstract
Keywords
benign biliary strictures; biliary calculi; biliary obstruction; biliary tract interventions; cholangiography; jaundice; metallic stents; percutaneous cholecystostomy; plastic stent
Percutaneous biliary intervention has been practiced since the early reports of percutaneous transhepatic cholangiography in the 1960s and is now a common procedure for the interventional radiologist. However, biliary tract interventions still present some of the most demanding and complex problems in interventional radiology. Advances in both technology and techniques over the last three decades have lead to a continued expansion in the scope of biliary interventional practice. In particular, biliary stent design has undergone major changes, and continues to develop, extending the role of stents in both benign and malignant disease. Interventional radiology therefore has an established and expanding role in the diagnosis and management of patients with both benign and malignant biliary disease. This review summarizes the established biliary interventional techniques and outlines both current state-ofthe- art practice and likely future directions in the light of recent advances.
Basic established techniques
The current biliary interventional techniques can be divided at the most basic level into endoscopic procedures or percutaneous procedures. Combined procedures, where both endoscopic and percutaneous biliary approaches are employed, are used less frequently.
Endoscopic retrograde cholangiopancreatography
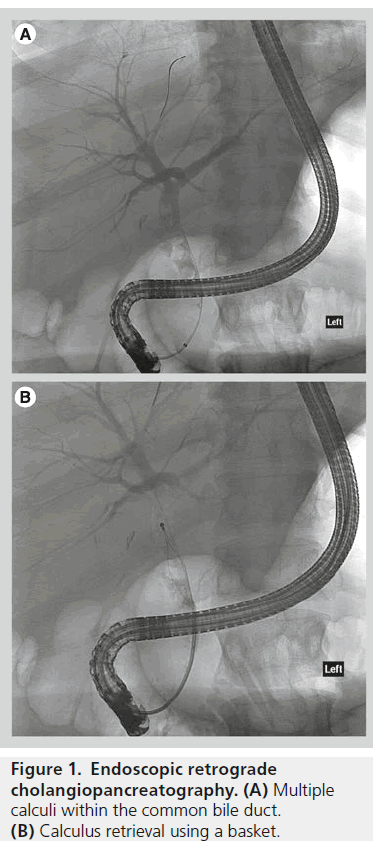
Endoscopic retrograde cholangiopancreatography (ERCP) is usually the preferred method of accessing the biliary system if possible. A side-viewing endoscope allows visualisation of the ampulla and cannulation of the bile duct. Cholangiography is performed, and wires and catheters can be passed into the bile ducts. A large range of instrumentation can then be deployed, including balloon catheters and baskets for removing stones, brushes for taking bile duct wall specimens, and plastic and metallic biliary stents (Figure 1).
Percutaneous transhepatic cholangiography
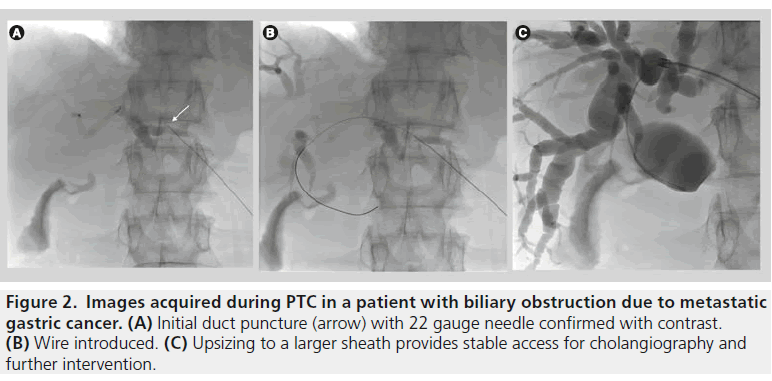
Fine needle percutaneous transhepatic cholangiography (PTC) became a commonly performed procedure in the 1980s to evaluate and treat biliary tract obstruction. Typically, a 22 gauge needle is passed through the skin into the liver and then into a bile duct and contrast is injected to opacify the biliary system. For therapeutic procedures a wire is then passed down the needle and into the bile duct; this then allows a large variety of instruments to be used (Figure 2).
For instance, balloon catheters can be inserted to dilate strictures, biliary drain tubes can be placed to relieve obstruction or infection, and stents can be deployed.
Although advances in cross-sectional imaging and ERCP mostly have replaced diagnostic PTC, percutaneous transhepatic interventional techniques still play an important role in the treatment of both benign and malignant biliary disease. Current indications include the treatment of biliary obstruction when endoscopic techniques are not appropriate or have failed. These include complex hilar or intrahepatic bile duct obstruction, interventions involving biliary-enteric anastomoses (where the normal transampullary ERCP access is lost), the evaluation and treatment of post-surgical bile duct injuries and as access for treatment of biliary stone disease [1].
Percutaneous cholecystostomy
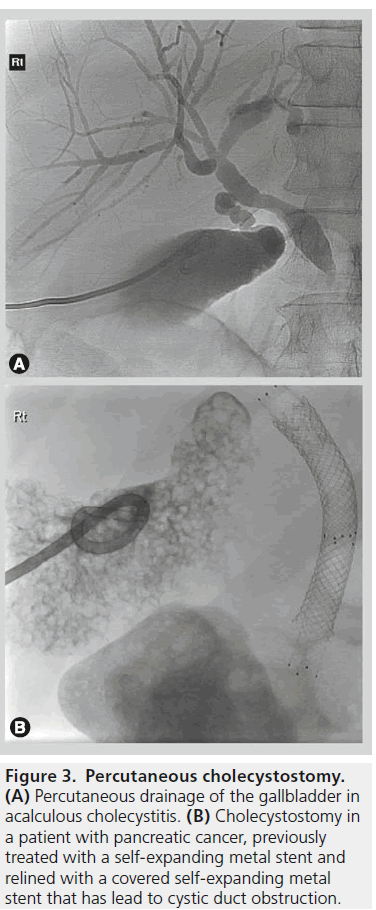
Percutaneous cholecystostomy is a minimally invasive method of gallbladder access that can be performed using a transhepatic or transperitoneal approach. The procedure is usually for gallbladder drainage but can also be used for additional procedures such as trans-cystic cholangiography and bile duct drainage, stone extraction and lithotripsy [2].
The two most frequent indications are in patients with acute calculous cholecystitis who are poor surgical candidates and in critically ill patients with acalculous cholecystitis. Acute cholecystectomy in very the elderly or patients with a high surgical risk due to comorbid conditions carries a high morbidity (41%) and mortality (4.5%) rate [3]. In contrast, percutaneous cholecystostomy in a high surgical risk group has a high reported technical success rates (95–100%) with low procedure-related mortality of (0–2%) (Figure 3) [2].
Overview of recent technological developments
One of the most important changes in recent years has been the rapid development and evolution in metallic biliary stent design. For many years metal stents designed for the vascular system were the only option for the biliary interventionalist, but there is now a variety of specialised stent systems designed specifically for biliary use. This section describes the most important developments.
Metallic stent technology
Stent technology has undergone a rapid series of advances over the last two decades. The introduction of nitinol, a nickel-titanium alloy, which exhibits the properties of superelasticity and thermal shape memory has lead to the introduction of a new generation of stents [4]. These stents demonstrate increased flexibility and conformability compared to their stainless steel predecessors, without significant compromise in stent radial force. Nitinol's good biocompatibility and suitability for MR imaging are additional benefits. Stent properties such as the radial force, radio-opacity and flexibility can be modified by the use of similar alloys such as Platinol®.
Figure 2.Images acquired during PTC in a patient with biliary obstruction due to metastatic gastric cancer. (A) Initial duct puncture (arrow) with 22 gauge needle confirmed with contrast. (B) Wire introduced. (C) Upsizing to a larger sheath provides stable access for cholangiography and further intervention.
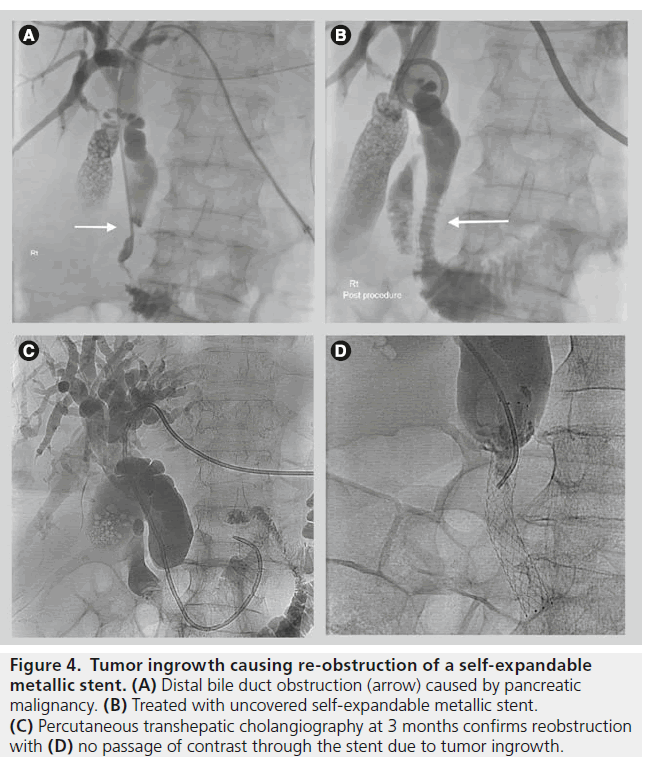
Originally only uncovered self-expandable metallic stents (USEMS) were available. Covered SEMS (CSEMS) are now available; these stents are lined with a material that prevents passage of materials between the metal interstices of the stent. These stents can be used to treat bile leaks, or to treat malignant obstruction. Covered stents are also now manufactured either fully covered or partially covered (PCSEMS) for different indications. The introduction of both PCSEMS and fully covered SEMS has further improved stent patency rates especially in malignant disease by reducing or eliminating the problem of tumor ingrowth (Figure 4).
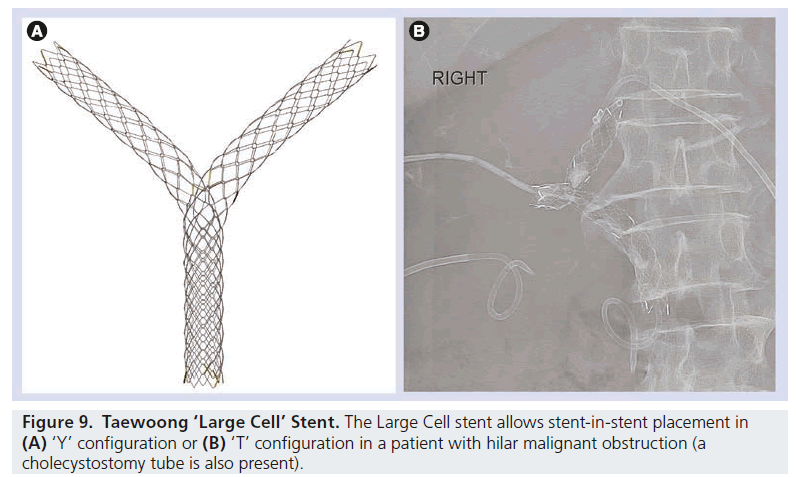
The design of the metallic structure of the stent can also be varied. Metallic biliary stents are usually uniform in structure along their length, but stents are now available that have larger gaps in the metallic material at certain points. For instance, the Taewoong Niti-S ‘Large Cell’ stent has a more open structure to allow an additional stent(s) to be passed through this area, for treating hilar malignant strictures. This open design allows the end result of a ‘T’ or ‘Y’ stent pattern by passing the second stent through the wall of the first.
The same manufacturer, Taewoong Medical (Gyeonggi-do, Korea) has developed a covered “Bumpy” stent, where the external surface of the stent has a non-uniform shape; this model has been designed to allow fluids from adjacent structures such as the pancreatic or cystic duct to drain around the outer surface of the stent. This design aims to avoid complications caused by stents obstructing the gallbladder and pancreas such as acute cholecystitis and acute pancreatitis (Figure 5).
Removable metallic stents
Metallic stents are also now available in removable forms. This potentially broadens the clinical applications of metallic stents into treatment of benign strictures. Removable stents are available for both percutaneous and endoscopic insertion and percutaneous stents can be retrieved either percutaneously or endoscopically.
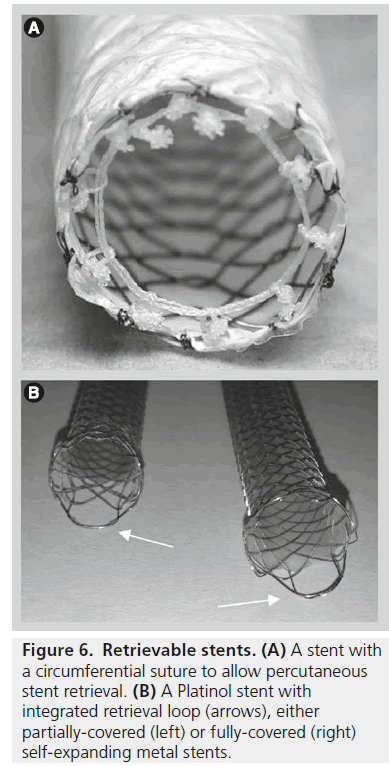
Endoscopic covered stent removal can be performed using snare forceps to grip the tip of the stent, which is then pulled towards the working channel of the duodenoscope. Some stents, for example, Niti-S covered biliary stents, contain a removal suture that collapses the stent when pulled. This can be gripped with biopsy forceps endoscopically or ‘hooked’ and withdrawn into a sheath using a specifically designed device for percutaneous removal. This allows the stent to be pulled inside-out easily during removal (Figure 6). The timing of removal is dependent on a number of technical and clinical issues, but in the setting of benign biliary strictures covered stent removal is usually performed approximately 6–8 weeks following insertion [5]. When used to treat bile leaks, stents are often left for longer and are usually removed approximately 3 months after insertion [6].
Figure 3.Percutaneous cholecystostomy. (A) Percutaneous drainage of the gallbladder in acalculous cholecystitis. (B) Cholecystostomy in a patient with pancreatic cancer, previously treated with a self-expanding metal stent and relined with a covered self-expanding metal stent that has lead to cystic duct obstruction
Advanced endoscopic access
A percutaneous transhepatic approach has been the usual option for patients in whom endoscopic access to the biliary system has been unsuccessful and in patients who have undergone previous surgery such as Roux-en-Y hepaticojejunostomy or choledochojejunostomy. However, a combination of newer techniques and most importantly technological developments in both the enteroscopes and endoscopic equipment have widened the scope of biliary endoscopic practice. Examples include using the variable stiffness colonoscope, single and double balloon enteroscopes, and spiral enteroscopy, which have increased ERCP success rates in reaching areas previously thought to be out of endoscopic range [7].
Figure 4.Tumor ingrowth causing re-obstruction of a self-expandable metallic stent. (A) Distal bile duct obstruction (arrow) caused by pancreatic malignancy. (B) Treated with uncovered self-expandable metallic stent. (C) Percutaneous transhepatic cholangiography at 3 months confirms reobstruction with (D) no passage of contrast through the stent due to tumor ingrowth.
Another recent advance has been in the use of new intraductal endoscopy/cholangioscopy technologies such as the SpyGlass (Boston Scientific, MA, USA) direct visualization system. This allows visualization of the inner walls of the biliary tree and targeted tissue sampling. A range of therapeutic interventions can also be performed as part of intraductal endoscopy. These include lithotripsy (electrohydraulic or laser), photodynamic therapy and argon plasma coagulation [8].
Endoscopic ultrasound
Ampullary cannulation failure rates at ERCP are around 3-5%, usually due to either tumor infiltration or challenging post-surgical anatomy. In most centres, the majority of these patients have traditionally been referred for percutaneous biliary drainage. Over the last decade, technological advances in endoscopic ultrasound (EUS) have seen this technique develop from a purely diagnostic tool to a therapeutic one. EUS-guided cholangiopancreatography was first performed in 1996 [9]. There are now several series in the literature describing a variety of EUS-guided biliary drainage techniques including EUS-guided rendezvous procedures, choledochoduodenostomy and hepaticogastrostomy [10,11], however, these are evolving techniques.
Percutaneous approach for malignant disease: state-of-the-art
In malignant disease, the primary indication for percutaneous biliary intervention remains the palliation of biliary obstruction in patients who are either unfit for surgery or who have an unresectable tumor. Malignant biliary obstruction is usually due to a primary pancreatico-biliary malignancy or less commonly external biliary compression by lymphadenopathy or metastatic disease. Other indications for PTC in malignant disease include percutaneous endobiliary biopsy and biliary drainage as an adjunct to surgical intervention.
Percutaneous biliary stenting using SEMS became established in the late 1980s following the first percutaneous placement in a canine model in 1985 [12]. There continues to be a role for both percutaneous and endoscopic techniques depending on the anatomical location of the biliary obstruction, local expertise and postsurgical anatomy following any previous upper gastrointestinal surgery. The use of USEMS is well established. SEMS have been shown to be superior to plastic stents with lower complication rates, longer patency rates and require fewer re-interventions. Numerous studies have shown that patients with malignant biliary obstruction, unsuitable for surgery and with a life expectancy of greater than 4–6 months should be treated with USEMS rather than endoscopic plastic stents [13,14].
Confirming the diagnosis: bile duct biopsy
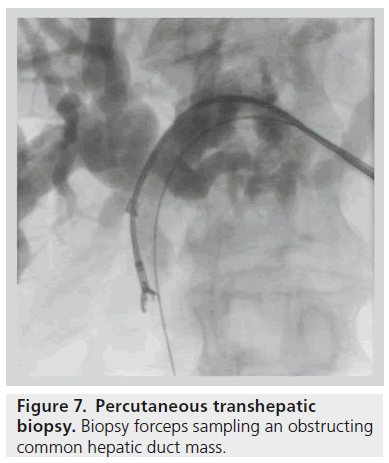
Imaging findings in patients with suspected malignant biliary obstruction are often nonspecific. As pathology guides treatment regimes and prognosis, an attempt should be made to obtain a tissue diagnosis in patients undergoing transhepatic drainage. Cytological evaluation of bile aspirated at the time of PTC is rarely performed as it has been shown to have a very poor sensitivity of 15% [15]. Endobiliary brushings done at the time of PTC have sensitivities of up to 75% but poor negative predictive values of around 12.5% [16].
The reported sensitivity of biopsy using percutaneous biliary forceps biopsy is higher (78%) than for cytology, and is even higher for cholangiocarcinoma (94%) (Figure 7) [17].
Management of distal bile duct malignancies
Percutaneous biliary drainage with crossing of the stricture, at which time a stent(s) can be deployed, is usually possible, although occasionally an external drain only is required at first attempt and, in the presence of significant biliary sepsis, this may be initially safer. A one stage PTC and stenting with either placement of a temporary small access catheter or rarely immediate removal of percutaneous access and tract embolization, has obvious benefits for patients in terms of hospital stay and quality of life [18].
Figure 6.Retrievable stents. (A) A stent with a circumferential suture to allow percutaneous stent retrieval. (B) A Platinol stent with integrated retrieval loop (arrows), either partially-covered (left) or fully-covered (right) self-expanding metal stents.
Although still advocated by some authors, routine predilation prior to stent insertion has been shown to have no effect on stent patency rates or patient survival and incurred a 19% cost increase [19], although it is technically sometimes helpful for tight strictures. The newer lower profile stent delivery systems reduce the occasions when predilation is required.
Figure 7.Percutaneous transhepatic biopsy. Biopsy forceps sampling an obstructing common hepatic duct mass
Covered or uncovered for distal bile duct malignancies?
USEMS used to treat malignant biliary obstruction are prone to re-obstruction due to sludge, stone formation and tumor obstruction (both overgrowth at the ends of the stent and ingrowth through the interstices of the stent). In an attempt to reduce tumor ingrowth, prolong stent patency and avoid reintervention, there has been increasing interest in the use of both PCSEMS and CSEMS.
The majority of studies comparing covered and uncovered stents have demonstrated improved patency rates in the covered stent group. A study comparing PCSEMS to USEMS showed that the covered stent group had improved patency rates in malignant extra-hepatic biliary obstruction (76 vs 57% at 12 months). However, no significant difference in patient survival between the two groups was observed [20].
Another study randomized patients to CSEMS or USEMS for treating biliary obstruction caused by unresectable pancreatic carcinoma [21]. Complications and cost were similar in both groups. Importantly, patency rates were significantly higher in the CSEMS group. Reinterventions were significantly lower in the CSEMS group with stent dysfunction in 30% of the bare-stent group after a mean period of 82.9 days compared with 10% after a mean period of 126.5 days for the CSEMS group. This suggests that the use of CSEMS could play an important role in reducing reinterventions and maintaining quality of life.
Others have found no difference in patency rates between USEMS and covered stents. In one study, time to recurrent obstruction and patient survival were not significantly different but adverse events, particularly migration, were much higher in the partially covered group (12 vs 0%) [22].
A recent meta-analysis of studies comparing uncovered and covered metal stents in malignant distal biliary obstruction shows improved patency rates in the covered group. Earlier studies had shown higher rates of cholecystitis and pancreatitis when using CSEMS but this meta-analysis demonstrated these complications were not significantly higher in the covered stent group [23].
Patient selection remains of key importance in planning percutaneous biliary drainage. Patients with very advanced or rapidly progressive cancer may have such limited survival that a CSEMS is unlikely to provide any benefit.
The value of being able to remove a covered biliary stent has the potential to make a major impact on current clinical practice, particularly in patients who have a suspected but unconfirmed bile duct malignancy; placement of such a stent is not an irrevocable step and options are preserved.
Biliary drainage pre-pancreaticoduodenectomy
A lack of consensus remains regarding use of stents in preoperative biliary drainage both in patients who are candidates for pancreaticoduodenectomy or hepatic resection. Most clinical studies have failed to support experimental data which suggests preoperative drainage would lead to improved surgical outcomes [24,25]. A large recent retrospective analysis showed no difference in serious complications, 30-day mortality and hospital stay, concluding that SEMS is not contraindicated in patients requiring preoperative drainage [26]. A recent review of the literature concludes that preoperative drainage should be reserved for patients with proximal obstruction, cholangitis, renal failure, or who are planned for neoadjuvant chemotherapy [27].
Management of Hilar bile duct malignancies
Hilar malignancy includes all tumors involving the confluence of the left and right hepatic ducts or the proximal 2cm of the common hepatic duct. Cholangiocarcinoma is the most common malignancy in this region, but intrahepatic and perihilar metastatic disease as well as gall bladder carcinoma can also cause anatomically similar biliary obstruction.
Malignant hilar biliary obstruction is a particularly challenging area in interventional biliary radiology; obtaining a tissue diagnosis and achieving longterm palliation in hilar cholangiocarcinoma are both often difficult. Accurate preprocedure imaging is of critical importance, and good quality MRCP is an invaluable tool for procedural planning.
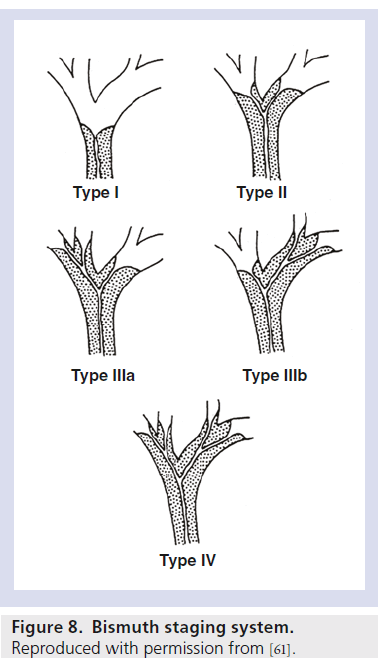
The Bismuth system of classification of hilar cholangiocarcinoma is a well-established method of describing these lesions (Figure 8).
Accurate imaging, most often a combination of CT and MRI and MRCP, helps determine potential resectability which provides the main opportunity for longterm survival. In the majority of patients, surgery is not possible and palliation in most centres involves biliary stenting. Whilst endoscopic stenting is superior to a percutaneous approach in distal malignant biliary obstruction, the optimal approach for hilar lesions is less clear with both techniques having a role, partially influenced by local expertise [28].
Published technical success rates for SEMS insertion for hilar malignant obstruction are similar for both approaches: 100% for the percutaneous approach and 98.8% endoscopically [29,30]. However percutaneous biliary drainage and stenting (sometimes using ultrasound guidance) has the distinct advantage over ERCP in hilar disease in that one or more appropriate segments for drainage can be selected with confidence. This has the added benefit of avoiding injection of contrast medium into segments that are too small to be drained.
The exact details of a malignant hilar stenting procedure can be complex. The operator can choose to place a single unilobar stent, or can choose to drain both sides of the liver. If multiple stents are used, one stent can be placed through the interstices of another, or the stents can be placed alongside each other. The stent(s) can be passed all the way into the duodenum, or the inferior part of the stent can be left within the normal portion of the common duct. However, there is no strong evidence that routine bilateral stenting confers benefit over unilateral stenting in Bismuth II–IV strictures, and involves potentially more discomfort, cost and hospitalization. There are cases, however, based on the need to drain infected segments or a larger volume of liver, where bilateral stenting is preferable. These decisions are often made based on anatomical considerations, patient factors and individual operator preference and experience (Figure 9).
Benign disease: state-of-the-art
Bile duct injuries
The majority of bile duct injuries are due to biliary tract surgery, in particular laparoscopic cholecystectomy, with traumatic bile duct injuries, both blunt and penetrating, accounting for only a small percentage. Anastomotic bile leaks after pancreaticoduodenectomy may also require biliary intervention.
The widespread use of the laparoscopic approach to cholecystectomy resulted in a rise in the incidence of iatrogenic bile duct injuries from 0.2 to 0.5% [31]. The type and site of the injury determines whether surgical, endoscopic or percutaneous intervention or a combined approach is appropriate. Both percutaneous and endoscopic techniques have a role, both as definitive treatments for bile duct injuries and as adjuncts to surgery.
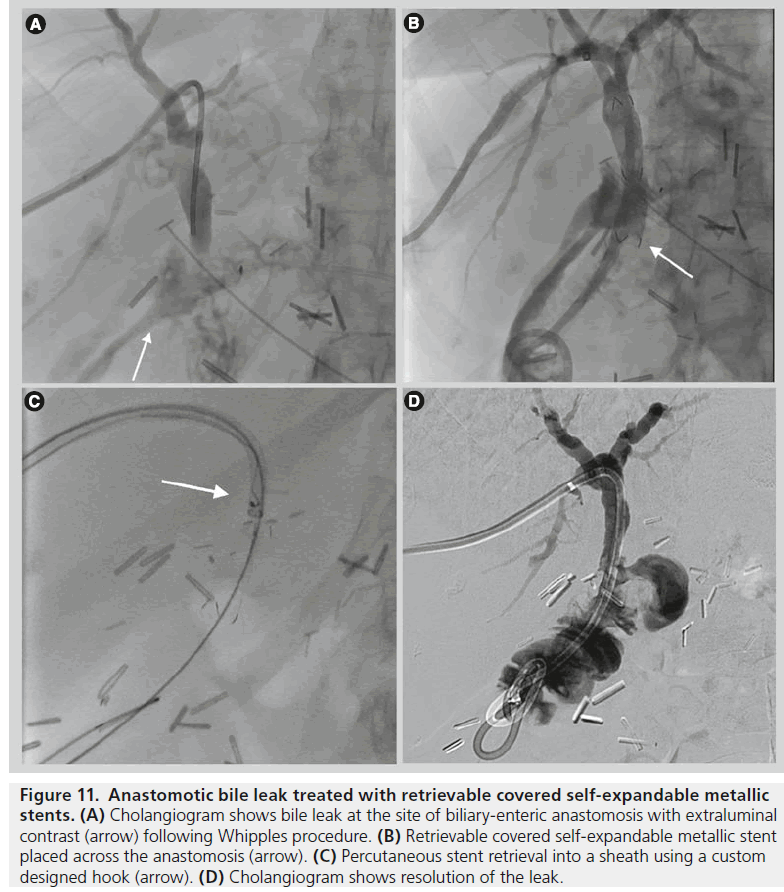
Figure 11.Anastomotic bile leak treated with retrievable covered self-expandable metallic stents. (A) Cholangiogram shows bile leak at the site of biliary-enteric anastomosis with extraluminal contrast (arrow) following Whipples procedure. (B) Retrievable covered self-expandable metallic stent placed across the anastomosis (arrow). (C) Percutaneous stent retrieval into a sheath using a custom designed hook (arrow). (D) Cholangiogram shows resolution of the leak.
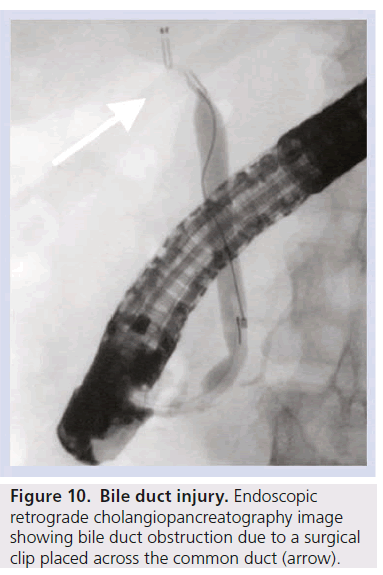
If possible, the location and extent of the injury should be characterized using non-invasive imaging such as computed tomography, ultrasonography and magnetic resonance cholangiopancreatography. CT cholangiography using Biliscopin (Schering, Berlin) and MR cholangiography using hepatocyte-specific contrast agents have also been successfully used to confirm and locate bile leaks [32,33]. ERCP and PTC should rarely be required as purely diagnostic tests and are generally reserved for interventions (Figure 10).
Prompt percutaneous biloma drainage reduces the incidence of complications such as abscess formation and cholangitis [34]. PTC with biliary drainage is often required in cases where there is complete ductal transection or ligation, proximal duct injury or following injury to an aberrant right hepatic duct [35]. Ideally an internal-external catheter should be placed but if not technically possible then an external catheter allows drainage and serves as a useful landmark for subsequent surgery or allows further radiological intervention. Bilateral drainage is often required for hilar injuries and targeted segmental drainage for transection of an aberrant right hepatic duct. Ultrasound guided bile duct puncture is therefore essential for this type of targeted approach and allows highly accurate punctures to be performed.
Although the published literature is limited to a few studies, the use of removable CSEMS in the treatment of complex or persistent bile leaks after laparoscopic cholecystectomy or pancreaticoduodenectomy has shown promising results (Figure 11).
In the more specialised orthotopic liver transplant group with persistent bile leak after plastic stent placement, the use of CSEMS has been shown to successfully treat the leak but with high rates of post-stent-removal strictures (35%) [6].
Benign biliary strictures
Benign biliary strictures (BBS) may be due to postoperative duct injury, primary sclerosing cholangitis, anastomotic strictures, chronic pancreatitis or strictures secondary to biliary calculi. Strictures can lead to a broad spectrum of clinical presentations from mildly abnormal liver function tests to obstructive jaundice or cholangitis. The initial priority is restoration of biliary drainage, usually endoscopically in patients with distal strictures in native ducts or percutaneously in patients with proximal or biliary-enteric anastomotic strictures.
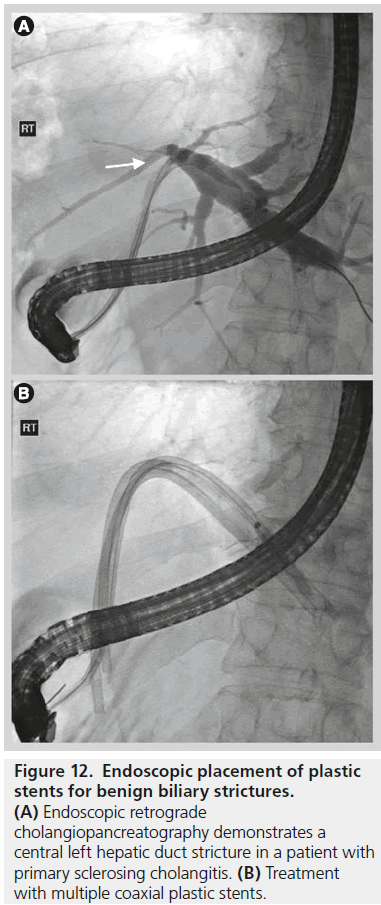
Endoscopic treatment has typically involved balloon dilatation and the placement of 10–12 Fr plastic biliary stents which are replaced every 3–4 months, usually for up to 1 year with reported successful dilatation of the stricture ranging from 74 to 100% [36]. Longer-term success rates are similar to those of surgery with stricture recurrence in 20% within 2 years of stent removal (Figure 12) [37]. In patients who are not suitable for endoscopic therapy, reported percutaneous transhepatic techniques have involved drainage, balloon dilatation and the placement of both uncovered and covered metal stents.
Decisions on how best to treat BBS depend on their cause and their anatomical location. The most widely practiced percutaneous transhepatic treatments of BBS have been either sequential balloon dilatations of the stenosis every 2–3 weeks with an access catheter left in situ between dilatations or sequential upsizing of an internalexternal biliary drain placed across the stenosis which allows slow tract dilatation. Balloon diameter selection depends on the site of stenosis but typically l0 mm balloons are used for common bile duct stenosis, while 6–8 mm balloons are suitable for intra-hepatic ducts stenoses. The balloon size should be guided by the size of the adjacent normal caliber duct. The use of cutting balloons has been described to treat BBS that respond poorly to initial conventional balloon dilatation either due to being markedly fibrotic or demonstrating significant elastic recoil [38]. However the relatively high stricture recurrence rates following balloon dilatation has lead to the more widespread use of metal stents. Uncovered metal stents should not be used in benign disease due to their poor long-term patency rates and the inability to remove them [39]. A number of studies have shown excellent results in the use of covered removable metal stents to treat balloon dilatation resistant BBS. Gwon et al reported the percutaneous temporary placement of covered removable stents in 29 patients with BBS. All stents were successfully removed after a mean of 6.7 weeks with excellent primary (90.6%) and secondary (97%) patency rates [5]. Kim et al demonstrated primary patency rates of 87% 3 years post-percutaneous CSEMS for BBS compared with only 44% in similar patients treated with balloon dilatation [40].
In the setting of non-transplant BBS, multiple studies have shown favourable results for the endoscopic placement of CSEMS with 100% success in attempted stent removal and stricture resolution following stent removal in 77–90% [41].
Transjejunal techniques
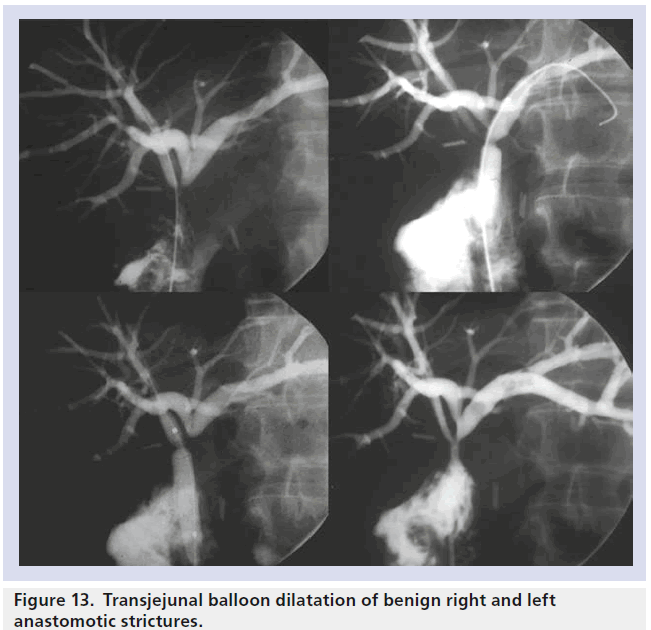
Percutaneous transjejunal biliary intervention is a technique developed to allow repeated percutaneous access to the biliary system for the treatment of benign biliary strictures and calculi. A Roux-en-Y loop created at the time of the biliary enteric anastomosis is surgical fixed to the anterior abdominal wall and marked with clips to allow subsequent percutaneous fluoroscopic puncture [42]. As post-operative benign biliary stricture recurrence requiring re-intervention ranges between 10–45%, repeated access to the biliary tree is required. The transjejunal procedure avoids the morbidity and discomfort of a percutaneous transhepatic approach and allows access to all segments of the biliary tree which can be repeated over a long period as needed, with high success rates and minimal complication rates [42]. The technique, using ballooning or stenting, can also be used in patients who have tumor recurrence or fibrotic stricturing following resection for malignant obstruction (Figure 13) [43].
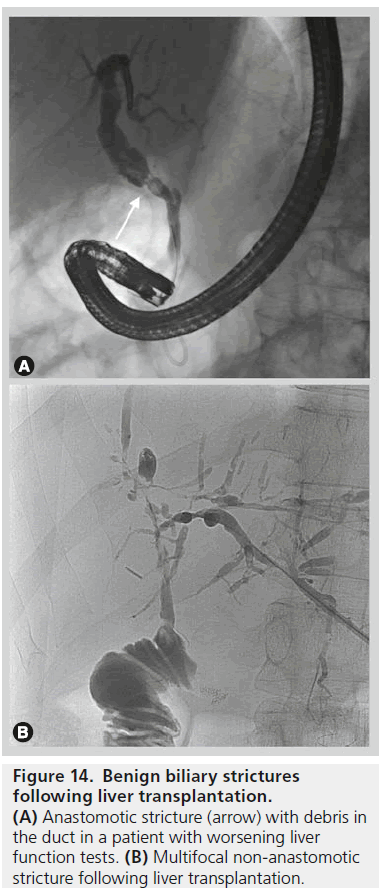
Biliary strictures after liver transplantation
Biliary strictures are the most frequent type of late biliary complication following liver transplant with an incidence of up to 15% [44]. Post transplant bile duct strictures are classified as anastomotic strictures (AS) or non-anastomotic strictures (NAS) with the latter showing poor response to treatment and a higher rate of graft loss of up to 50% at 10 years (Figure 14) [45].
Historically, the post-orthotopic liver transplant biliary strictures were treated surgically, usually with the formation of a Roux-en-Y hepaticojejunostomy. However, with advances in therapeutic endoscopy and the development of removable endoscopic and percutaneouslydelivered stents over the past two decades, these non-surgical approaches are now considered the treatment of choice in most centres. Endoscopic treatment is the preferred approach with either a combined rendezvous procedure or transhepatic techniques reserved for those in whom endoscopic treatment has not been possible.
Balloon dilatation and plastic stent placement (multiple plastic stents are often placed across the same stricture in order to achieve a good long term outcome) is the conventional endoscopic approach, repeated every 3–4 months for a period of 12–24 months with increasing stent diameter. Durable success in stricture treatment is seen in 75% of patients [44]. When conventional endoscopic treatments have failed to treat AS following orthotopic liver transplant, placement of a removable CSEMS can lead to successful treatment of the stricture in 71.8–95.5% but with a significant stent migration rate of 22.7–33.3% [46,47]. In patients treated by a percutaneous transhepatic approach, clinical success rates of approximately 70% and primary patency rates of 92.9% are comparable with those achieved by endoscopic plastic stenting or percutaneous balloon dilatation. One significant advantage of using covered removable stents is a marked reduction in the average duration of treatment, in one study, 197 days for removable CSEMS compared with 278 days for serial balloon dilatation [48].
Stone disease
Biliary calculi may be located in either the intrahepatic or extrahepatic biliary tree. Intrahepatic calculi, located proximal to the confluence of the right and left hepatic ducts are commonly associated with strictures and have a much higher incidence in far eastern Asian populations where parasitic infections play an important role in etiology [49]. Endoscopic retrograde treatments for intrahepatic stones can be very challenging and therefore percutaneous techniques play a significant role, particularly in the patient who presents with cholangitis. Management usually involves establishing effective percutaneous transhepatic drainage and then subsequent cholangioscopy with stone removal or fragmentation.
In contrast to intrahepatic stones, between 85–95% of extrahepatic stones can be successfully extracted using an endoscopic approach. This usually involves sphincterotomy and stone removal using a basket or balloon catheter. Mechanical lithotripsy and newer advanced endoscopic techniques such as cholangioscopy guided laser or electrohydraulic lithotripsy are reserved for more difficult cases [50]. Endoscopic treatment may be unsuccessful due to challenging access to the bile duct, large or impacted stones or intrahepatic stones.
If endoscopic retrograde techniques fail, two nonsurgical alternatives for stone clearance are either a rendezvous procedure or a complete percutaneous procedure. If the papillary region can be reached endoscopically, a rendezvous using a percutaneous transhepatic guidewire advanced into the duodenum allows easy localization and cannulation of the papilla.
If the duodenum cannot be reached endoscopically, percutaneous treatment is the only alternative to surgery. A variety of percutaneous methods for stone extraction have been described including the use of forceps or Dormia baskets both of which have success rates of nearly 95% but require large transhepatic tract diameters. Alternative percutaneous techniques have also been described, including antegrade papillary balloon dilatation with a reported stone clearance rate of 96% [51]. Technological advances such as smaller caliber cholangioscopes and improvements in electrohydraulic lithotripsy, have lead to improved results for percutaneous transhepatic cholangioscopic lithotomy which achieves excellent stone clearance rates of up to 100%. When compared with endoscopic techniques, percutaneous stone clearance is associated with a higher rate of complications (18 vs 51%); the majority of these are minor and transient [52,53].
The transjejunal approach is very useful if there has been a biliary-enteric anastomosis with the jejunal loop surgically fixed. Stone clearance may take more than one session and stones have a tendency to recur so this long-term access approach is particularly appealing, either for stone disease alone or stone disease associated with strictures [42,43].
Future perspective
Stents
Technological advances need to address problems of current stents such as migration, tumor in-growth and stent obstruction. Two recent developments in stent design are the introduction of drug-eluting stents and biodegradable stents. Drug-eluting stents are impregnated with a chemotherapeutic agent to achieve locally high doses of agents whose antitumoral effects are proposed to reduce tumor growth and prolong stent patency. Initial studies have shown drugeluting metal stents to be safe with acceptable complication rates [54]. However, a clear clinical benefit has not been shown and further studies possibly with a combination of systemic and local chemotherapy are required before they can be recommended.
Biodegradable biliary stents have been developed to minimize the problems of a long-term foreign body or the need for a further procedure to remove stents. Initial studies in animal models demonstrate that these stents appear safe and do not migrate [55]. Combination biodegradable, drug-eluting stents are also undergoing clinical trials [56].
Magnetic compression anastomosis
Magnetic compression anastomosis (MCA) is a nonsurgical technique for luminal recanalization in the digestive or biliary system using a pair of specially designed rare earth metal magnets. These are placed on either side of the obstruction and the magnet attraction and approximation causes tissue compression creating an effective luminal anastomosis.
In the small group of patients in whom conventional percutaneous or endoscopic methods have both failed, this technique has been used as an alternative to either surgery or lifelong external drainage. In the biliary system MCA has been shown to be both safe and effective in the setting of duct-to-duct anastomotic strictures following liver transplantation. One study reported successful recanalization of anastomotic strictures in 83.3% of patients with no major complications and a stricture recurrence rate of 10% [57].
MCA has also been used in the palliative setting to treat obstructive jaundice caused by malignant disease by the creation of a biliaryenteric anastomosis. One magnet is placed transhepatically within the bile duct, above the level of obstruction and the second magnet delivered into the duodenal lumen. Tissue compression and necrosis results in the formation of a biliary-enteric anastomosis with one study showing no procedural complications and a significant fall in bilirubin levels in all patients at 1 week [58].
Endoscopic technological advances
Expansion of interventional biliary endoscopy has been due in part to improvements in the design and capabilities of endoscopes and the more recent rapid expansion in the range of stents and other endoscopic devices.
One area of current rapid development is the new generation of peroral cholangioscopes. Peroral cholangioscopy has been limited in its clinical use until recently due to factors including the need for two endoscopists to perform the procedure, poor image resolution and fragile, inflexible cholangioscopes with inadequate small-calibre accessories. New, single-operator, small calibre, multi-channel devices such as the SpyGlass and Polyscope have overcome many of these problems. They allow direct visualization of the bile ducts, tissue sampling and therapeutic intervention such as stone fragmentation using EHL or laser [59,60].
Conclusion
Radiological biliary intervention has evolved significantly, and will continue to do so as the continuing technological advances allow more anatomically complex and challenging cases to be effectively treated. Both percutaneous and endoscopic approaches will continue to be important and best outcomes will continue to be achieved with effective cooperation between all specialties and careful pretreatment diagnostic imaging workup.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
* of interest
- Pomerantz BJ.Biliary tract interventions. Tech. Vasc. Interv. Radiol. 12(2), 162–170 (2009).
- Ginat D, Saad WE. Cholecystostomy and transcholecystic biliary access. Tech. Vasc. Interv. Radiol. 11(1), 2–13 (2008).
- Kortram K, van Ramshorst B, Bollen TL et al. Acute cholecystitis in high risk surgical patients: percutaneous cholecystostomy versus laparoscopic cholecystectomy (CHOCOLATE trial): study protocol for a randomized controlled trial. Trials 13, 7 (2012).
- Stoeckel D, Pelton A, Duerig T. Selfexpanding nitinol stents: material and design considerations. Eur. Radiol. 14(2), 292–301 (2004).
- Gwon DI, Shim HJ, Kwak BK. Retrievable biliary stent-graft in the treatment of benign biliary strictures. J. Vasc. Interv. Radiol. 19(9), 1328–1335 (2008).
- Martins FP, Phillips M, Gaidhane MR, Schmitt T, Kahaleh M. Biliary leak in post-liver-transplant patients: is there any place for metal stent? HPB Surgery 684172 (2012).
- Moreels TG. Altered anatomy: enteroscopy and ERCP procedure. Best Pract. Res. Clin. Gastroenterol. 26(3), 347–357 (2012).
- Judah JR, Draganov PV. Intraductal biliary and pancreatic endoscopy: an expanding scope of possibility. World J. Gastroenterol. 14(20), 3129–3136 (2008).
- Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest. Endosc. 43(2 Pt 1), 102–106 (1996).
- Horaguchi J, Fujita N, Noda Y et al. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig. Endosc. 21(4), 239–244 (2009).
- Sarkaria S, Lee HS, Gaidhane M, Kahaleh M. Advances in endoscopic ultrasound-guided biliary drainage: a comprehensive review. Gut Liver 7(2), 129–136 (2013).
- Carrasco CH, Wallace S, Charnsangavej C et al. Expandable biliary endoprosthesis: an experimental study. Am. J. Roentgenol. 145(6), 1279–1281 (1985).
- Kaassis M, Boyer J, Dumas R et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest. Endosc. 57(2), 178–182 (2003).
- Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur. J. Gastroenterol. Hepatol. 19(12), 1119–1124 (2007).
- Savader SJ, Lynch FC, Radvany MG et al. Single-specimen bile cytology: a prospective study of 80 patients with obstructive jaundice. J. Vasc. Interv. Radiol. 9(5), 817–821 (1998).
- Xing GS, Geng JC, Han XW, Dai JH, Wu CY. Endobiliary brush cytology during percutaneous transhepatic cholangiodrainage in patients with obstructive jaundice. HBPD INT. 4(1), 98–103 (2005).
- Tapping CR, Byass OR, Cast JE. Cytological sampling versus forceps biopsy during percutaneous transhepatic biliary drainage and analysis of factors predicting success. Cardiovasc. Intervent. Radiol. 35(4), 883–889 (2012).
- Thornton RH, Frank BS, Covey AM et al. Catheter-free survival after primary percutaneous stenting of malignant bile duct obstruction. Am. J. Roentgenol. 197(3), W514–518 (2011).
- Inal M, Aksungur E, Akgul E, Oguz M, Seydaoglu G. Percutaneous placement of metallic stents in malignant biliary obstruction: one-stage or two-stage procedure? Pre-dilate or not? Cardiovasc. Intervent. Radiol. 26(1), 40–45 (2003).
- Gwon DI, Ko GY, Kim JH. A comparative analysis of PTFE-covered and uncovered stents for palliative treatment of malignant extrahepatic biliary obstruction. Am. J. Roentgenol. 195(6), W463–W469(2010).& A good prospective study from a group leading research in the field of biliary intervention. Direct clinical applications and adding to the literature in terms the safety and efficacy of covered and uncovered biliary stents in malignant biliary obstruction.
- Krokidis M, Fanelli F, Orgera G et al. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/ FEP-covered versus uncovered nitinol biliary stents. Cardiovasc. Intervent. Radiol. 34(2), 352–361 (2011).
- Telford JJ, Carr-Locke DL, Baron TH et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest. Endosc. 72(5), 907–914 (2010).
- Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest. Endosc. 74(2), 321–327 (2011).
- Mezhir JJ, Brennan MF, Baser RE et al. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J. Gastrointest. Surg. 13(12), 2163–2169 (2009).
- van der Gaag NA, Rauws EA, van Eijck CH et al. Preoperative biliary drainage for cancer of the head of the pancreas. New Engl. J. Med. 362(2),129–137 (2010).
- Cavell LK, Allen PJ, Vinoya C et al. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am. J. Gastroenterol. 108(7), 1168–1173 (2013).
- Iacono C, Ruzzenente A, Campagnaro T, Bortolasi L, Valdegamberi A, Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann. Surg. 257(2), 191–204 (2013).
- George C, Byass OR, Cast JE. Interventional radiology in the management of malignant biliary obstruction. World J. Gastrointest. Oncol. 2(3), 146–150 (2010).
- van Delden OM, Lameris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur. Radiol. 18(3), 448–456 (2008). & A good review of the literature of the role and efficacy of percutaneous techniques in the palliation of malignant biliary obstruction.
- Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 12, 103 (2012).
- Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA 290(16), 2168–2173 (2003).
- Dinkel HP, Moll R, Gassel HJ et al. Helical CT cholangiography for the detection and localization of bile duct leakage. Am. J. Roentgenol. 173(3), 613–617 (1999).
- Alegre Castellanos A, Molina Granados JF, Escribano Fernandez J, Gallardo Munoz I, Trivino Tarradas Fde A. Early phase detection of bile leak after hepatobiliary surgery: value of Gd-EOB-DTPA-enhanced MR cholangiography. Abdom. Imaging 37(5), 795–802 (2012).
- Lee CM, Stewart L, Way LW. Postcholecystectomy abdominal bile collections. Arch. Surg. 135(5), 538–542 (2000).
- Thompson CM, Saad NE, Quazi RR, Darcy MD, Picus DD, Menias CO. Management of iatrogenic bile duct injuries: role of the interventional radiologist. Radiographics 33(1), 117–134 (2013).&An excellent overview of bile duct injuries covering assessment and management with a focus on image-guided interventional techniques.
- Shimada H, Endo I, Shimada K, Matsuyama R, Kobayashi N, Kubota K. The current diagnosis and treatment of benign biliary stricture. Surg. Today 42(12), 1143–1153 (2012).
- Bergman JJ, Burgemeister L, Bruno MJ et al. Long-term follow-up after biliary stent placement for postoperative bile duct stenosis. Gastrointest. Endosc.. 54(2),154–161 (2001).
- Atar E, Bachar GN, Eitan M, Graif F, Neyman H, Belenky A. Peripheral cutting balloon in the management of resistant benign ureteral and biliary strictures: long-term results. Diagn. Interv. Radiol. 13(1), 39–41 (2007).
- Siriwardana HP, Siriwardena AK. Systematic appraisal of the role of metallic endobiliary stents in the treatment of benign bile duct stricture. Ann. Surg. 242 (1), 10–19 (2005).
- Kim JH, Gwon DI, Ko GY et al. Temporary placement of retrievable fully covered metallic stents versus percutaneous balloon dilation in the treatment of benign biliary strictures. J. Vasc. Interv. Radiol. 22(6), 893–899 (2011).&A good comparison of a more established technique (balloon dilation) and a new and innovative approach (retrievable covered metal stents) to treat benign biliary strictures demonstrating the superiority of the covered stents.
- Baron TH. Covered self-expandable metal stents for benign biliary tract diseases. Curr. Opin. Gastroenterol. 27(3), 262–267 (2011).
- McPherson SJ, Gibson RN, Collier NA, Speer TG, Sherson ND. Percutaneous transjejunal biliary intervention: 10-year experience with access via Roux-en-Y loops. Radiology 206(3), 665–672 (1998).
- Fontein DB, Gibson RN, Collier NA et al. Two decades of percutaneous transjejunal biliary intervention for benign biliary disease: a review of the intervention nature and complications. Insights Imaging 2(5), 557–565 (2011).
- Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver 5(2), 133–142 (2011).
- Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am. J. Transplant. 13(2), 253–265 (2013).&A thorough overview covering the etiology, manifestations, assessment and management of the range of biliary complications following liver transplantation.
- Tarantino I, Traina M, Mocciaro F et al. Fully covered metallic stents in biliary stenosis after orthotopic liver transplantation. Endoscopy 44(3), 246–250 (2012).
- Garcia-Pajares F, Sanchez-Antolin G, Pelayo SL et al. Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant. Proc. 42(8), 2966–2969 (2010).
- Kim J, Ko GY, Sung KB et al. Percutaneously placed covered retrievable stents for the treatment of biliary anastomotic strictures following living donor liver transplantation. Liver Transplant. 16(12), 1410–1420 (2010).
- Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am. J. Gastroenterol. 90(4), 540–548 (1995).
- Trikudanathan G, Navaneethan U, Parsi MA. Endoscopic management of difficult common bile duct stones. World J. Gastroenterol. 19(2), 165–173 (2013).
- Szulman C, Gimenez M, Sierre S. Antegrade papillary balloon dilation for extrahepatic bile duct stone clearance: lessons learned from treating 300 patients. J. Vasc. Interv. Radiol. 22(3), 346–353 (2011).
- Lee JH, Kim HW, Kang DH et al. Usefulness of percutaneous transhepatic cholangioscopic lithotomy for removal of difficult common bile duct stones. Clin. Endosc. 46(1), 65–70 (2013).
- Arya N, Nelles SE, Haber GB, Kim YI, Kortan PK. Electrohydraulic lithotripsy in 111 patients: a safe and effective therapy for difficult bile duct stones. Am. J. Gastroenterol. 99(12), 2330–2334 (2004).
- Song TJ, Lee SS, Yun SC et al. Paclitaxeleluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: a prospective comparative pilot study. Gastrointest. Endosc. 73(4), 727–733 (2011).
- Yamamoto K, Yoshioka T, Furuichi K et al. Experimental study of poly-L-lactic acid biodegradable stents in normal canine bile ducts. Cardiovasc. Intervent. Radiol. 34(3), 601–608 (2011).
- Shi J, Lv Y, Yu L. Interest of a new biodegradable stent coated with paclitaxel on anastomotic wound healing after biliary reconstruction. Eur. J. Gastroenterol. Hepatol.25 (12 ), 1415 –1423 (2013)
- Jang SI, Kim JH, Won JY et al. Magnetic compression anastomosis is useful in biliary anastomotic strictures after living donor liver transplantation. Gastrointest. Endosc.. 74(5), 1040–1048 (2011).
- Avaliani M, Chigogidze N, Nechipai A, Dolgushin B. Magnetic compression biliary-enteric anastomosis for palliation of obstructive jaundice: initial clinical results. J. Vasc. Interv. Radiol. 20(5), 614–623 (2009).
- Cennamo V, Luigiano C, Fabbri C et al. Cholangioscopy using a new type of cholangioscope for the diagnosis of biliary tract disease: a case series. Endoscopy 44(9), 878–881 (2012).
- Balderramo D, Sendino O, Miquel R et al. Prospective evaluation of single-operator peroral cholangioscopy in liver transplant recipients requiring an evaluation of the biliary tract. Liver Transplant. 19(2), 199–206 (2013).& A prospective study evaluating the feasibility and efficacy of a new technique made possible by ongoing technological advances in the equipment available in the assessment and treatment of biliary disease.
- McQueen AS, Grant LA, Findlay JL, Sharma S, Shrivastava VS, McDonald SMM. Grainger and Allison’s Diagnostic Radiology 5th Edition. Adam A, Dixon AK (Eds). Churchill Livingstone, London, UK, 775 (2008).