Special Report - Interventional Cardiology (2015) Volume 7, Issue 6
Intracardiac echocardiography for guiding biopsy of cardiac tumors: a novel noninvasive technique
- Corresponding Author:
- Jae-Hwan Lee
Division of Cardiology, Department of Internal Medicine, Chungnam National University Hospital
Chungnam National University School of Medicine, South Korea
Tel: +82 42 280 8237
Fax: +82 42 280 8238
E-mail: myheart@cnu.ac.kr
Submitted: 10 July 2015; Accepted: 21 August 2015; Published online: 12 November 2015
Abstract
Since intracardiac echocardiography (ICE) was introduced into clinical practice about 20 years ago, the application of ICE in cardiac procedure has gradually expanded. Currently, ICE is most prevalent in electrophysiologic procedures, percutaneous device closures and the emerging transcatheter aortic valve implantation technique. Considering the various advantages of ICE shown in other cardiac procedures, these could also be applied to biopsy for cardiac tumors. Although cardiac biopsy is not a common procedure, the prognosis of cardiac tumor presents critical variation according to the primary pathology, and tissue diagnosis is essential for the treatment plan. Therefore, we should improve the current quality of cardiac biopsy, and the novel imaging modality; ICE could be important for this aim.
Since intracardiac echocardiography (ICE) was introduced into clinical practice about 20 years ago, the application of ICE in cardiac procedure has gradually expanded. Currently, ICE is most prevalent in electrophysiologic procedures, percutaneous device closures and the emerging transcatheter aortic valve implantation technique. Considering the various advantages of ICE shown in other cardiac procedures, these could also be applied to biopsy for cardiac tumors. Although cardiac biopsy is not a common procedure, the prognosis of cardiac tumor presents critical variation according to the primary pathology, and tissue diagnosis is essential for the treatment plan. Therefore, we should improve the current quality of cardiac biopsy, and the novel imaging modality; ICE could be important for this aim.
Keywords
cardiac biopsy, cardiac tumor, imaging, intracardiac echocardiography (ICE), pathology
Because the cardiac tumors manifest with nonspecific clinical features, those are usually detected on cardiac imaging performed for other causes. However, owing to recent wide use of echocardiography and other imaging modalities, cardiac tumor is not very unusual in a real clinical practice. As the definite diagnoses of most tumors are made by pathologic confirmation, the golden standard for diagnosis and treatment is complete excision. However, it is unavailable for many reasons, and the cardiac biopsy is considered as alternative approach. According to the American Heart Association/American College of Cardiology/ European Society of Cardiology (AHA/ ACC/ESC) 2007 guidelines [1]; the cardiac biopsy is reasonable if the diagnosis cannot be established by noninvasive modality, and if the pathologic diagnosis influence the treatment plans. There is critical variation in prognosis among malignant primary cardiac tumors; the life expectancy of sarcoma is less than 1 year, whereas it could be up to 5 years in lymphoma [2]. Metastatic cardiac tumor is much common as the previous autopsy series reported the prevalence of intracardiac metastasis to be above 10% [3]. In metastatic setting, occasionally the cardiac biopsy is useful; it helps to plan a systemic treatment and to avoid invasive biopsy for another site. Basically, cardiac biopsy is the intraluminal access, the potential risk of bleeding – in other words, extravasation – would be much reduced compared with the explorations for other sites.
However, there have been several technical challenges in the cardiac biopsy. First, the fluoroscopy is actually very limited in spatial information, but it only shows cardiac silhouette. Therefore, it is insufficient to ensure the precise location and depth in sample acquisition [4]. Second, as the target is constantly moving with heart beats, respiration and blood flow; the temporal resolution of image is another requirement [5]. Third, the wall thickness is variable (especially, 2–5 mm in left atrium [6]) that the operator should be cautious to perforation or laceration. Also the operator should be cautious to the neighboring structures such as ascending aorta and lung. In this background, there has been a demand for a delicate imaging modality, and some operators use the echocardiography in combination with fluoroscopy [1]. This brief report is focused on intracardiac echocardiography (ICE) with overview for the basic concepts, technical advantages and current limitations. To the present, firm evidence about the application of ICE in cardiac biopsy is lacking; it has only been shown in several case reports, not in large-sized patient group. Therefore in this emerging field, it may be a practical way to review the lessons from other noncoronary procedures using ICE.
Overview of ICE
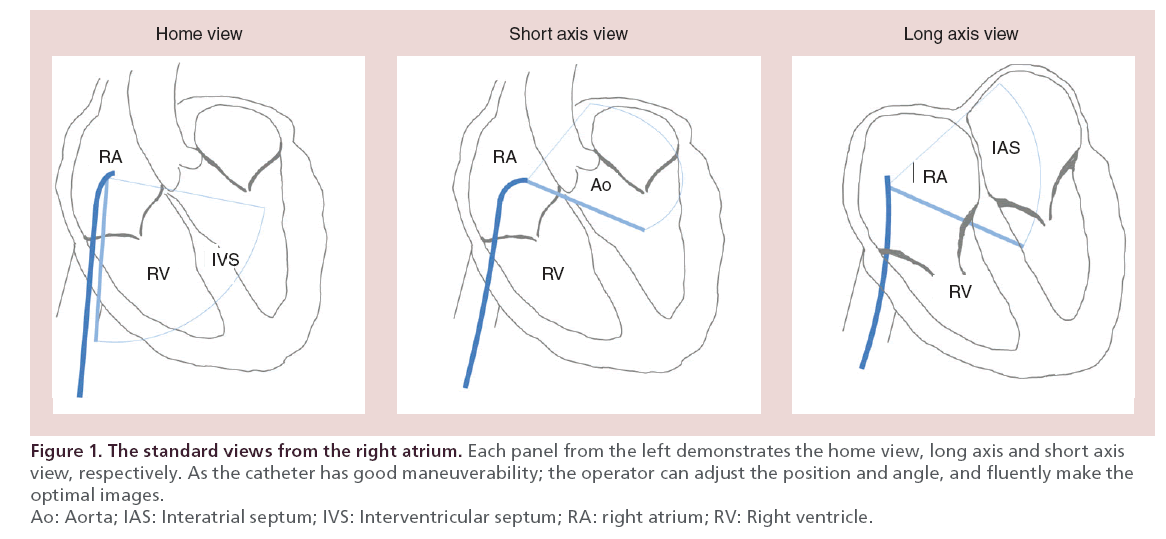
Usually, the ICE catheters access though femoral or jugular vein and the tips are placed in right atrium (RA) in which the navigation starts [7]. The operator can adjust the catheter for the position, orientation and angle to acquire the optimal images (Figure 1). The first commercially available system was the UltraICE® (Boston Scientific, MA, USA), a rotational catheter with a 9 MHz single element transducer [8,9]. It provides imaging sector of 360° with depth up to 5 cm [8,9]. This system is not steerable, and lacks of Doppler imaging [8,9]. The following models are the AcuNav® (Siemens-Acuson, Inc., CA, USA) and ViewFlex Plus® (St. Jude Medical, MN, USA) those are 64-element phased-array systems. The AcuNav has 5.0–10.0 MHz transducers, imaging sector of 90° with tissue penetration up to 16 cm. The ViewFlex Plus has similar profile with tissue penetration up to 21 cm. These two systems have Doppler capabilities and excellent maneuverability (anterior-posterior and left-right) for optimal imaging [9,10].
The ICE is distinctive to the computed tomography scan (CT) and MRI because of the real-time imaging during the procedure. Therefore, ICE provides real-time feedback for the device manipulation and the relationships with target structure [8]. Although transthoracic echocardiography (TTE) is also a real-time imaging, it is limited by various factors as obesity, emphysema, patient’s position, respiratory cooperation and shadowing by echogenic materials. And inherently, visualization of structure far from the apex is not satisfactory. The anatomic information of interatrial septum (IAS) is insufficient, as it is only shown as a linear structure. In contrast, the ICE can be placed in the nearest point or in neighboring chamber where it exhibits the full advantages of near field imaging.
Lessons from other noncoronary procedures using ICE
For the procedural guiding, the transesophageal echo-cardiography (TEE) is generally accepted as the best imaging modality. Still, there is no evidence that ICE is better than TEE in imaging aspect. However, the unique advantages of ICE in variable situations made it another efficient guiding modality. In reports about percutaneous ASD closure, freedom from the general anesthesia was emphasized as the major advantage of ICE compared with TEE [11,12]. Not only it avoided complications of general anesthesia, but Boccalandro et al. [12] reported that ASD closures using ICE presented shorter procedural time (47 ± 8 vs 35 ± 6 min, p < 0.001) and catheterization laboratory time (92 ± 18 vs 50 ± 12 min, p <0.001) compared with those using TEE. And a TEE specialist was not needed thus to save a professional fee [11,12]. Moreover, occasionally TEE machine can block the fluoroscopic motion, and the probe hide fluoroscopic images; whereas the ICE is free from all these interruptions to the procedural flow. In reports about transcatheter aortic valve replacement, the ICE helped precise positioning of balloon and prosthetic valve, thus to reduce procedural time, radiation exposure and use of radiocontrast agents [13]; therefore, patients using ICE presented significantly lower incidence of acute kidney injury [14]. For the paravalvular leakage, currently the best imaging modality is TEE. Because the targets are usually tiny and occasionally concealed in acoustic shadow of prosthetic valve, it is technically demanding to affirm the wire passage and adequate closure. Although Ruiz et al. [15] reported the successful deployment of occluder in 42 of 49 (86%) lesions, Sorajja et al. [16] presented the moderate to severe remnant leakage in 30 of 126 (25%) patients. In this challenging area, ICE has some potentials; it would be suitable to visualize the orifice at the anterior side of prosthetic aortic valve (opposite side of TEE probe) [17], could be placed in nearest point to the orifice to ensure complete closure. All these are yet to be firm; however, the featured advantages of ICE to complement the TEE is noticeable in emerging procedures. The percutaneous mitral commissurotomy (PMC) including trans-septal puncture (TSP) is usually performed only under fluoroscopic guidance. The ICE also showed advantages in TSP, the standard views from RA provided powerful near field images of IAS [18]. The ICE presented the detailed anatomic feature of IAS (aneurysm, lipomatous hypertrophy, double layered septum and presence of patent foramen ovale [PFO]) and specified the puncture point [9]. Accordingly, it helped to avoid the potential risk of injury in adjacent structures (1–4% [19]). Therefore, the TSP seems to be the best indication of ICE; currently, the TSP under ICE is expanded to ablation for atrial fibrillation and MitraClip® implantation [7]. Ultimately, it would be applicable to biopsy of the left heart tumor.
Advantages of ICE specific to cardiac biopsy
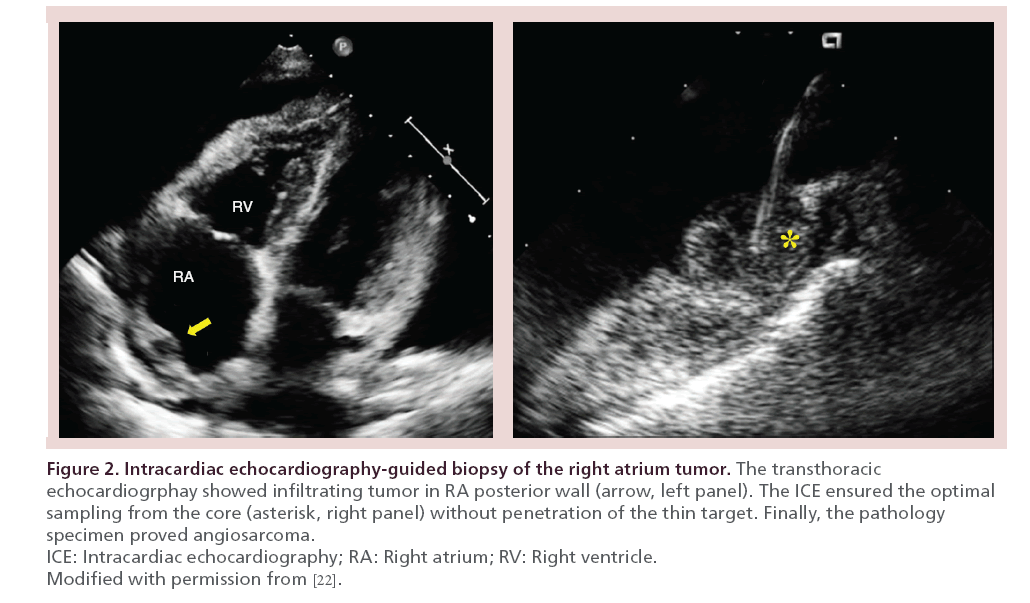
As the imaging capability of ICE is best for the near filed, it is the key to place the catheter tip at the nearest point to the target. Once ICE is placed in optimal position, it presents specific advantages for cardiac biopsy. First, it points for the site of tissue sampling considering extent and thickness of tumor and surrounding anatomies [8]. Marrouche et al. [20] reported in their patients underwent pulmonary vein isolation under fluoroscopy that the catheter locations were apart from true ostium by 5 ± 3 mm. For an example, lymphoma presents rapid proliferation and degeneration; occasionally, the biopsy is nondiagnostic according to location and depth of tissue sampling. Also enough tissue volume is good to special staining. Therefore, the ICE would be advantageous to enhance the diagnostic power of cardiac biopsy. Second, the ICE is the real-time imaging for the direction, curve and angle of devices; helps to deliver the operator’s manipulation. Remind that ‘biting or slippage’ determines ‘success or failure’ of whole biopsy procedure. Third, for an example of angiosarcoma with predilection to RA and infiltrating nature [2,21]; the operator should be extremely cautious to mural laceration and cardiac tamponade. In the previous report of our group [22], the ICE helped operator to avoid perforation of RA wall, and at the same time it ensured the adequate depth of tissue sampling (Figure 2). Bedside TTE is generally used for emergent evaluation, however, it needs at least several minutes for a personnel and machine to be available. Also it contaminates the sterile procedural field. Using ICE, the operator may adjust the depth of sampling, monitor the complication in real-time and make prompt decision for pericardiocentesis if needed.
Figure 2: Intracardiac echocardiography-guided biopsy of the right atrium tumor.
Safety, technology & feasibility issues
Although the benefits are thought to outweigh, there are also potential risks in ICE inherent to catheter based modality. The overall complication rate was reported to be 4% in all cases [23]. Femoral or jugular venipuncture can cause vascular injury or bleeding. Manipulation of catheter can irritate endocardium and provoke arrhythmias. And the introduction of catheter can cause endothelial damage and thrombosis leads to venous and pulmonary thromboembolism, and even systemic embolism in presence of PFO. In technical aspect, far field imaging is another limitation. The left atrial far wall, appendage and left ventricular lateral walls are challenging sites for ICE. However, new phased-array systems provide imaging depth of 16–21 cm and good maneuverability to adjust the imaging fields [8–10], and the recently introduced intracoronary sinus ICE will be helpful for left heart imaging. Although the real-time 3D imaging in TTE and TEE presented benefits in terms of procedural success, complication and procedural times [7], it is still developing in ICE. The economic burden is another issue. Alborias et al. [11] reported in their 40 patients underwent ASD device closure that the ICE group presented higher hospital charges compared with the TEE group (USD 24,595 ± 4616 vs 19,473 ± 2613, p < 0.001). They also pointed out that the ICE group saved professional fee (for general anesthesia and TEE), therefore the global charges including hospital and professional charges were balanced. However, surely the cost of catheter system (USD 2000–2500) is an important barrier to the general use of ICE [8,11]; there should be individual consideration for various national circumstances regarding professional fee, health insurance system, and strategy for re-use of device. In educational aspect, general standard has not been established as those in TTE or TEE. Instead, the vendors provide some educational courses including hands-on training.
Conclusion
ICE is an emerging imaging modality that has potential to fulfill various needs for guiding cardiac biopsy It provides clear intracardiac imaging for target and devices, therefore helps adequate tissue sampling which enhances the diagnostic power. Continuous monitoring for complication and freedom from general anesthesia are important advantages. Also it helps to reduce procedural time and radiation exposure. Although there are technical and economic issues to be solved, and large-sized clinical evidence is still lacking; the prospect of ICE in cardiac biopsy is encouraging in a near future as the evidence accumulates.
Future perspective
• With the rapid technical advances in devices, the far field imaging and real-time 3D imaging of ICE will be improved.
• Accompanied with trans-septal puncture and intracoronary sinus ICE, the experiences for the left heart biopsy will be increased.
• The interventional cardiologist and trainees will have the chance for more generalized and standardized education.
• As the benefits are shown in more clinical evidences, ICE will become a standard imaging modality in the biopsy of cardiac tumor in the near future.
Executive summary
Background
• Cardiac biopsy is essential in both primary and secondary (metastatic) cardiac tumors.
• Primary cardiac tumors show variable prognosis according to the pathology, also the metastatic cardiac tumor is treated according to the pathologic diagnosis.
• Fluoroscopy is an insufficient imaging modality for cardiac biopsy, therefore intracardiac echocardiography (ICE) is expected to be key to enhance this procedure.
Overview of device & advantages
• The commonly available systems are the AcuNav® and ViewFlex Plus® with 64-element phased-array beam, 5.0–10.0 MHz, and penetration up to 16–21 cm.
• ICE surpasses transthoracic echocardiography, which is limited by obesity, emphysema, respiration and acoustic shadowing by echogenic materials.
• ICE helps to avoid general anesthesia, to reduce procedural time and radiation exposure, and to save professional fees.
• ICE provides clear near field images of the tumor and surrounding structures for precise targeting.
• ICE provides real-timing feedback for device manipulation for adequate tissue sampling.
• ICE provides continuous monitoring for complications during procedure.
Safety & economic issues
• The overall complication of ICE was reported as 4% including vascular injury, bleeding, arrhythmias and thrombosis.
• The cost of the catheter system (US$ 2000–2500) is an important economic barrier.
Conclusion
• ICE-guided biopsy for cardiac tumor has potential benefits outweighing the risk and cost, therefore its role will expand as the clinical evidence accumulates in a near future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Cooper LT, Baughman KL, Feldman AM et al. The Role of Endomyocardial Biopsy in the Nanagement of Cardiovascular Disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 28(24), 3076–3093 (2007).
- Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 6(4), 219–228 (2005).
- Wee JO, Sepic JD, Mihaljevic T, Cohn LH. Metastatic carcinoid tumor of the heart. Ann. Thorac. Surg. 76(5), 1721–1722 (2003).
- Scholte AJ, Frissen PH, Van Der Wouw PA. Transesophageal echocardiography-guided transvenous biopsy of an intracardiac tumor. Echocardiography 21(8), 721–723 (2004).
- Sze DY, Lee DP, Hofmann LV, Petersen B. Biopsy of cardiac masses using a stabilized intracardiac echocardiography-guided system. J. Vasc. Interv. Radiol. 19(11), 1662–1667 (2008).
- Ho SY, Cabrera JA, Sanchez-Quintana D. Left atrial anatomy revisited. Circ. Arrhythm. Electrophysiol. 5(1), 220–228 (2012).
- Bartel T, Muller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur. Heart J. 35(2), 69–76 (2014).
- Kim SS, Hijazi ZM, Lang RM, Knight BP. The use of intracardiac echocardiography and other intracardiac imaging tools to guide noncoronary cardiac interventions. J. Am. Coll. Cardiol. 53(23), 2117–2128 (2009).
- Ali S, George LK, Das P, Koshy SK. Intracardiac echocardiography: clinical utility and application. Echocardiography 28(5), 582–590 (2011).
- Hijazi ZM, Shivkumar K, Sahn DJ. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation 119(4), 587–596 (2009).
- Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am. J. Cardiol. 94(5), 690–692 (2004).
- Boccalandro F, Baptista E, Muench A, Carter C, Smalling RW. Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. Am. J. Cardiol. 93(4), 437–440 (2004).
- Bartel T, Bonaros N, Muller L et al. Intracardiac echocardiography: a new guiding tool for transcatheter aortic valve replacement. J. Am. Soc. Echocardiogr. 24(9), 966–975 (2011).
- Bartel T, Bonaros N, Edlinger M et al. Intracardiac echo and reduced radiocontrast requirements during tavr. JACC Cardiovasc. Imaging 7(3), 319–320 (2014).
- Ruiz CE, Jelnin V, Kronzon I et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J. Am. Coll. Cardiol. 58(21), 2210–2217 (2011).
- Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J. Am. Coll. Cardiol. 58(21), 2218–2224 (2011).
- Rihal CS, Sorajja P, Booker JD, Hagler DJ, Cabalka AK. Principles of percutaneous paravalvular leak closure. JACC Cardiovasc. Interv. 5(2), 121–130 (2012).
- Epstein LM, Smith T, Tenhoff H. Nonfluoroscopic transseptal catheterization: safety and efficacy of intracardiac echocardiographic guidance. J. Cardiovasc. Electrophysiol. 9(6), 625–630 (1998).
- Hahn K, Gal R, Sarnoski J, Kubota J, Schmidt DH, Bajwa TK. Transesophageal echocardiographically guided atrial transseptal catheterization in patients with normal-sized atria: incidence of complications. Clin. Cardiol. 18(4), 217–220 (1995).
- Marrouche NF, Martin DO, Wazni O et al. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation 107(21), 2710–2716 (2003).
- Maraj S, Pressman GS, Figueredo VM. Primary cardiac tumors. Int. J. Cardiol. 133(2), 152–156 (2009).
- Park KI, Kim MJ, Oh JK et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ. J. 45(2), 165–168 (2015).
- Earing MG, Cabalka AK, Seward JB, Bruce CJ, Reeder GS, Hagler DJ. Intracardiac echocardiographic guidance during transcatheter device closure of atrial septal defect and patent foramen ovale. Mayo Clin. Proc. 79(1), 24–34 (2004).
• Overview the meaning of cardiac biopsy and current imaging modalities.
•• Explains the basic concepts of intracardiac echocardiography (ICE) and variable clinical applications.
•• Explains the variable clinical applications.
• Concisely review the current technology of devices.
•• Concisely review the evolution and current technology of devices.
• Potentials of ICE to complement the transesophageal echocardiography, which is the current best imaging modality.