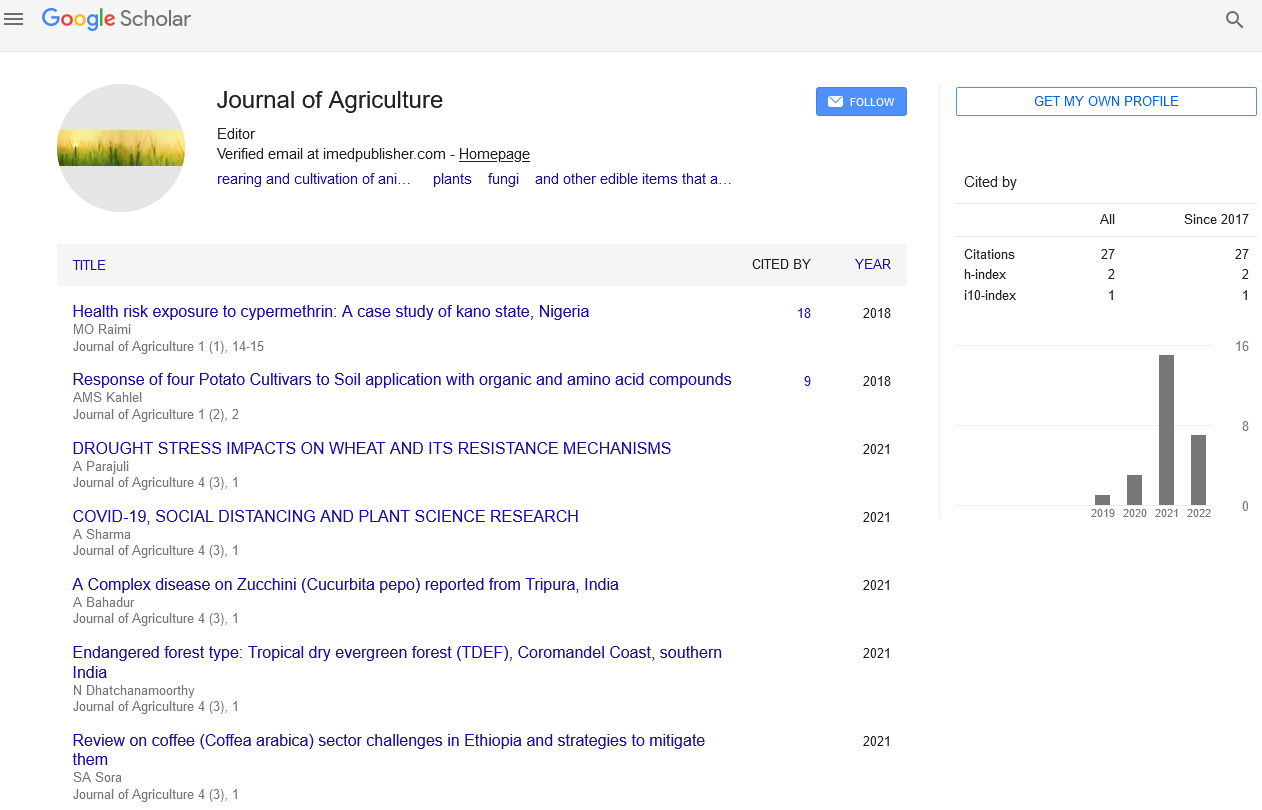
Mini Review - Journal of Agriculture (2023) Volume 6, Issue 1
Introducing the Journal of Agriculture and Food Research, a brand-new venue for multidisciplinary studies
Anil Mishra*
Department of Chemical and Environmental Engineering, University of South Carolina, Columbia
Department of Chemical and Environmental Engineering, University of South Carolina, Columbia
E-mail: AnilMishra54@gmail.com
Received: 01-Feb-2023, Manuscript No. jagri -23-95380; Editor assigned: 06-Feb-2023, Pre-QC No. jagri-23-95380 (PQ); Reviewed: 19- Feb-2023, QC No. jagri -23-95380; Revised: 24-Feb-2023, Manuscript No. jagri -23-95380 (R); Published: 28ss-Feb-2023; DOI: 10.37532/ jagri.2023.6(1).17-19
Abstract
The Journal of Agriculture and Food Research is a new venue for interdisciplinary, cuttingedge, and creative research in all facets of agriculture and food, and we are happy to introduce it. This journal will publish all types of publications, including full-length research articles, reviews, quick communications, viewpoints, and commentaries. It is peerreviewed and completely open-access. The journal welcomes various submissions from all disciplines and sectors, including but not limited to academic institutions, international research centers, and public and private research organisations. Its goal is to serve as a comprehensive and trustworthy source of information on current developments in the intricate and intertwined agricultural and food systems. The development of the world’s agricultural and food systems has coincided with a revolution in the globe and a change in our way of life brought about by improvements in science, engineering, and technology. Such Research developments in agriculture and food are not science fiction or fantasy, but rather extremely practical practises and tools that have a significant impact on how we live our daily lives. Numerous innovations are being developed and put into practise to transform farm animals and field crops into useful food products, not only to feed the world’s expanding population but also to enhance the general public’s overall wellness. As a result, experts in the fields of agriculture and food are convening and working together more than ever. Massive research efforts are currently being made to find strategies to guarantee sustainable agriculture and food products with increased effectiveness, quality, and safety. In this section, we’d like to quickly highlight a few of the important breakthroughs that are revolutionising our food and agriculture systems. Robotics, plant phenotyping, the agricultural internet of things, intelligent agricultural machinery, agricultural big data, and plant breeding are some of the technological advancements that are revolutionising our food and agricultural systems to increase food security and promote human and environmental health.
Introduction
Numerous potyviruses are harming crop microorganisms. In all temperate and tropical regions, they infect the majority of angiosperm taxa. When aphids move from plant to plant in search of their preferred host species, they are carried by aphids, particularly species of the Aphidian family. Additionally, some are carried by seeds to infected plants’ offspring [1]. Why are so many potyviruses present? Is their power recent or long-standing? To answer such questions, we must first determine the date of the potyvirus eradication. Infections leave no fossils and their transformative rates must be assessed by connecting highlights of their phylogenies to dates got in alternate ways. Some animal viruses, like orthomyxoviruses and lent viruses, evolve so quickly that it is possible to estimate their evolutionary rates by comparing samples taken at different times during an epidemic [2]. The co-evolutionary congruence is sometimes complete, indicating that the virus group is as old as its hosts, and other viruses have phylogenies that are congruent with those of their 3hosts. Tobamoviruses, a genus of plant viruses, have this kind of relationship, which suggests that it first appeared around 100 million years ago, when the asteroid and rosid plant lineages diverged [3]. Potyvirus taxonomy does not match that of their hosts, and species from the same lineage may have hosts that are very different from one another. Aroids, cucurbits, legumes, orchids, and pass floras, for instance, serve as the primary hosts for various species of the bean common mosaic lineage. Wheat streak mosaic tritimovirus’s nucleotide substitutions from rice yellow mottle virus and tomato yellow leaf curl begomovirus are the only other published estimates of plant virus evolution rates [4]. These rates are like those revealed for certain populaces of infections of creatures. We examine the evolution of potyviruses over time in this paper. An active area of research at the moment is the development of methods for inferring and dating phylogenies from gene sequences [5].
Materials and Methods
The Neighbor-joining tree option was used for the initial sequence collation and alignment. TREEVIEW was used to view the resulting trees. The DnDscan method was used to estimate the similarities and differences between sequences, and EXCEL was used to present and graph the results. Using SBHistogram, some distance estimates were presented as histograms [6]. For phylogenetic comparisons, only the “coherently evolving” region of the potyvirus coat protein genes was used; at one end, the cCP region of the potato virus Y genome contains the sequence DVNAG and at the other, the VP’s C-terminus. All alignments were based on a representative set of 47 different cCP sequences, with the ryegrass mosaic rymovirus serving as an outlier to the out group when necessary. The alignment of the cCPs of these “out group potyviruses” will be provided upon request. These sequences were aligned using their encoded amino acid sequences using the Trans align program, which was generously provided by Georg Weiler and CLUSTALX. The default parameters produced an alignment with 720 nucleotides and gaps [7]. The adjusted out group groupings were checked for incongruent connections, which could have come about because of recombination, utilizing the RDP bundle rendition with default settings and a Bonferroni revised P-esteem cut off of 0.05, and they were likewise checked by the PHYLPRO program. Most of the time, the maximum likelihood approach was used to infer and compare the sequences’ phylogenetic relationships. BEAST was also used to conduct a few tests to see if Bayesian methods produced trees with different sizes or topologies than ML methods did. We also checked to see if the tree branch reconnection and Neighbor-joining methods, as well as the maximum parsimony method PAUP, produced trees with similar topologies. Except where otherwise specified, all analyses utilized the default settings. To look at the transformative rates acquired utilizing different developmental models and techniques we gathered four little delegate sets of adjusted cCP arrangements [8]. The premise of each set was the 47 out group potyvirus cCPs, which accommodated each set a similar unsettled root. One set contained only the 47 species sequences; another set contained the 29 cCP sequences of the cowpea aphid-borne mosaic virus (CAbMV); a third set contained the 30 representative cCP sequences of the papaya rings pot virus (PRSV) (List S2); and the fourth set contained all three sets—the out group, CAbMV, and PRSV sequences. Thus, there were 47, 76, 77, and 106 sequences in the four test sets, but they all had the same root. Using both the Akaike information criterion (AIC) and the likelihood ratio test, MODELTEST favoured the Hasegawa, Kishina, and Yano model for each of the four test sequence sets, as well as the general time-reversible model with gammadistributed rate variation and a proportion of invariable sites. The clock likelihood ratio test was used to compare likelihood scores obtained in PAUP using models with branch-specific clocks or strict clocks for all branches. BEAST was used to obtain trees with either “strict” or “relaxed” clocks in order to evaluate the impact of this difference on dating; for the ‘casual’ clock the rates for various branches were disseminated by a lognormal conveyance [9]. At least five million Monte Carlo Markov Chain generations were used in each BEAST analysis, samples were taken every 1000, and at least 50 ESS values were evaluated by Tracer 1.4; the results of the duplicate analyses were less than 2% off the mean on average. By comparing pairwise distances inferred as ML estimates by PhyML and date estimates by BEAST employing the GTR+I+G model for both, divergence times were estimated from the complete sets of cCP sequences aligned with the out group cCPs. PATRISTIC was used to convert the Newick format trees from PhyML or BEAST into distance matrices [10].
Major Radiation and Potyvirus Dating
As a result, we looked for isolated and outbreak events in the past that were linked to the phylogenetic relationships of potyvirus cCP sequences and could provide estimates of the radiation’s date on longer time scales. Four distinct lines of evidence were discovered. The majority of Prunus species have lengthy historical records of the plum pox virus. Chinese writing records development of the peach, Prunus persica, throughout the course of recent years. Twelve distinct plum varieties were mentioned by Pliny, some of which were brought to Great Britain by the Romans and recorded in William Langland’s Piers Ploughman. However, it is significant that the very obvious and harmful symptoms of the plum pox virus (PPV) were not recorded until around 1915 in Bulgaria. In a well-documented epidemic that affected the majority of cultivated and wild Prunus species, plum pox, or “Sharka” in Slavic, then spread north and east across Europe; it has recently reached the Americas. In 1992, it was first found in Chile, and in 1999, it was discovered in North America. The majority of the 67 cCP genes found in PPV isolates collected from the region affected by this outbreak fell into two main clusters when compared. Before gene sequencing became common, serological tests were used to distinguish these two clusters, which are known as strains of PPV D and PPV. The cCP sequences for the D strain were derived from isolates collected in Europe, Eurasia, and the Americas, whereas the cCP sequences for the M strain were derived solely from Europe. Additionally, there are four cCP sequences derived from three other distinct strains. All pairs of sequences linked by the M strain cluster’s basal node had a mean difference of 0.028+/-0.004 nucleotide substitutions per site (ns/s), but only 0.009+/- 0.002 ns/s for the D strain. It might appear that the D strain, which spreads more widely, was the cause of the 90-year epidemic. However, the M strain “is considered to be the epidemic form of the virus,” whereas the D strain “may have spread further, but more recently, merely because infected plants show few or ephemeral symptoms and so plants chosen for propagation and dispersal may have been infected with this strain until more sensitive detection methods stopped its inadvertent spread in horticultural stock.” The M strain, on the other hand, causes symptoms that are much more severe and “is considered to be the epidemic Plum pox illness previously showed up in Bulgaria a long time back, yet the pestilence in stone fruits presumably began quite a while previously. Assuming we expect thusly that the variety of the PPV-M populace has been procured throughout recent years in addition to an ‘overshadow’ time of 10 years during which the meaning of the sickness was not perceived or recorded, then the transformative pace of PPV-M will have been.
References
- Smith DJ, Lapedes AS, Bestebroer TM et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 305, 371–376 (2004).
- Lukashov VV, Goudsmit J. Evolutionary relationships among parvoviruses: virus-host coevolution among autonomous primate parvoviruses and links between adeno-associated and avian parvoviruses. Journal of Virology.75, 2729–2740 (2001).
- Ong CK, Chan SY, Campo MS et al. Evolution of human papillomavirus type 18: an ancient phylogenetic root in Africa and intertype diversity reflect coevolution with human ethnic groups. Journal of Virology. 67, 6424–6431 (1993).
- Duffy S, Holmes EC. Phylogenetic Evidence for Rapid Rates of Molecular Evolution in the Single-Stranded DNA Begomovirus Tomato Yellow Leaf Curl Virus. Journal of Virology. 82, 957–965 (2008).
- Jenkins GM, Rambaut A, Pybus OG. Rates of Molecular Evolution in RNA Viruses: A Quantitative Phylogenetic Analysis. Journal of Molecular Evolution. 54, 156–165 (2002).
- Saitou N, Nei M. The Neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 4, 406–425 (1987).
- Guindon S, Gascuel O. PhyML - A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology.52, 696–704 (2003).
- Lanave C, Preparata G, Saccone C. A new method for calculating evolutionary substitution rates. Journal of Molecular Evolution. 20, 86–93 (1984).
- Fourment M, Gibbs MJ. PATRISTIC: a program for calculating patristic distances and graphically comparing the components of genetic change. BMC Evolutionary Biology. 6, 1 (2006).
- Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 418, 700–707 (2002).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref