Perspective - Imaging in Medicine (2013) Volume 5, Issue 2
Is there a place for ultrasound in neuraxial anesthesia?
Silke Brinkmann1, Geneviève Germain*1, Andrew Sawka1, & Himat Vaghadia11University of British Columbia Department of Anesthesiology, Pharmacology & Therapeutics, Vancouver General Hospital, Room 2449, Jim Pattison Pavilion, 899 West 12th Avenue, Vancouver, BC, V5Z 1M9, Canada
- Corresponding Author:
- Geneviève Germain
University of British Columbia Department of Anesthesiology
Pharmacology & Therapeutics, Vancouver General Hospital
Room 2449, Jim Pattison Pavilion, 899 West 12th Avenue
Vancouver, BC, V5Z 1M9, Canada
Tel: +1 604 875 4304
Fax: +1 604 875 5209
E-mail: genevieve.germain@vch.ca
Abstract
Keywords
epidural anesthesia ▪ neuraxial anesthesia ▪ spinal anesthesia ▪ ultrasonography ▪ ultrasound
The clinical use of ultrasound has gained increased popularity among anesthesia providers in the last decade. Its use has become commonplace to facilitate central venous catheterization, arterial line placement and difficult venous access [1]. It is now the preferred technique in some centers for the establishment of central venous access [2] and an integral part of anesthetic training and practice [3]. It has also revived the popularity and use of peripheral nerve blockade [4–7]. La Grange et al. first described an ultrasound-facilitated brachial plexus block in 1978 using a Doppler ultrasound to facilitate a supraclavicular block [8]. The first direct use of ultrasound for a regional block appears later in the literature, in 1994, when Kapral et al. described their use of ultrasound again for a supraclavicular brachial plexus block [9]. Since that time, the ultrasound image quality has greatly improved and the machines have become portable, relatively inexpensive and simple to use. Ultrasound machines are now an integral part of modern anesthesia practice.
Ultrasonography has been shown to increase the overall success rate of peripheral nerve blocks when compared with other methods of nerve localization [10] and to decrease the procedural time required to achieve anesthesia [11,12]. In addition, because ultrasound guidance increases the ability to place the needle close to the nerve, it has permitted a significant reduction in volumes and doses of local anesthetics [13]. Use of ultrasound has been shown in comparative studies to produce faster onset times and longer duration of regional blocks when compared with other nerve localization techniques [7,14]. A recent study has even demonstrated the cost– effectiveness of ultrasoundguided regional anesthesia in daily clinical practice [15].
Ultrasonography permits the anesthesia provider to visualize the neural target, the surrounding structures, needle advancement and the spread of local anesthetic. However, there is still debate as to whether this reduces the complications associated with regional anesthesia such as inadvertent intravascular injection and pleural or neurological injury [16–19]. The advent of ultrasound guidance has helped the proliferation of regional anesthetic techniques as well as increased its use among anesthesiologists.
Conversely, ultrasonography has been relatively underutilized in neuraxial anesthesia, mainly due to the fact that the conventional surface landmark-based techniques are mastered by the vast majority of anesthesiologists, with success rates approaching 96% for spinal anesthesia [20]. In addition, there is less familiarity and greater perceived difficulty of spinal sonoanatomy compared with other blocks [21]. Consequently, the adoption of ultrasound for neuraxial anesthesia will certainly be slower and not likely as widespread as with peripheral blocks.
This article will briefly review the literature on the use of ultrasonography in neuraxial anesthesia for determining landmarks and more recently, its use in real-time ultrasound-guided techniques [22,23].
Indications for ultrasound-assisted neuraxial blockade
Central neuraxial blocks are widely performed for anesthesia and analgesia, and success relies upon the ability to direct a needle to the epidural or subarachnoid space. The standard technique of spinal anesthesia that is currently practiced is essentially a ‘blind’ technique based on palpable landmarks. The spinal needle is advanced towards the dura with reliance on tactile feedback, pops, clicks and bony resistance. If the initial needle pass fails, success with subsequent passes relies on the ability to appropriately redirect the needle based on the operator’s anatomical knowledge and experience. This technique has a long track record of reliability and safety, and most anesthesiologists are able to become experts in spinal anesthesia.
Traditional techniques can be challenging in patients with poorly palpable surface landmarks, age-related changes or abnormal spinal anatomy. Although a higher BMI is not an independent indicator of difficult neuraxial anesthesia, those with poorly palpable bony landmarks are predictive of multiple needle passes and longer procedural times [24–26]. In addition, neuraxial anesthesia in elderly patients may be challenging owing to the presence of osteophytes and narrowing of the disc spaces [27].
Early evidence suggests that ultrasound-assisted spinals may be particularly beneficial in patients with poor bony landmarks [28]. In addition, ultrasonography in patients with normal anatomy [29,30] or abnormal anatomy [31] allows for a more accurate determination of the level of needle insertion than palpation of surface landmarks alone, and enables the prediction of depth with a high degree of accuracy [32,33]. Preprocedural ultrasonography has also been shown to facilitate needle insertion in a number of populations including obstetric patients [34–36], older orthopedic patients [37] and patients with complex anatomy [38,39]. Recently, ultrasonography has also been used to estimate intrathecal volume and dural sac length; in the future this information may assist in determining the optimal dosage for spinal anesthesia [40]. However, further research is needed to clearly establish the impact of ultrasound on procedure success and safety profile, particularly in the adult nonobstetric population [32].
A reduction in needle passes in neuraxial anesthesia with ultrasonography may have some putative benefits and in actuality [28,41], practitioners often underestimate the number of needle passes performed [42]. The risk of epidural hematoma is rare in all patient populations, but the risk of this devastating complication does increase with the number of needle passes required for neuraxial blockade [43,44]. Multiple needle passes are also a risk factor for minor or major hemorrhagic complications [43–45] and the development of postdural puncture headaches [46], and may cause unnecessary patient discomfort. Multiple needle passes can also theoretically increase the risk for paresthesias and Horlocker et al. have shown that paresthesias during needle placement significantly increases the risk of persistent paresthesias [47].
Fortunately, the risk of all major complications with spinal anesthesia is extremely low and we expect this would be the case for ultrasoundguided spinals as well. For this reason, it may be impossible to demonstrate increased safety with the use of ultrasound for neuraxial block, as the sample size required in such a study would be prohibitive.
In patients with abnormal spinal anatomy, surface landmarks may be difficult to interpret. In a retrospective review on ankylosing spondylitis patients and neuraxial anesthesia, Schelew et al. found that spinal anesthesia was only possible in 76.2% of cases (ten out of 13 attempts) and that epidural anesthesia was unsuccessful in each attempt (three out of three) [48]. In addition, patients with congenital abnormalities of the spine [39], scoliosis [49] or previous spinal surgery [31,38,50–54], with or without instrumentation, may have challenging anatomy for neuraxial techniques. With ankylosing spondylitis and significant scoliosis, ultrasonography may allow for the visualization of the spinal anatomy to determine if spinal anesthesia is feasible and if so, potentially predict the optimal needle trajectory to achieve success. However, there is no evidence that ultrasound would actually improve quality or success of neuraxial block in this specific population.
There is increasing interest in the use of ultrasonography to improve precision, accuracy and safety in the performance of neuraxial blocks by trainees. Spinal ultrasonography is also a relatively new topic of interest among anesthesiologists and the teaching of this technique is only now starting to enter residency training curriculums. A preliminary study by Grau et al. shows promising results on the utilization of ultrasound imaging for teaching epidural anesthesia in obstetric patients, as they found a higher rate of success with its use in new anesthesia residents [35]. Vallejo et al. also found that ultrasonography decreased the rate of epidural catheter replacement for failed labor analgesia and reduced the number of epidural attempts when performed by first year residents compared with traditional techniques [55].
Equipment
The resolution of most modern ultrasound units is capable of imaging the key structures of the spine and boundaries of the neuraxial space, although current ultrasound fidelity cannot clearly identify nerves and other structures within the neuraxial space (the space appears dark or hypoechoic) [56]. Unlike most anesthesiarelated uses of ultrasound for peripheral nerve blockade and vascular access, the actual target structure for neuraxial blockade is largely hidden by the bony structures of the laminae, spinous processes and facet joints. With realtime ultrasound-guided needle insertion, there is also poor needle tip visibility owing to narrow interlaminar spaces with bony shadows and the need for steep needle insertion angles. Despite these limitations, researchers using standard and novel equipment have described a number of feasible ultrasound-assisted techniques for neuraxial blockade.
High-frequency linear transducers may be used to assist in imaging the spinous processes and identifying the midline. However, a lowfrequency curvilinear transducer is required to image deeper structures such as the laminae, transverse processes and the dura [1]. Lowfrequency curvilinear transducers yield a slightly reduced image quality for nerves and vessels compared with high-frequency linear transducers, but provide a greater field of view, which is necessary to determine the ideal needle trajectory.
When preparing for preprocedural ultrasound scanning, the needle entry point may be marked with indelible ink prior to performing a sterile preparation of the area. For real-time ultrasound-guided neuraxial techniques, a sterile sheath for the transducer and sterile ultrasound conductive gel is necessary. Care must also be taken to ensure the needle entry point is free of gel, as there may be tracking into the neuraxial space [57] with unclear clinical consequences [58]. Mixing with chlorhexidine preparation solution should also be strictly avoided [59].
One limitation for real-time ultrasoundguided neuraxial blocks is the ability to visually track the needle due to bony shadows and steep angles. Use of fixed needle guides attached to the transducer have been described to maintain the needle within the ultrasound plane [60], as well as a novel split array transducer to accommodate a needle [101].
Recently, several other technologies have been investigated in various ex vivo and in vivo studies to improve needle localization and provide additional anatomical information. These include echogenic Tuohy needles that have a textured needle surface in order to improve ultrasound beam reflectivity [23,61]; Tuohy needles with an embedded highfrequency ultrasound transducer [62]; 3D/4D ultrasound systems that provide more detailed anatomical information [63,64]; and piezoelectric needle and catheter designs that permit distal tip visualization using color f low Doppler [65]. Most recently, a novel needle tracking system that uses an electromagnetic emitter and sensors in the needle and transducer, has been shown to allow for real-time tracking of the needle position relative to the ultrasound plane for neuraxial blockade [66,67]. Predicted trajectory and location of the needle tip are displayed digitally in real-time and overlaid on the ultrasound image.
Preprocedural ultrasound scanning
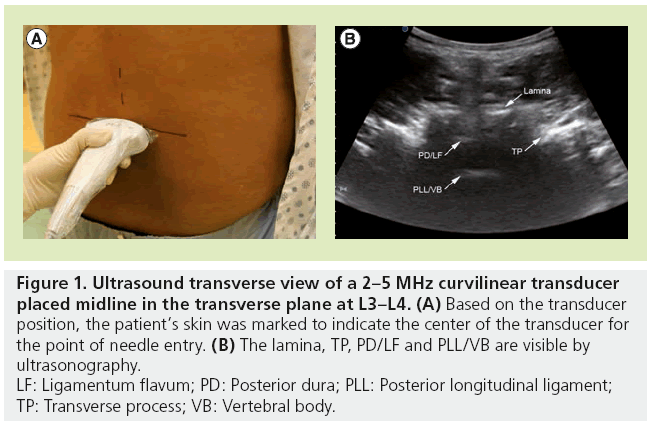
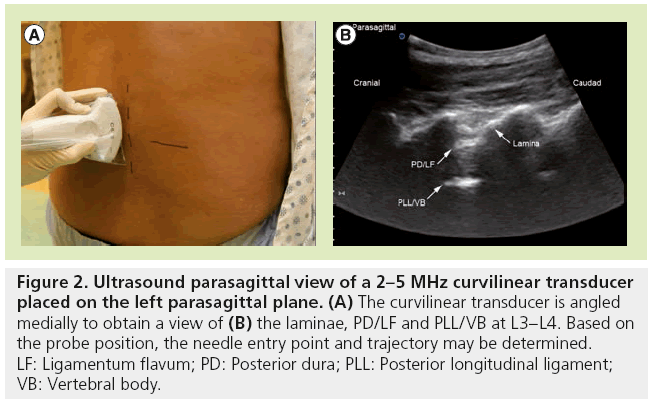
Preprocedural ultrasound scanning involves identification of the spinous processes, laminae and dura and provides useful information on the depth and optimal angle from the skin to the neuraxial space. The two standard views are the transverse (Figure 1) and parasagittal (Figure 2) views that would allow for identification of an ideal trajectory to the dura. Once the target is centered on the image, the skin is marked at the estimated center of the transducer. The needle is then inserted at this point with a trajectory similar to the transducer angle in the transverse and sagittal planes.
Figure 1: Ultrasound transverse view of a 2–5 MHz curvilinear transducer
placed midline in the transverse plane at L3–L4. (A) Based on the transducer
position, the patient’s skin was marked to indicate the center of the transducer for
the point of needle entry. (B) The lamina, TP, PD/LF and PLL/VB are visible by
ultrasonography.
LF: Ligamentum flavum; PD: Posterior dura; PLL: Posterior longitudinal ligament;
TP: Transverse process; VB: Vertebral body.
Figure 2: Ultrasound parasagittal view of a 2–5 MHz curvilinear transducer placed on the left parasagittal plane. (A) The curvilinear transducer is angled medially to obtain a view of (B) the laminae, PD/LF and PLL/VB at L3–L4. Based on the probe position, the needle entry point and trajectory may be determined. LF: Ligamentum flavum; PD: Posterior dura; PLL: Posterior longitudinal ligament; VB: Vertebral body.
Although preprocedural ultrasound imaging of the lumbar spine can help by providing additional anatomical information, the actual needle insertion remains a ‘blind’ technique. This approach requires the operator to accurately determine the center of the transducer head for the point of needle entry. In addition, the needle trajectory must accurately replicate the transducer angle in two planes and requires excellent visuospatial skills. If there is patient movement between the time of ultrasonography and needle insertion, the skin markings may be less useful; however, commercially available positioning systems may help limit this movement. Several investigative groups are now pursuing techniques that may make real-time ultrasound-guided neuraxial blocks feasible.
Real-time ultrasound scanning
With recent improvements in ultrasound technology, there is significant momentum to develop and apply clinically viable real-time ultrasound-guided neuraxial techniques. Case reports [50] and a limited number of studies [22,23,60,68,69] have described real-time ultrasound guidance for neuraxial blocks; however, these techniques have not yet been widely adopted for a number of reasons.
The neuraxial space is deep and access is limited by narrow interlaminar spaces. Therefore, there is limited space in the area around the spinous processes and laminae to accommodate a bulky ultrasound transducer and needle. With exception to the described technique where the patient is in the prone position [22], most realtime techniques have the patient in the sitting or lateral position. This requires the transducer to be precisely maintained on a vertical surface while manipulating a needle, which is challenging. In addition, needle visibility is poor due to steep insertion angles and bony acoustic shadows. To overcome some of these constraints, many described real-time techniques have required two operators [68], complex needleguide brackets [60], specialized loss of resistance devices [23,70] or the patient in a prone position [22]. Although these techniques may be useful in some difficult patients, they are not practical enough to be easily applied in everyday practice.
Unlike preprocedural scanning, which is often done in the midline for spinals, a midline ultrasound-guided approach to real-time neuraxial blockade has not been described. Although many anesthesiologists may favor the midline approach, particularly at lumbar levels, real-time techniques are best done via a paramedian approach with the transducer placed parasagittally. This is because the interlaminar window is larger from this angle compared with the midline, therefore, providing space to accommodate a transducer and needle. Longitudinal views along the midline tend to be of poor quality owing to shadowing caused by the spinous processes and poor contact with the skin over the spinous processes in slim patients.
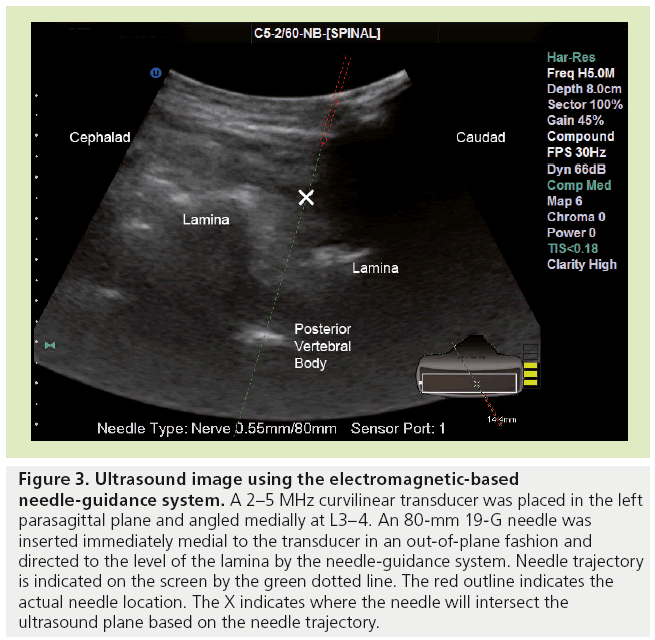
Recent work with an ultrasound-based electromagnetic needle tracking system has demonstrated that real-time neuraxial blocks are feasible using this technology [66]. This system was found to be successful in performing simulated spinal blocks in cadavers [66] and in a patient with previous spinal surgery [67]. Using the needle-guidance system, a proprietary introducer needle containing a sensor filament could be accurately placed along a trajectory towards the dura to the level of the lamina (Figure 3). After removing the sensor from the needle, a spinal needle could be inserted using a needle-through-needle technique towards the intrathecal space. This was performed using an out-of-plane technique in the cadaver study, with the transducer held in a parasagittal plane and the needle introduced immediately medial to the transducer [66]. An in-plane technique using the same system was described in a recently published case report [67].
Figure 3: Ultrasound image using the electromagnetic-based needle-guidance system. A 2–5 MHz curvilinear transducer was placed in the left parasagittal plane and angled medially at L3–4. An 80-mm 19-G needle was inserted immediately medial to the transducer in an out-of-plane fashion and directed to the level of the lamina by the needle-guidance system. Needle trajectory is indicated on the screen by the green dotted line. The red outline indicates the actual needle location. The X indicates where the needle will intersect the ultrasound plane based on the needle trajectory.
A novel real-time in-plane technique has recently been described in 100 orthopedic patients with a 97% success rate [69]. The authors used an oblique view of the spine which maximized the interlaminar window to allow space for manipulating the needle to the dura, and they predominantly used 22-gauge spinal needles to improve needle visibility.
Echogenic needles have allowed for increased visibility in peripheral nerve blocks, and the use of an echogenic Tuohy has been described for neuraxial blocks [23,61]. In the future, this needle may be useful for the placement of epidural catheters under real-time ultrasound guidance; however, this needle is not ideal for spinal anesthesia. Needles for spinal anesthesia are limited by the need for a small gauge and it will be interesting to see if the technology of echogenic needles can be applied to such needles. Other technologies to enhance needle visibility, such as piezoelectric needles, which emit vibrations from the needle tip that can be detected by Doppler ultrasound, are still in developmental stages [65].
With the standard technique of epidural placement, two hands are required, as one is required to manipulate the needle and the other to test for a loss of resistance. Owing to this, a real-time ultrasound technique requires another operator to hold the transducer. One described method to overcome this problem is the use of automated loss of resistance devices affixed to the needle, which indicates a change in the resistance that would allow a single operator [23,70].
Limitations of ultrasound for neuraxial techniques
■ Learning curve
Training in advanced ultrasonography is required and access to appropriate programs that utilize cadavers, animal models and volunteers, as well as live video demonstration are helpful in acquiring these skills. A study on skill acquisition for the performance of ultrasoundguided peripheral nerve blocks by Barrington et al. demonstrated the need for 28 supervised blocks before a trainee becomes competent [71]. Similar numbers of neuraxial blocks may be required for proceduralists to learn image optimization and recognition of normal and abnormal sonographic anatomy.
Despite the advantage previously stated of the use of ultrasound for teaching obstetrical epidural to residents, a study by Margarido et al. demonstrated that ultrasound-guided spinal anesthesia might be a skill more difficult to teach and learn than was previously thought and special attention should be given to the meticulous measurement of landmarks and distances [72]. This group also concluded that, in addition to the educational material, achieving competence in spinal ultrasound assessment requires a lecture, a demonstration workshop and more than 20 supervised trials.
■ Neuraxial ultrasonography
Appreciation of the problems that are unique to imaging the neuraxial region is critical to the safe and effective performance of ultrasoundguided spinal anesthesia. Acoustic shadowing occurs when superficial structures with a larger attenuation coefficient than deeper structures cause the latter to appear less echogenic than normal. This phenomenon is commonly encountered when the target area lies deep to the bone. Therfore, the spinous processes and laminae in adults impede penetration of ultrasound waves and results in decrement of signal from the ligamentum flavum and reduced ability to visualize it clearly.
Bony structures, such as the spinous processes and laminae, act as strong specular reflectors and create a ‘drop-out’ hypoechoic acoustic shadow deep to the bone that impairs the ability to visualize the neuraxial space clearly [73]. However, ultrasonography can help identify acoustic shadows from the spinous processes and, therefore, the midline in patients with challenging anatomy. This would at least offer the operator a starting point for needle entry, as well as the expected depth that the dura and any bony structures can be encountered.
■ Single-operator techniques
Since traditional neuraxial techniques only require one operator, a feasible real-time ultrasound approach should only require a single operator for it to be widely adopted in daily anesthesia practice. Several described techniques for spinal anesthesia only require one operator [22,23,60,69,70]; however, this may be difficult in epidural placement because the loss of resistance technique whilst holding the transducer seemingly requires a third hand. Described real-time epidural techniques have necessitated a second operator [68], needle guide brackets [60] or specialised loss-of-resistance devices that allow the Tuohy to be inserted with one hand [23,70].
■ Transducer design
Current neuraxial sonography requires the use of large curvilinear transducers to achieve adequate depth and field of view. The size of these transducers make them cumbersome to use in real-time procedures and difficult to maintain in one position on the back of a sitting patient. In addition, the size of the transducer limits the potential needle entry points to outside the transducer head. Unfortunately, with current technology, the reduction in transducer size is limited by the physics of ultrasonography.
■ Needle design & needle-tracking technology
The introduction of echogenic needles [23,61] and the electromagnetic needle-tracking technology [66,67] appears to show promise towards the development of single operator techniques that are real-time. In addition, it will be interesting to review the results of the clinical trial on the novel split array transducer, which appears to have a smaller profile and may accommodate a needle [101].
■ Cost & development of new technology
Although the costs of ultrasound equipment have decreased, the initial and maintenance costs, as well as technical support, may restrict some centers from its use. In addition, use of specialized novel equipment, such as the electromagnetic-based needle-guidance system, 3D/4D ultrasound systems, or ultrasound transducers embedded within the needle may be limited at this time.
Future of ultrasonography in neuraxial anesthesia
Ultrasonography has become an invaluable tool in anesthesia for the performance of procedures. Not long ago, there was little interest in performing peripheral nerve blocks because of the difficulty of locating nerves with traditional landmark-based techniques, the length of time required to perform the techniques and the relatively low success rates, except when done by experts. With the introduction and dissemination of ultrasoundguided techniques, there has been a significant increase in the interest in regional anesthesia, as evidenced by the relative increase in the size of regional anesthesia groups and growth in the membership of American Society of Regional Anesthesia, which is now the largest subspecialty anesthesia society.
The cost of acquiring high-quality ultrasound technology has decreased dramatically and ultrasound machines have become commonplace in operating rooms in most countries. Ultrasonography skills are being taught earlier in anesthesia training programs and these skills are a prerequisite for regional anesthesia fellowship programs [74].
However, this is not yet the case with ultrasound-guided neuraxial techniques, where almost all spinals and epidurals are still performed with traditional landmark-based techniques. In order for an ultrasound-based technique to be widely adopted, it must be shown either to improve safety or improve efficiency. Demonstrating an improvement in safety for neuraxial anesthesia is not feasible; however, there is now evidence to support the use of preprocedural ultrasonography to assist in patients with difficult anatomy.
In the future, a more widespread single-operator real-time technique may be developed, but this is contingent on several technical improvements. This will rely on modifications of the current transducer and needle design. The transducer shape and size needs to be reduced in order to improve ergonomics. Needles appropriate for neuraxial blockade with better shaft and tip visualization at acute angles need to be developed. Ridges in the needle design have improved visualization of peripheral nerve block needles and Tuohy needles [23,61]. However, the need for small gauge needles for spinal anesthesia may limit the applicability of this technology. With the electromagnetic needletracking system [66,67] and with the embedded high-frequency ultrasound transducer [62], the needle size is also limited by the need for a filament sensor or ultrasound crystal within the needle. However, these technologies may be applied to a Tuohy type needle for real-time ultrasound-guided placement of epidural catheters.
Where do we go from here?
Sonography of the neuraxial space with current ultrasound techniques yields 2D images in the transverse or sagittal planes. A coronal highresolution image that provides a ‘birds-eye’ view of the target area is not possible with 2D ultrasound. Preliminary investigations with 3D and 4D ultrasound of the paravertebral and neuraxial areas suggest interesting possibilities with this technology in the future [63,64]. Highresolution images in the transverse, sagittal and coronal planes provide anatomical information that is more detailed and not achievable with 2D ultrasound. The future application of such ‘volumetric’ ultrasound techniques of the neuraxial region may provide very detailed information on the relevant anatomy in a manner similar to CT. This type of detailed information may be helpful in planning approaches to spinal anesthesia in patients with challenging anatomy and in investigation of postoperative complications of neuraxial blocks.
3D/4D ultrasonography may increase image quality by overlapping images, but because the target structures are deep, it still relies on lowfrequency sones that cannot achieve the same resolution from a high-frequency transducer. Although one study has shown some promise for the use of 3D/4D ultrasonography for epidural catheter insertion [64], for the moment this technology is limited to retrospective image acquisition rather than prospective real-time imaging. One key limitation is the processing speed of the 3D/4D ultrasound units, which creates a delay in rendering the image from multiple slices. With faster processors in the units, real-time 3D-guided blocks may be possible in the future.
Conclusion
There have been numerous technological advancements over the past century that have made anesthesia safer and more reliable. The introduction of ultrasound is arguably the single biggest advancement in regional anesthesia after the basic understanding of neural anatomy and the introduction of local anesthetics. It is quite possible that in the not too distant future landmark- and nerve stimulator-based peripheral nerve blocks will be completely replaced by ultrasound- based techniques.
The future of ultrasonography in neuraxial anesthesia is less clear. With our current technology, ultrasound-based neuraxial anesthesia is limited to a relatively small number of practitioners, many of whom have been self-taught. Preprocedural scanning has been demonstrated to reduce the number of needle passes required for difficult spinals, but it is limited by the need to replicate an estimated needle trajectory after the removal of the ultrasound transducer. Patient movement may also limit the success of this technique.
The future research in ultrasound-guided neuraxial anesthesia may be in improvements in real-time techniques. The ideal technique must be practical; easy to learn and use; improve efficiency; require a single operator; have a minimal set up time; and offer the same reliability and safety as conventional spinal and epidural anesthesia, without greatly increased cost. In addition, the needle sizes available for spinal techniques must be similar to the currently available spinal needles. Currently, none of the described real-time techniques meet all these criteria; however, it is an evolving field dependent on technological improvements. It is not yet clear which of these technologies will emerge as the best option for this field.
Future perspective
Having seen the positive effects ultrasonography has had on improving peripheral nerve blocks, there is now significant momentum to apply this technology to neuraxial blocks. We believe the most significant change in this area will be in the development of a singleoperator real-time ultrasound-guided approach that could be widely applied to patients with normal and abnormal anatomy. The technique will utilize a paramedian or oblique view of the spine and enhanced needle visualization from improvements in needle or needletracking technology. Such a system will allow for a reduction in needle passes and improve operating room efficiency.
Financial & competing interests disclosure
A Sawka has received equipment and travel support from Ultrasonix. R Tang has received equipment and travel support for being a speaker at the Canadian Anesthesia Society Meeting 2012. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Sandhu NS. Ultrasound imaging in anesthesia: an overview of vascular access and peripheral nerve blocks. Semin. Anesth. Perioperat. Med. Pain 26(4), 197–209 (2007).
- Rupp SM, Apfelbaum JL, Blitt C et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology 116(3), 539–573 (2012).
- Kessler J, Bolger AF, Gray AT. An essential skill. Reg. Anesth. Pain Med. 31(6), 498–500 (2006).
- Sites BD. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol. Scand. 52, 456–466 (2008).
- Marhofer P, Chan VW. Ultrasound-guided regional anesthesia: current concepts and future trends. Anesth. Analg. 104(5), 1265–1269 (2007).
- Sites BD, Brull R. Ultrasound guidance in peripheral regional anesthesia: philosophy, evidence-based medicine, and techniques. Curr. Opin. Anaesthesiol. 19, 630–639 (2006).
- Marhofer P, Kettner SC, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: part 1. Br. J. Anaesth. 104(5), 538–546 (2010).
- la Grange P, Foster PA, Pretorius LK. Application of the Doppler ultrasound bloodflow detector in supraclavicular brachial plexus block. Br. J. Anaesth. 50(9), 965–967 (1978).
- Kapral S, Krafft P, Eibenberger K, Fitzgerald R, Gosch M, Weinstabl C. Ultrasoundguided supraclavicular approach for regional anesthesia of the brachial plexus. Anesth. Analg. 78(3), 507–513 (1994).
- Gelfand HJ, Jean-Pierre O, Lesley MR et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J. Clin. Anesth. 23(2), 90–96 (2011).
- Brull R, Lupu M, Perlas A, Chan VW, McCartney CJ. Compared with dual nerve stimulation, ultrasound guidance shortens the time for infraclavicular block performance. Can. J. Anesth. 56(11), 812–818 (2009).
- Antonakakis JG, Ting PH, Sites BD. Ultrasound-guided regional anesthesia for peripheral nerve blocks: an evidence-based outcome review. Anesthesiol. Clin. 29(2), 179–191 (2011).
- Koscielniak-Nielsen ZJ, Dahl JB. Ultrasoundguided peripheral nerve blockade of the upper extremity. Curr. Opin. Anaesthesiol. 25(2), 253–259 (2012).
- Abrahams MS, Aziz MF, Fu RF, Horn J. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and metaanalysis of randomized controlled trials. Br. J. Anaesth. 102(3), 408–417 (2009).
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br. J. Anaesth. 103(3), 428–433 (2009).
- Neal JM. Ultrasound-guided regional anesthesia and patient safety. Reg. Anesth. Pain Med. 35(2), 59–67 (2010).
- Hebl J. Ultrasound-guided regional anesthesia and the prevention of neurologic injury: fact or fiction? Anesthesiology 108, 186–188 (2008).
- Neal JM, Bernards CM, Hadzic A et al. ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine. Reg. Anesth. Pain Med. 33(5), 404–415 (2008).
- Orebaugh SL, Kentor ML, Williams BA. Adverse outcomes associated with nerve stimulator-guided and ultrasound-guided peripheral nerve blocks by supervised trainees: update of a single-site database. Reg. Anesth. Pain Med. 37(6), 577–582 (2012).
- Munhall RJ, Sukhani R, Winnie AP. Incidence and etiology of failed spinal anesthetics in a university hospital: a prospective study. Anesth. Analg. 67(9), 843–848 (1988).
- Chin KJ, Perlas A. Ultrasonography of the lumbar spine for neuraxial and lumbar plexus blocks. Curr. Opin. Anaesthesiol. 24(5), 567–572 (2011).
- Lee PJ, Tang R, Sawka A, Krebs C, Vaghadia H. Brief report: real-time ultrasound-guided spinal anesthesia using Taylor’s approach. Anesth. Analg. 112(5), 1236–1238 (2011).
- Brinkmann S, Mitchell CH, Hocking G. Assessment of a single-operator real-time ultrasound-guided epidural technique in a porcine phantom. Can. J. Anesth. 59(3), 323–324 (2012).
- Atallah MM, Demian AD, Shorrab AA. Development of a difficulty score for spinal anaesthesia. Br. J. Anaesth. 92(3), 354–360 (2004).
- Ellinas EH, Eastwood DC, Patel SN, Maitra-D’Cruze AM, Ebert TJ. The effect of obesity on neuraxial technique difficulty in pregnant patients: a prospective, observational study. Anesth. Analg. 109(4), 1225–1231 (2009).
- Sprung J, Bourke DL, Grass J et al. Predicting the difficult neuraxial block: a prospective study. Anesth. Analg. 89(2), 384–389 (1999).
- Tessler MJ, Kardash K, Wahba RM, Kleiman SJ, Trihas ST, Rossignol M. The performance of spinal anesthesia is marginally more difficult in the elderly. Reg. Anesth. Pain Med. 24(2), 126–130 (1999).
- Chin KJ, Perlas A, Chan V et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology 115(1), 94–101 (2011).
- Furness G, Reilly M, Kuchi S. An evaluation of ultrasound imaging for identification of lumbar intervertebral level. Anaesthesia 57(3), 277–280 (2002).
- Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth. Analg. 106(2), 538–540 (2008).
- Yamauchi M, Honma E, Mimura M, Yamamoto H, Takahashi E, Namiki A. Identification of the lumbar intervertebral level using ultrasound imaging in a postlaminectomy patient. J. Anesth. 20(3), 231–233 (2006).
- Perlas A. Evidence for the use of ultrasound in neuraxial blocks. Reg. Anesth. Pain Med. 35(2), S43–S46 (2010).
- Grau T, Horter J, Conradi R, Martin EO, Motsch J. Paramedian access to the epidural space: the optimum window for ultrasound imaging. J. Clin. Anesth. 13, 213–217 (2001).
- Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth. Analg. 104(5), 1188–1192 (2007).
- Grau T, Bartusseck E, Conradi R, Martin E, Motsch J. Ultrasound imaging improves learning curves in obstetric epidural anesthesia: a preliminary study. Can. J. Anesth. 50(10), 1047–1050 (2003).
- Carlos J, Carvalho A. Ultrasound-facilitated epidurals and spinals in obstetrics. Anesthesiol. Clin. 26, 145–158 (2008).
- Chin KJ, Perlas A, Singh M et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can. J. Anesth. 56(9), 643–650 (2009).
- Prasad A, Tumber PS, Lupu CM. Correspondance: ultrasound guided spinal anesthesia. Can. J. Anesth. 55(10), 716–717 (2008).
- Schisler T, Huttunen H, Tang R, Vaghadia H. Ultrasound-assisted spinal anaesthesia in a patient with Wildervanck syndrome and congenital abnormalities of the lumbar spine. Br. J. Anaesth. 109(2), 290–291 (2012).
- Fanning N, Arzola C, Balki M, Carvalho JC. Lumbar dural sac dimensions determined by ultrasound helps predict sensory block extent during combined spinal-epidural analgesia for labor. Reg. Anesth. Pain Med. 37(3), 283–288 (2012).
- Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol. Scand. 45(6), 766–771 (2001).
- Weed J, Finkel K, Beach ML, Granger CB, Gallagher JD, Sites BD. Spinal anesthesia for orthopedic surgery: a detailed video assessment of quality. Reg. Anesth. Pain Med. 36(1), 51–55 (2011).
- Li SL, Wang DX, Ma D. Epidural hematoma after neuraxial blockade: a retrospective report from China. Anesth. Analg. 111(5), 1322–1324 (2010).
- Lerner SM, Gutlerman P, Jenkins F. Epidural hematoma and paraplegia after numerous lumbar punctures. Anesthesiology 39(5), 550–551 (1973).
- Horlocker TT, Wedel DJ, Schroeder DR et al. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth. Analg. 80(2), 303–309 (1995).
- Harrison DA, Langham BT. Spinal anaesthesia for urological surgery. A survey of failure rate, postdural puncture headache and patient satisfaction. Anaesthesia 47(10), 902–903 (1992).
- Horlocker TT, Schroeder DR, McGregor DG, Matsushige DK, Besse JA. A retrospective review of 4767 consecutive spinal anesthetics: central nervous system complications. Perioperative Outcomes Group. Anesth. Analg. 84(3), 578–584 (1997).
- Schelew BL, Vaghadia H. Ankylosing spondylitis and neuraxial anaesthesia – a 10 year review. Can. J. Anesth. 43(1), 65–68 (1996).
- McLeod A, Roche A, Fennelly M. Case series: ultrasonography may assist epidural insertion in scoliosis patients. Can. J. Anesth. 52(7), 717–720 (2005).
- Chin KJ, Chan VW, Ramlogan R, Perlas A. Real-time ultrasound-guided spinal anesthesia in patients with a challenging spinal anatomy: two case reports. Acta Anaesthesiol. Scand. 54(2), 252–255 (2010).
- Minville V, Lavidalle M, Bayoumeu F, Parrant O, Fourcade O. Rachianesthésie assistée par l’échographie chez une parturiente avec une scoliose corrigée. Ann. Fr. Anesth. Reanim. 29(6), 501–502 (2010).
- Chin KJ, Macfarlane AJ, Chan V, Brull R. The use of ultrasound to facilitate spinal anesthesia in a patient with previous lumbar laminectomy and fusion: a case report. J. Clin. Ultrasound 37(8), 482–485 (2009).
- Yeo ST, French R. Combined spinal–epidural in the obstetric patient with Harrington rods assisted by ultrasonography. Br. J. Anaesth. 83(4), 670–672 (1999).
- Costello JF, Balki M. Cesarean delivery under ultrasound-guided spinal anesthesia, in a parturient with poliomyelitis and Harrington instumentation. Can. J. Anesth. 55(9), 606–611 (2008).
- Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int. J. Obstet. Anesth. 19(4), 373–378 (2010).
- Tsen LC. The all-seeing eye? Ultrasound technology for neuraxial techniques. Anesthesiology 114(6), 1274–1276 (2011). 57 Belavy D. Brief reports: regional anesthesia needles can introduce ultrasound gel into tissues. Anesth. Analg. 111(3), 811–812 (2010).
- El-Dawlatly A, Kathiry K, Al Rikabi A, Hajjar W, Al Obaid O, Alzahrani T. Ultrasound gel–nerve contact: an experimental animal histologic study. Anesth. Analg. 113(3), 657–659 (2011).
- Killeen T, Kamat A, Walsh D, Parker A, Aliashkevich A. Severe adhesive arachnoiditis resulting in progressive paraplegia following obstetric spinal anaesthesia: a case report and review. Anaesthesia 67(12), 1386–1394 (2012).
- Tran D, Kamani AA, Al-Attas E, Lessoway VA, Massey S, Rohling RN. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can. J. Anesth. 57, 313–321 (2010).
- Hebard S, Hocking G. Echogenic technology can improve needle visibility during ultrasound-guided regional anesthesia. Reg. Anesth. Pain Med. 36(2), 185–189 (2011).
- Chiang HK, Zhou Q, Mandell MS et al. Eyes in the needle: novel epidural needle with embedded high-frequency ultrasound transducer – epidural access in porcine model. Anesthesiology 114(6), 1320–1324 (2011).
- Karmakar MK, Li X, Li J, Hadzic A. Technical communication: volumetric threedimensional ultrasound imaging of the anatomy relevant for thoracic paravertebral block. Anesth. Analg. 115(5), 1246–1250 (2012).
- Belavy D, Ruitenberg MJ, Brijball RB. Feasibility study of real-time three-/fourdimensional ultrasound for epidural catheter insertion. Br. J. Anaesth. 107(3), 438–445 (2011).
- Klein SM, Fronheiser MP, Reach J, Nielsen KC, Smith SW. Piezoelectric vibrating needle and catheter for enhancing ultrasound-guided peripheral nerve blocks. Anesth. Analg. 105(6), 1858–1860 (2007).
- Brinkmann S, Tang R, Vaghadia H, Sawka A. Assessment of a real-time ultrasound-guided spinal technique using SonixGPSTM in human cadavers. Can. J. Anesth. 59(12), 1156–1157 (2012).
- Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can. J. Anesth. 60(1), 50–53 (2013).
- Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur. J. Anaesthesiol. 21, 25–31 (2004).
- Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol. Res. Prac. doi:10.1155/2013/525818 (2013) (Epub ahead of print).
- Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br. J. Anaesth. 102(6), 845–854 (2009).
- Barrington MJ, Wong DM, Slater B, Ivanusic JJ, Ovens M. Ultrasound-guided regional anesthesia: how much practice do novices require before achieving competency in ultrasound needle visualization using a cadaver model. Reg. Anesth. Pain Med. 37(3), 334–339 (2012).
- Margarido CB, Arzola C, Balki M, Carvalho JCA. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can. J. Anesth. 57(2), 120–126 (2010).
- Sites BD, Brull R, Chan VWS et al. Artifacts and pitfall errors associated with ultrasoundguided regional anesthesia. Part II: a pictorial approach to understanding and avoidance. Reg. Anesth. Pain Med. 32(5), 419–433 (2007).
- Regional Anesthesiology and Acute Pain Medicine Fellowship Directors Group. Guidelines for fellowship training in regional anesthesiology and acute pain medicine: second edition, 2010. Reg. Anesth. Pain Med. 36(3), 282–288 (2011).
- A trial of the use of ultrasound to aid the insertion of combined spinal epidural anaesthesia. http://clinicaltrials.gov/ct2/show/ NCT00166699
• Describes the application of ultrasonography of the spine to facilitate neuraxial and lumbar plexus blockade.
• • Randomized controlled trial demonstrating that ultrasound guidance significantly reduces technical difficulty of spinal anesthesia in the older patient population with poor-quality surface landmarks.
• Letter to the editor describing that an electromagnetic needle-tracking system facilitated real-time ultrasound-guided neuraxial injection in cadavers.
• • Case study describing a novel approach to single-operator real-time ultrasound-guided spinal anesthesia using an electromagnetic needle tracking system.
■ Website