Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Is there a role for embolic protection during treatment of critical limb ischemia?
- Corresponding Author:
- Robert Lookstein
Green Lane Cardiovascular Service, Auckland City Hospital Park Road, Auckland, New Zealand
Tel: +1 212 241 7409
Fax: +1 212 348 7403
E-mail: robert.lo okstein@mssm.edu
Abstract
Keywords
angioplasty, critical limb ischemia, distal embolization, embolic protection device, endovascular, stent, thrombolysis
Critical limb ischemia & role of lower extremity arterial interventions
Patients with critical limb ischemia (CLI) represent a subset of patients with peripheral arterial disease presenting with chronic ischemic rest pain persisting for more than 2 weeks for ischemic skin lesions, including gangrene and ulcers. Although the natural history of CLI is not well delineated, a diagnosis of CLI imparts a poor prognosis. Within 1 year of diagnosis, a quarter of CLI patients will die and a quarter will require amputation. Overall treatment strategies are designed to prolong survival, improve patient function and quality of life, relieve pain, heal ulcers and prevent limb loss. Revascularization is a mainstay of management and is the treatment of choice in this patient population in order to restore a functional and pain-free extremity [1].
Endovascular therapy has gained acceptance and popularity as a highly valuable treatment modality for patients with CLI. Many patients suffering from CLI may otherwise be poor surgical candidates owing to concomitant cardiovascular disease and other medical comorbidities, thus rendering minimally invasive endovascular intervention highly desirable. The gold standard for revascularization is surgical bypass utilizing autologous vein. Some patients may not have a suitable conduit to permit surgical bypass with the autologous vein. Therefore, these patients are also candidates for endovascular management. Available and widely used endovascular interventions include balloon angioplasty, stent placement, directional atherectomy, pharmacologic thrombolysis and mechanical thrombectomy.
Based on the original TransAtlantic Inter- Society Consensus (TASC) recommendations, bypass surgery was more commonly performed for long segment disease and critical limb ischemia, while angioplasty was reserved for patients with intermittent claudication and limited disease [2]. Owing to this, very few studies examined the outcomes of bypass surgery compared with endovascular therapy for the management of infrainguinal arterial disease. There is now strong evidence to support the role of endovascular intervention as an acceptable treatment option for patients with severe limb ischemia [3]. The Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial prospectively compared the outcome of primary bypass surgery with primary balloon angioplasty in 452 patients presenting with severe limb ischemia due to infrainguinal arterial disease over a period of 5.5 years. The primary end points of the study were amputation of the trial leg or death. It was shown that there is no significant difference in patient outcomes between the two study groups after 6 months. Additional benefits of primary endovascular intervention included reduced short-term morbidity, reduced hospital costs and similar health-related quality of life [3]. The Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) document cites the BASIL study as evidence to support the role of endovascular treatment of infrainguinal disease in patients with intermittent claudication. Given the associated low morbidity and mortality, endovascular interventions such as angioplasty are considered to be the first-line therapy for patients with limited disease including short segment stenoses or occlusions (<10 cm) [1]. The conclusion may be drawn that endovascular management represents an established treatment modality in patients who are not otherwise ideal candidates for surgical intervention.
Incidence & significance of distal thromboembolism during routine intervention
An important complication of lower extremity peripheral arterial intervention is distal embolization of liberated or generated material. The consequence of distal embolization resulting in occlusion of the vascular bed in patients with poor arterial inflow, poor collateralization or poor run-off may be devastating. This complication may require the use of additional intervention including thrombectomy or thrombolysis, resulting in longer procedure time, greater volumes of contrast administered and increased radiation exposure [4]. If subsequent attempts at thrombolysis or thrombectomy are unsuccessful, amputation may be necessary.
The incidence of distal embolization during lower extremity arterial intervention is higher than initially reported. The incidence of distal embolization detected angiographically or clinically was initially reported as 4–5%; however, contemporary studies have demonstrated a much higher incidence of 3.8–67% [4–11]. Limb-threatening distal embolization occurred in approximately 2% of patients during routine intervention [12,13]. The greatest amount of distal embolization occurred with the use of directional atherectomy devices, mechanical thrombectomy and pharmacologic thrombolysis [4,7–9]. This stark rise in the incidence of distal embolization may be due to the increased utilization of contemporary endovascular tools, including atherectomy and thrombectomy devices. These devices have been studied and reviewed by core angiographic laboratories in order to support approval of such devices and which has subsequently resulted in the demonstration of a much higher incidence of distal embolization. The quality of conventional digital subtraction angiography continues to improve with technological advances, resulting in improved resolution and better detection of small emboli.
Distal embolic protection devices (EPDs) have been effectively employed for carotid, coronary and renal arterial interventions, and have become the standard of care in many clinical settings [14–16]. EPDs have been used with success during renal artery stenting, and have been associated with an improvement in glomerular filtration rate when used in combination with glycoprotein IIb/IIIa inhibitors [17]. A recent meta-analysis by Garg et al. demonstrated that the use of EPDs was associated with a reduced risk of perioperative stroke in patients undergoing carotid artery stenting [18]. This great success has led to the application of EPD technology to the lower extremity arterial circulation. It is worth noting that there are no currently available EPDs that are approved or designed for infrainguinal use.
Currently available EPDs
Two general types of EPDs are currently available for use. Balloon-based devices provide wall contact and achieve improved seal compared with filter-based devices. The disadvantage of the balloon-based device is that they provide complete arterial occlusion, which is not ideal in patients with CLI and compromised arterial inflow. Filter-based devices are more commonly used and function by deploying a perforated membrane or Nitinol mesh over a guidewire distal to the lesion. The benefit of this device is that small pores in the filter device maintain arterial inflow. The drawback is a larger profile, risk of vessel spasm and incomplete seal, which may prove problematic in tortuous diseased arteries with eccentric lumens [19]. The large pore size of currently available filters may also prove problematic. Currently available filter devices include the PercuSurge GuardWire® (Medtronic, MN, USA), TriActiv FX® (Kensey Nash, PA, USA), AngioGuard™ (Cordis, NJ, USA), Filterwire EZ™ (Boston Scientific, MA, USA), Spider FX® (ev3, MN, USA), Rubicon (Boston Scientific, MA, USA), Interceptor Plus (Medtronic), RX ACCUNET™ (Abbott, IL, USA), FiberNet® (Lumen Biomedical, MN, USA) and Emboshield® Pro (currently NAV6; Abbott, IL, USA; previously NeuroShield™, MedNova, Ireland).
FiberNet is a recently approved device that incorporates novel technology, providing for both excellent filtration and preservation of physiologic inflow. The FiberNet filter uses a 3D structure comprised of numerous strands of polymer fibers that deploy in a radial fashion through which there are many pathways for blood to f low distally. Debris as small as 40 μm may be captured while preserving adequate forward flow. This device may be used in lesions requiring a short landing zone and a low crossing profile while providing for excellent wall apposition, even at sites of vessel angulation. Prior to catheter retrieval, suction of debris at the base of the filter is initially performed in addition to continuous suction during subsequent filter wire removal. Henry et al. demonstrated the success and feasibility of this technology in the carotid circulation without evidence of wall damage [20].
Previous experience with EPD in chronic limb ischemia: a review of the literature
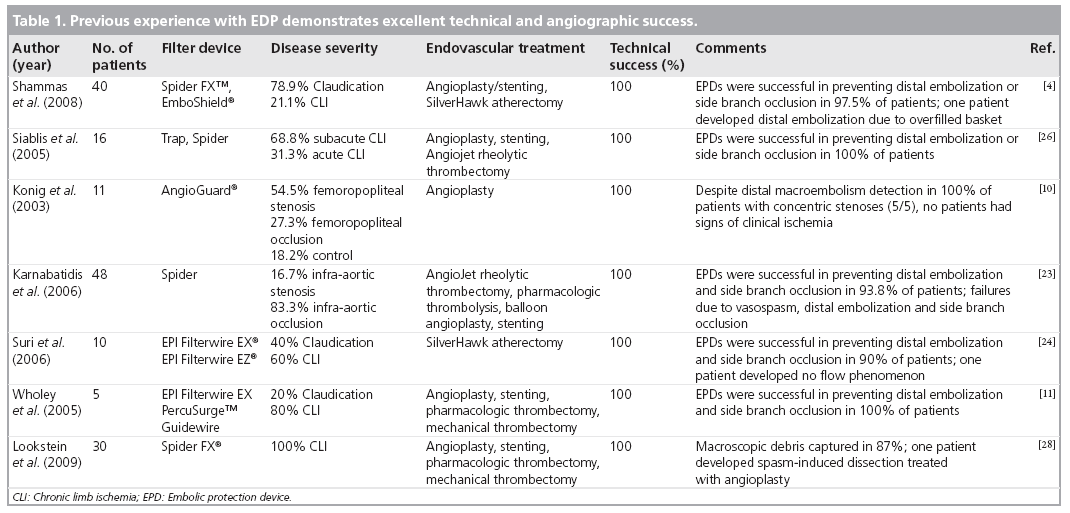
Preliminary work has demonstrated the safety and feasibility of the use of EPDs in the infrainguinal arterial system (Table 1). Clinical use is increasing despite a lack of a clear consensus regarding which patients are at the highest risk for distal embolization and which anatomy is appropriate for use of this technology.
Defining which features of a lesion impart a higher risk for distal embolization remains unclear and a source of controversy. Features separating patients with stable claudication from those with ongoing ischemia include the length of the stenosis and the length of the lesion, presence of distal run–off and chronicity of disease [21,22]. The peripheral microcirculation in ischemic patients has been compromised, and any further downstream embolization may result in worsening or persistent ischemia. This is supported by the findings of Karnabatidis in which histologic analysis demonstrated a greater amount of collected particles in the filter baskets positively correlating with increased lesion length and reference vessel diameter, acute thrombosis and total vessel occlusion [23]. Embolism has also been reported in patients with concentric stenoses [10,24]. These factors highlight the importance of EPD use in ischemic patients [11]. Furthermore, thrombus that may be dislodged during the intervention poses a significant risk [22]. Freeman et al. defines high-risk patients as those with limited distal run–off, vulnerable or unstable plaque, history of thromboembolic disease or aneurysmal disease [19]. Shammas et al. state that predicting which vessels will embolize based on lesional characteristics is not possible [4]. In a study by Lam et al., there was no difference in sonographically detected embolic signals between TAIC classifications [25]. It seems clear that patients with pre-existing thrombi would undoubtedly benefit from EPD use, and that further research is necessary to clarify which atherosclerotic lesions are at greatest risk for distal embolization.
It has been well established that patients undergoing pharmacologic or mechanical thrombectomy are at a significantly higher risk for distal embolization. The rate of distal embolization during thrombolytic therapy in limb-threatening ischemia has been reported as 3.8–37% [7–9]. Wholey et al. examined several patients, many of whom underwent either mechanical or pharmacologic thrombolysis, and determined that the use of EPD allowed for earlier intervention (in cases that otherwise would have required additional thrombolytic treatment or in cases in which the thrombus did not lyse) and provided further security when performing angioplasty and stent placement in these patients [11]. In a report by Siablis et al., the safety and feasibility of EPD during revascularization procedures utilizing angioplasty/ stenting and pharmacologic thrombolysis was demonstrated. The authors experienced a 100% success in deployment and utilization of the device and 100% recanalization rate without clinical or angiographic evidence of periprocedural distal embolization [26]. The Prophylaxis of Thromboembolism in Critical Care Trial (PROTECT) evaluated the safety and effectiveness of EPD in reducing distal embolization during percutaneous lower extremity interventions including angioplasty/ stenting and directional atherectomy. All patients undergoing directional atherectomy with the SilverHawk device demonstrated significant macroembolization, and 37.9% of patients undergoing angioplasty and or stenting demonstrated significant macroembolization [4]. The use of the directional atherectomy device was shown to be the strongest predictor of distal embolization [4,23]. The use of the EPD was shown to be effective in capturing the debris and was associated with a good angiographic outcome [4]. These findings were further supported by a recent study by Suri et al., in which ten patients with compromised run–off underwent directional atherectomy using the SilverHawk device. A significant amount of embolic debris was created by the atherectomy device, which was successfully captured by the EPD [24]. The use of embolic protection during atherectomy should be considered as an important adjunct.
Experience at our institution
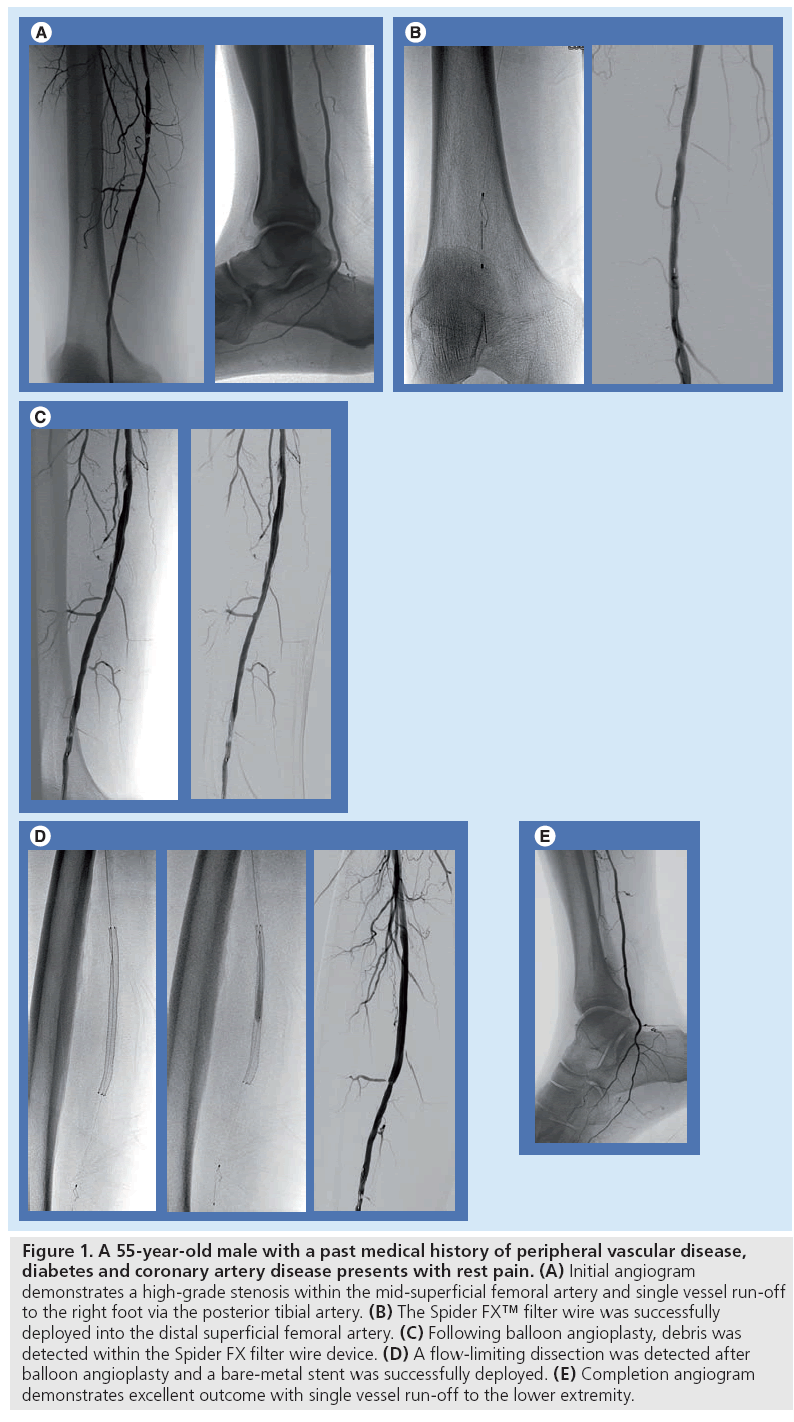
At our institution, we identify patients with CLI who are most vulnerable to major adverse sequella of thromboembolism using noninvasive modalities, including ankle-brachial indices, pulse volume recording, computerized tomographic angiography or magnetic resonance angiography. These studies illustrate which patients have compromised arterial run–off, poor arterial collateralization, extensive atherosclerotic disease characterized by dense calcification, vulnerable plaque and total occlusions. We have preferentially used the Spider distal protection device since it may be delivered over an independent 0.014” guidewire. To date, we have successfully used Spider EPD for embolic protection during endovascular procedures in the infrainguinal arterial system for CLI in 35 patients. The patients included 19 men and 16 women, aged 47–84 years. The Rutherford scale was used to assess a group of 18 patients with ischemic rest pain (Rutherford scale 4), 12 patients with minor tissue loss include toe or heel ulcers (Rutherford scale 5) and five patients with gangrene of the affected limb (Rutherford scale 6). A total of 64 lesions including 38 stenoses and 26 occlusions were treated with angioplasty and stent placement (n = 50) and/or mechanical thrombectomy (n = 14) under distal embolic protection. Distal filters were deployed in the popliteal artery during treatment of femoropopliteal disease in 24 patients and in the infrapopliteal arteries above the ankle to treat tibial occlusive disease in 11 patients. Mean lesion length was 37 mm (10–270 mm). All procedures were technically successful and macroscopic debris was captured in 87% of cases (Figure 1). No patients had clinical or angiographic evidence of distal embolization to the infrapopliteal vessels at the completion of the procedure. In three patients, the filters were completely filled with debris resulting in loss of distal flow to the extremity. Flow was restored in each case after the filters were retrieved. As a result of these encouraging initial results, our standard protocol for endovascular intervention in a patient with severely compromised infrapopliteal run–off is to preferentially utilize EPD during revascularization. We have found this technology applicable during revascularization involving the femoropopliteal arterial system as well as the infrapopliteal arterial segments.
Figure 1: A 55-year-old male with a past medical history of peripheral vascular disease, diabetes and coronary artery disease presents with rest pain. (A) Initial angiogram demonstrates a high-grade stenosis within the mid-superficial femoral artery and single vessel run-off to the right foot via the posterior tibial artery. (B) The Spider FX™ filter wire was successfully deployed into the distal superficial femoral artery. (C) Following balloon angioplasty, debris was detected within the Spider FX filter wire device. (D) A flow-limiting dissection was detected after balloon angioplasty and a bare-metal stent was successfully deployed. (E) Completion angiogram demonstrates excellent outcome with single vessel run-off to the lower extremity.
Indications for EPD in peripheral intervention
Based on previous work and the experience at our institution, there are several indications for use of EPD in peripheral arterial intervention. In patients whom preprocedure imaging evaluation demonstrates the presence of poor arterial inflow, collateral circulation or arterial run–off, the use of EPD should be considered during lower extremity revascularization procedures.
The use of a filter device is especially important in patients undergoing revascularization interventions with a higher risk of distal thromboembolism, including mechanical or pharmacologic thrombolysis, directional atherectomy, angioplasty or stenting.
While it has not been clearly established which features and characteristics of an atherosclerotic lesion portend a higher risk of distal thromboembolism, certain factors should be taken into consideration when making the decision to use an EPD. Common sense indicates that EPD use may be of value in a lesion with vulnerable or unstable plaque, acute thromboses, chronic total occlusions or aneurismal disease.
While many indications for EPD use in lower extremity peripheral arterial intervention exist, additional considerations including reference vessel diameter, lesion location and the presence or absence of a suitable landing zone need to be taken into account prior to the procedure. Further work is necessary to establish clear indications for the use of such technology in patients undergoing endovascular peripheral intervention.
Inherent limitations of the use of embolic protection devices in CLI
There are several inherent limitations to the use of EPD in the lower extremity arterial circulation in patients with CLI. While in many circumstances, EDP may be placed through a 4F or 5F catheter and easily advanced distally, gaining access to selected vessels and through selected lesions including chronic total occlusions may pose a challenge. The EPD devices are routinely mounted on medium support wires, which are not intended to function as primary crossing wires. This may necessitate the need for lesion crossing with a separate or smaller wire and vessel predilation, during which embolic debris may be generated and liberated [10,23].
Embolic protection devices typically require a rather long length of vessel for safe implantation, which may pose a challenge since many patients with CLI have extensive and diffuse atherosclerotic disease. Incomplete apposition of the device to the vessel wall may allow side escape of engendered debris, thus resulting in distal embolization. Distal embolization has been reported during filter deployment [10,23]. Furthermore, arterial spasm, arterial injury (including dissection) and de novo thrombus may occur as a result of the EPD device itself [23]. Muller-Hulsbeck et al. evaluated ex vivo porcine carotid arteries in order to evaluate the extent of vessel wall damage caused by EPD designed for carotid angioplasty. The devices studied included the Angioguard™, Filterwire EX™, TRAP (which is no longer commercially available), Neurosurge and PercuSurge®. All devices were shown to cause histologic vessel wall damage [27].
Revascularization procedures often result in the production of large amounts of macroscopic debris. The size of the filter basket may be inadequate for collection of debris. A large amount of collected debris can result in loss of antegrade blood flow [10]. Should this occur, aspiration of engendered debris or expeditious device removal is necessary in order to restore flow in patients with compromised arterial inflow. Extreme care must be taken since filter wire retrieval may also result in dislodgement of debris due to squeezing of the basket. Larger clots may also remain outside the struts of the filter and are too large to be removed by filter closing and standard resheathing techniques.
Embolic protection devices provide no protection to the collateral circulation. Therefore, the collateral vessels are vulnerable to the sequella of distal embolization should it occur. It is imperative that the operator be aware of these risks, and he/she must frequently assess the EPD with angiography and address potential complications promptly.
Conclusion
Many initial studies have demonstrated that the incidence of distal embolization during peripheral arterial intervention is higher than originally believed. The current role in lower extremity arterial intervention is not well established; however, initial studies suggest that embolic protection be considered in high-risk patients, including patients with CLI and patients undergoing interventions with high risk of distal thromboembolism, such as atherectomy. Although clearly identifying which patients and which lesional characteristics are at highest risk for distal embolization remains to be determined, the outcome of such events may prove disastrous in patients with compromised distal run–off and poor collateralization. Given the well-documented distal thromboembolism occurring in thrombolysis procedures, EPD protection allows for a more aggressive approach to revascularization in patients with acute limb ischemia. Prospective randomized trials are necessary in order to determine the clinical relevance of distal embolization and to determine the benefit of EPD use in patients with peripheral artery disease, and more specifically, CLI.
Future perspective
In order to routinely employ EPDs during lower extremity peripheral arterial intervention, a number of outstanding questions need to be addressed. Randomized trials are necessary in order to compare the outcomes of infrainguinal revascularization procedures in patients with CLI, with and without the use of an EPD, with particular attention paid to the incidence of adverse events. A benefit of such a study is that information regarding the frequency of devastating distal thromboembolism with compromised arterial inflow can be determined in the control arm. Through additional work, it may be possible to better define which patients and which anatomic or lesion characteristics may be considered highest risk for distal thromboembolism.
Further trials are needed to determine the ideal device design and characteristics best suited for the lower extremity circulation. Many of the inherent limitations of the application of the currently available EPDs approved for other vascular beds may be addressed, including the type of wire that the device is mounted on, improving the ability to safely remove a large amount of captured debris, ensuring appropriate wall apposition and maintaining adequate intraprocedural arterial inflow. A device design unique for the infrapopliteal circulation may also need to be developed. The ideal EPD for the lower extremity circulation would require a low crossing profile in order to be used in all cases of stenosis or occlusion. Given the broad number of proposed indications for EPD use in lower extremity peripheral intervention, the ideal device would have to be able to be used in vessels of varying sizes and in both healthy and heavily diseased arterial segments. The device would probably have a short ‘footprint’ in order to enable successful deployment in tortuous anatomy.
Finally, it has been well demonstrated that endovascular peripheral arterial interventions often result in the development of de novo thrombosis, release of mural plaque and in many circumstances, intimal injury. This raises the question of whether there is a role for the administration of adjuvant pharmacologic agents, including antiplatelet and anticoagulant medications. The glycoprotein IIb/IIIa inhibitors should be evaluated in particular given that initial platelet binding to damaged vascular surfaces occurs while there is concurrent blockade of the final common pathway of platelet aggregation. Randomized control trials are thus necessary to determine whether there is a potential to further minimize acute thrombus formation and distal thromboembolism. In summary, there is tremendous potential for further research to better define the role of EPD in patients with CLI, to improve on device design tailored best to the femoropopliteal and infrapopliteal arterial beds and to assess whether there is a role for adjuvant pharmacologic therapy.
Financial & competing interests disclosure
Robert Lookstein is a consultant for MedRad Interventional/ Possis and receives Honoraria as a speaker for educational activities from Cordis Cardiac and Vascular Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Critical limb ischemia & role of lower extremity arterial interventions
▪ Endovascular therapy is a highly valuable and desirable treatment modality for patients with chronic limb ischemia (CLI) who may otherwise be poor surgical candidates owing to concomitant cardiovascular disease and other medical comorbidities. Minimally invasive endovascular techniques commonly employed include balloon angioplasty, stent placement, directional atherectomy, pharmacologic thrombolysis and mechanical thrombectomy.
Incidence & significance of distal thromboembolism during routine intervention
▪ The consequence of distal embolization of liberated or generated material resulting in occlusion of the vascular bed in patients with poor arterial inflow or poor collateralization is potentially devastating. Recent studies have demonstrated a much higher incidence of distal embolization than was previously reported (3.8–67%), with the greatest degree of distal embolization occurring with the use of directional atherectomy devices, mechanical thrombectomy and pharmacologic thrombolysis.
Currently available embolic protection devices
▪ Although balloon-based devices provide wall contact and achieve improved seal, filter-based devices maintain arterial inflow and are therefore more commonly used and desirable for endovascular peripheral arterial intervention.
Previous experience with embolic protection devices in chronic limb ischemia
▪ Preliminary work has demonstrated the safety and feasibility of the use of embolic protection devices (EPDs) in the infrainguinal arterial system. There is controversy regarding which patients or which anatomic or lesional characteristics impart a higher risk of distal thromboembolism. However, many authors have established that certain interventions, including thrombectomy, thrombolysis and directional atherectomy, are associated with a significant amount of engendered material and distal thromboembolism.
Experience at our institution
▪ Standard protocol for endovascular intervention in patients with CLI is EPD during revascularization. We have preferentially used the Spider FX™ device with great success at capturing macroscopic debris and preventing the devastating complication of distal thromboembolism. This technology is applicable during revascularization involving both the femoropopliteal and infrapopliteal arterial segments.
Inherent limitations of the use of embolic protection devices in chronic limb ischemia
▪ Limitations of the use of EPDs include challenges in initial lesion crossing in patients with chronic total occlusions and device deployment in patients with extensive long segment disease. Arterial spasm, arterial injury and de novo thrombus may occur as a result of the EPD itself. The size of the filter basket may be inadequate for collection of debris, which can result in loss of antegrade flow, debris dislodgement and difficulty in device removal. Finally, EPDs provide no protection to collateral circulation.
Conclusion
▪ The incidence of distal embolization during peripheral arterial intervention is higher than was originally believed. Initial studies suggest that embolic protection can be considered in high-risk patients, including patients with CLI and patients undergoing high-risk interventions, such as atherectomy. EPD protection allows for a more aggressive approach to revascularization in patients with CLI.
Future perspective
▪ Further research is needed in order to better define the role of EPD in CLI and to determine which patients and which anatomic or lesion characteristics portend a higher risk of distal thromboembolism, as well as to improve on device design so it is tailored to the femoropopliteal and infrapopliteal arterial beds and to assess whether there is a role for adjuvant pharmacologic therapy.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Norgren L, Hiatt WR, Dormandy JA et al.; TASC II Working Group: Inter- Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 45(Suppl. S), S5–S67 (2007).
- TransAtlantic Inter-Society Consensus (TASC). Management of peripheral arterial disease (PAD). Eur. J. Vasc. Endovasc. Surg. 19(Suppl. A), S1–S250 (2000).
- BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomized controlled trial. Lancet 366, 1925–1934 (2005).
- Shammas NW, Dippel EJ, Coiner D et al.: Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J. Endovasc. Ther. 15(3), 270–276 (2008).
- McDermott JC, Crummy AB: Complications of angioplasty. Semin. Interv. Radiol. 11, 145–149 (1994).
- Becker GJ, Katzen BT, Dake MD: Noncoronary angioplasty. Radiology 170, 921–940 (1989).
- Rickard MJ, Fisher CM, Soong CV et al.: Limitations of intra-arterial thrombolysis. Cardiovasc. Surg. 5, 634–640 (1997).
- Chalmers RT, Hoballah JJ, Kresowik TF et al.: Late results of a prospective study of direct intra-arterial urokinase infusion for peripheral arterial and bypass graft occlusions. Cardiovasc. Surg. 3, 293–297 (1995).
- Wholey MH, Maynar MA, Wholey MH et al.: Comparison of thrombolytic therapy for lower extremity acute, subacute, and chronic arterial occlusions. Cathet. Cardiovasc. Diagn. 44, 159–169 (1998).
- König CW, Pusich B, Tepe G et al.: Frequent embolization in peripheral angioplasty: detection with an embolism protection device (AngioGuard®) and electron microscopy. Cardiovasc. Intervent. Radiol. 26(4), 334–339 (2003).
- Wholey MH, Toursarkissian B, Postoak D et al.: Early experience in the application of distal protection devices in treatment of peripheral vascular disease of the lower extremities. Catheter Cardiovasc. Interv. 64(2), 227–235 (2005).
- Belli AM, Cumberland DC, Knox AM et al.: The complication rate of percutaneous peripheral balloon angioplasty. Clin. Radiol. 41(6), 380–383 (1990).
- Matsi PJ, Manninen HI: Complications of lower limb percutaneous transluminal angioplasty: a prospective analysis of 410 procedures on 295 consecutive patients. Cardiovasc. Intervent. Radiol. 21(5), 361–366 (1998).
- Bartorelli AL, Koh TH, Di Pede F et al.: Distal embolic protection during percutaneous coronary intervention in patients with acute coronary syndromes: the RUBY study. Acute Card. Care 8, 148–154 (2006).
- Kastrup A, Nagele T, Groschel K et al.: Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke 37, 2312–2316 (2006).
- Holden A, Hill A, Jaff MR et al.: Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int. 70, 948–955 (2006).
- Cooper CJ, Haller ST, Colyer W et al.: Embolic protection and platelet inhibition during renal artery stenting. Circulation 117(21), 2752–2760 (2008).
- Garg N, Karagiorgos N, Pisimisis GT et al.: Cerebral protection devices reduce periprocedural strokes during carotid angioplasty and stenting: a systematic review of the current literature. J. Endovasc. Ther. 16(4), 412–427 (2009).
- Freeman HJ, Rundback JH: Embolic protection in femoropopliteal artery intervention. Endovascular today 5, 65–69 (2006).
- Henry M, Polydorou A, Henry I et al.: New distal embolic protection device the FiberNet 3 dimensional filter: first carotid human study. Catheter Cardiovasc. Interv. 69(7), 1026–1035 (2007).
- Shortell CK, Ouriel K: Thrombolysis in acute peripheral arterial occlusion: predictors of immediate success. Ann. Vasc. Surg. 8, 59–65 (1994).
- Sandbaek G, Staxrud LE, Rosen L et al.: Factors predicting the outcome of intraarterial thrombolysis in peripheral arterial and graft occlusions. Acta Radiol. 37(3 Pt 1), 299–304 (1996).
- Karnabatidis D, Katsanos K, Kagadis GC et al.: Distal embolism during percutaneous revascularization of infra-aortic arterial occlusive disease: an underestimated phenomenon. J. Endovasc. Ther. 13(3), 269–280 (2006).
- Suri R, Wholey MH, Postoak D et al.: Distal embolic protection during femoropopliteal atherectomy. Catheter Cardiovasc. Interv. 67(3), 417–422 (2006).
- Lam RC, Shah S, Faries PL et al.: Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J. Vasc. Surg. 46(6), 1155–1159 (2007).
- Siablis D, Karnabatidis D, Katsanos K et al.: Outflow protection filters during percutaneous recanalization of lower extremities’ arterial occlusions: a pilot study. Eur. J. Radiol. 55(2), 243–249 (2005).
- Müller-Hülsbeck S, Schäfer PJ, Hümme TH et al.: Embolic protection devices for peripheral application: wasteful or useful? J. Endovasc. Ther. 16(Suppl. 1), I163–I169 (2009).
- Lookstein RA, Stangl PA, Ellozy SH et al.: Distal embolic protection during endovascular revascularization procedures for critical limb ischemia. JVIR 20(2), S86–S87 (2009).
▪ Demonstrated distal macroembolism detection in 100% of patients with concentric stenoses undergoing endovascular intervention. Embolic protection devices (EPDs) were used, and no patients had signs of clinical ischemia.
▪▪ Examined patients, many of whom underwent either mechanical or pharmacologic thrombolysis, and determined that the use of embolic protection devices allowed for earlier intervention and provided further security when performing angioplasty and stent placement in these patients.
▪▪ Demonstrated a greater amount of collected particles in the filter baskets positively correlating with increased lesion length and reference vessel diameter, acute thrombosis and total vessel occlusion.
▪▪ Demonstrated a significant amount of embolic debris created by the SilverHawk atherectomy device could be successfully captured by the EPD in a series of patients with compromised arterial run-off.
▪▪ Demonstrated no significant difference in sonographically detected embolic signals between the TransAtlantic InterSociety Consensus classifications.
▪▪ Demonstrated the safety and feasibility of EPD during revascularization procedures utilizing angioplasty/stenting and pharmacologic thrombolysis. The authors experienced excellent recanalization rate and technical success without clinical or angiographic evidence of periprocedural distal embolization.
▪▪ Evaluated the safety and effectiveness of EPD in reducing distal embolization during percutaneous lower extremity interventions including angioplasty/ stenting and directional atherectomy. All patients undergoing directional atherectomy with the SilverHawk device demonstrated significant macroembolization, and 37.9% of patients undergoing angioplasty and or stenting demonstrated significant macroembolization.