Review Article - Interventional Cardiology (2009) Volume 1, Issue 2
Is there a role for transcatheter left atrial appendage occlusion?
- Corresponding Author:
- Peter C Block
Emory University Hospital F606
1364 Clifton Road, Atlanta, GA 30322, USA
E-mail: pblock@emory.edu
Abstract
Keywords
atrial fibrillation, left atrial appendage, stroke, warfarin
There is unequivocal benefit of chronic anticoagulation in the reduction of the risk of thromboembolism among patients with nonvalvular atrial fibrillation (AF). However, warfarin is difficult to administer successfully in clinical practice and is associated with a major risk of bleeding. In real-time clinical practice, close to 80% of patients coming to a hospital clinic who are taking warfarin have a subtherapeutic international normalized ratio (INR), approximately 17% are in the therapeutic range with an INR of 2–3, and 3.5% are over-anticoagulated with an INR higher than 3.2 [1,2]. In addition, there are multiple reasons for warfarin underutilization. Warfarin has an adverse drug–effect profile, with many drug and dietary interactions. It is difficult to administer, requires frequent blood testing and has a narrow therapeutic range. Taking warfarin can change a patient’s quality of life due to easy bruising and avoidance of minor daily-living trauma. Physicians are reluctant to prescribe warfarin to elderly patients primarily because of bleeding complications but also because of the risk of falling and compliance issues. Thus, in clinical practice, physicians who treat patients with AF both hate and love warfarin – depending on whether their latest patient has had bleeding problems or has suffered a thromboembolic stroke.
Rationale for transcatheter left atrial appendage occlusion
Since AF decreases left atrial velocities, left atrial transport and can cause enough stasis to produce echocardiographically observed ‘smoke’ in the left atrium, thrombus is prone to form in the fibrillating left atrium and can serve as a source of thromboembolism. Thrombus formation in the left atrium seems to be responsible for most thromboembolic strokes in a patient population with AF. Thrombus is not always seen by echocardiography in patients with nonrheumatic AF, but when it is seen it occurs approximately 90% of the time in the left atrial appendage (LAA) [3,4]. This fact serves as the basis for the notion that LAA obliteration may decrease stroke risk in patients with AF. In cardiac surgery, routine obliteration of the LAA at the time of mitral valve surgery is commonplace, and thoracoscopic LAA snaring has also been tried as a strategy to avoid thromboembolism in patients with AF, albeit with mixed results [5,6]. Despite the apparent logic in this strategy, the etiology of stroke in patients with AF is more complex. Intrinsic cerebrovascular disease, aortic atherosclerosis, ventricular thrombus or other sources of emboli within the left atrial body can all serve as contributors to stroke. Thus, obliteration of the LAA by any means does not guarantee that thromboembolic stoke will not occur. This disclaimer has not dampened the enthusiasm for LAA obliteration, especially now that percutaneous, transcatheter devices have been developed that can potentially replace surgical removal or ligation.
Two clinical trials have been completed to evaluate the safety and efficacy of percutaneous, transcatheter closure of the LAA.
PLAATO left atrial appendage occlusion
The Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) Feasibility Study was a nonrandomized, prospective, multicenter study in which patients with chronic continuous or paroxysmal AF that were at high risk for developing thromboembolic events [7,8] and who were not candidates for anticoagulation with warfarin were enrolled. A total of 64 patients were enrolled at ten clinical sites and 61 patients actually had implantation of the device.
Eligible subjects were patients with chronic continuous or paroxysmal AF that were at high risk for developing thromboembolic events or stroke, who were not candidates for long-term anticoagulation with warfarin. All patients had to have a total high-risk (CHADS2) [7,8] score of 2 or more, or presence of at least one high-risk echocardiographic risk factor. The primary study end point was the occurrence of major adverse events within 1 month of the index procedure, defined as new major or minor stroke, cardiac or neurological death, or myocardial infarction.
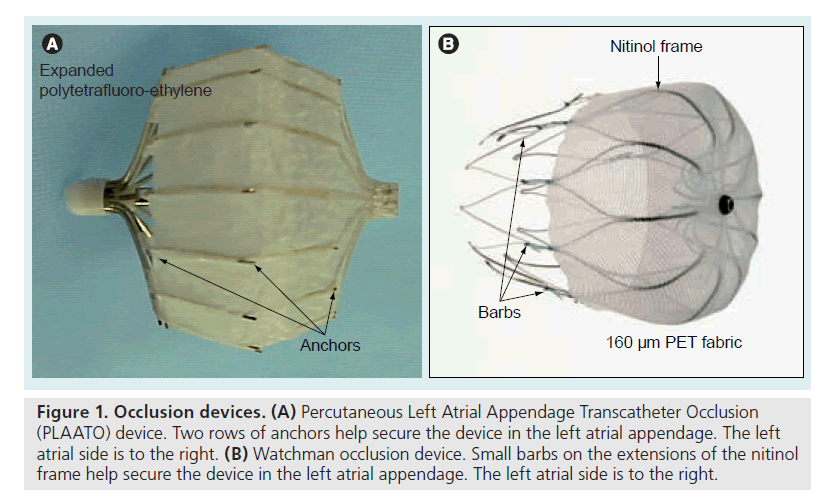
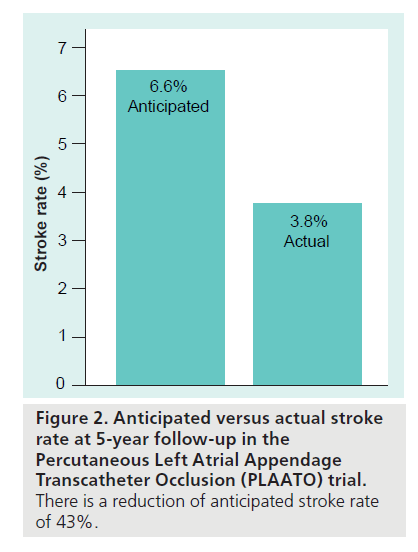
The PLAATO system [9–13] is seen in Figure 1A. It consists of a self-expanding nitinol frame covered with expanded polytetrafluoroethylene, with a double set of anchors on its perimeter. In the study, all subjects received enteric-coated aspirin and clopidogrel (or ticlopidine if they were clopidogrel intolerant) preprocedure. After transseptal puncture, an angiogram of the LAA was performed in two views and the appropriate- sized PLAATO implantation device was selected according to the measurement of the LAA orifice diameter. Study patients took clopidogrel 75 mg once daily (or ticlopidine 250 mg daily) for 4–6 weeks postprocedure, and aspirin 325 mg daily indefinitely. From this, 3‑year [14] and 5‑year follow-ups [15] have been reported. At 5‑year follow-up eight strokes occurred, of which five were major (present after 7 days or increased NIH Stroke Scale by >4) and three were minor (resolved completely within 7 days or increased NIH Stroke Scale by <3). The mean CHADS2 score in the study population was 2.6, resulting in an expected annual stroke rate of 6.6. There were 239.9 patient-years tallied in the study. Thus, the eight strokes reported resulted in an annualized stroke rate of 3.8%, a 43% reduction from expected (Figure 2). The PLAATO system was therefore shown to be safe and effective for LAA occlusion in patients with AF who were not candidates for warfarin. This was not a randomized study and although stroke rate in the study patients was less than expected, only guarded conclusions can be drawn concerning whether LAA occlusion using the PLAATO device impacted the incidence of stroke. There are no plans to pursue a pivotal study of the PLAATO system.
Figure 1. Occlusion devices. (A) Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device. Two rows of anchors help secure the device in the left atrial appendage. The left atrial side is to the right. (B) Watchman occlusion device. Small barbs on the extensions of the nitinol frame help secure the device in the left atrial appendage. The left atrial side is to the right.
Watchman device & the PROTECT-AF trial
The Phase II clinical trial evaluating the Watchman LAA occlusion device (Figure 1B) was the Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) trial [16]. The Watchman LAA occlusion device has a selfexpanding nitinol frame but is covered with 160 μm PET fabric that serves as a filter until it is endothelialized. It has barbs on extensions of the nitinol frame which fix the filter at the LAA orifice. The PROTECT-AF study was quite different from the PLAATO trial. First, it was a prospective, randomized study. Second, all study patients had to be able to take warfarin, since the trial was a noninferiority trial comparing placement of the Watchman device (followed by 45 days of warfarin therapy) with standard warfarin therapy. The composite primary end point was absence of ischemic and hemorrhagic stroke, cardiovascular and unexplained death, and systemic embolism. After 900 patient-years of observation, the primary event rate was 32% lower in the Watchman group than in the standard warfarin-therapy group [17]. This met the prespecified criterion for noninferiority. In addition, the group randomized to the Watchman device showed superiority in the outcome of hemorrhagic stroke.
Despite the fact that the trial showed noninferiority of the Watchman device compared with warfarin therapy, the design and even outcome of this trial has had detractors [18]. Difficulty with maintaining a therapeutic INR in trial participants, early learning curve procedural complications, need for warfarin therapy for 45 days after device implantation, questions about the incidence of subclinical stroke rate (routine CNS imaging was not part of the trial), the relatively low risk of enrolled patients and the fact that patients who are not candidates for warfarin were excluded have raised questions about the applicability of the results of the trial to patients with AF. Nonetheless, the Watchman device is designed to offer patients with AF an alternative to long-term warfarin therapy, which it does without apparent clinical penalty. Whether the application of this device to patients with AF will become widespread is not yet clear.
AGA amplatzer cardiac plug
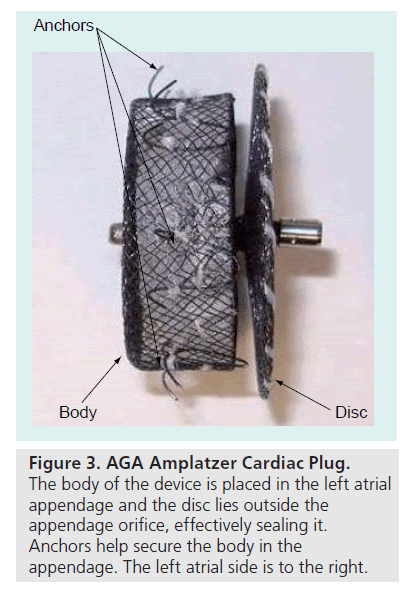
The AGA Amplatzer Cardiac Plug (ACP) (Figure 3) is a promising new device currently being implanted in patients in Europe who have AF and are at risk for stroke. It is a percutaneous transcatheter device intended for cardiac structures not involving the septal wall, which require closure or occlusion. One intended use is closure of the LAA to prevent thrombus embolization in subjects who have nonvalvular AF. The device utilizes materials and design elements common to the Amplatzer family of occluder devices. The ACP is a self-expanding device constructed from a nitinol mesh and polyester patch. It has two segments: a body and a disc connected by a central waist, and is available in eight body diameter sizes ranging from 16 to 30 mm. The body has stabilizing wires to improve device placement and retention in the LAA and is first deployed inside the opening of the LAA. The disc is then deployed just outside the orifice of the LAA with slight tension, thereby forming a cover over the LAA orifice. With endothelial ingrowth the cover forms a continuous endothelial barrier over the LAA orifice. The device has threaded screw attachments for connection to, and release from, the delivery cable. Early feasibility studies in Europe have shown that this device is easily deployed and released, and can be repositioned and retrieved, if needed. AGA Medical received CE Mark clearance in Europe in December 2008 and the ACP device is now being marketed in Europe. A prospective, postmarket registry has been initiated at 8 to 10 centers in Europe. AGA Medical has applied to the FDA for a clinical trial to support US approval of the ACP, with an initial indication for LAA occlusion.
Conclusion
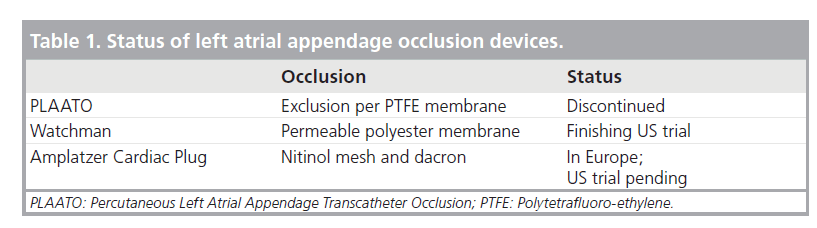
Since anticoagulation with warfarin is difficult in many patients with AF, alternative strategies for treatment have emerged. LAA occlusion using the PLAATO and Watchman devices has been shown to reduce anticipated stroke rates by 50% and also to be noninferior to warfarin therapy. Nevertheless, since the trials evaluating these devices have been carried out in differing patient populations, the exact role of transcatheter devices in the treatment of patients with AF is not yet determined. Future trials and ongoing data collection using the Watchman and other devices will hopefully allow a clearer definition of how these devices are best used (Table 1).
Future perspective
Since the PLAATO device has shown an important reduction in the anticipated stroke rate of patients with AF at high risk (CHADS2 score 1–2), it seems likely that transcatheter LAA occlusion devices offer a reasonable alternative to some patients with AF – especially those at risk of complications with warfarin therapy. The Watchman device showed noninferiority to warfarin in a randomized trial, but the patient population enrolled was different from those receiving the PLAATO device, which makes it difficult to project the best strategy for patients with AF who desire not to take warfarin. It is important to note that none of the trials using LAA occlusion devices have shown an overall reduction in stroke compared with standard anticoagulation therapy using warfarin. Any transcatheter device has some risk associated with its use. Whether in the long run the risk:benefit ratio of any LAA occlusion device warrants its widespread use remains to be seen as other trials are completed and experience of using these devices increases. Nevertheless, the availability of a clinical LAA transcatheter device will allow interventional cardiologists to selectively treat such patients to avoid anticoagulant therapy.
Executive summary
Primary reason for left atrial appendage occlusion
▪ Patients with atrial fibrillation (AF) are frequently difficult to treat clinically with warfarin.
Outcome of PLAATO device nonrandomized trial
▪ The Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device, used in patients at high risk for stroke but unable to take warfarin, successfully closed the left atrial appendage (LAA) and reduced the anticipated rate of stroke in a nonrandomized trial.
▪ However, no further trials are currently planned for this device.
PROTECT-AF shows Watchman device noninferiority
▪ The Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) trial established that the Watchman LAA occlusion device was noninferior to warfarin therapy for stroke (and superior for incidence of intracranial hemorrhage).
New devices under development
▪ The Amplatzer Cardiac Plug is a new device currently being marketed in Europe for LAA occlusion. A clinical trial to support US FDA approval of this device with an initial indication for LAA occlusion is planned.
Final strategies for LAA occlusion still unclear
▪ Strategies for clinical treatment of patients with AF either at risk for bleeding or desiring to avoid warfarin therapy are not yet defined, but ongoing data collection and the results of future trials should allow expanding selection of patients for transcatheter, LAA occlusive therapy.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT: Why do patients with atrial fibrillation not receive warfarin? Arch. Intern. Med. 160, 41–46 (2000).
- Bungard TJ, Ackman ML, Ho G, Tsuyuki RT: Adequacy of anticoagulation in patients with atrial fibrillation coming to a hospital. Pharmacotherapy 20(9), 1060–1065 (2001).
- Blackshear JL, Odell JA: Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61, 755–759 (1996).
- Odell JA, Blackshear JL, Davies E, Kollmorgen CF, Edwards WD, Orszulak TA: Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann. Thorac. Surg. 61, 565–569 (1996).
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I: Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiographic study. JACC 36(2), 468–471 (2000).
- Schneider B, Stöllberger C, Sievers HH: Surgical closure of the left atrial appendage – a beneficial procedure? Cardiology 104, 127–132 (2005).
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ: Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285(22), 2864–2870 (2001).
- Gage BF, van Walraven C, Pearce L et al.: Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 110(16), 2287–2292 (2004).
- Sievert H, Lesh MD, Trepels T et al.: Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 105(16), 1887–1889 (2002).
- Nakai T, Lesh M, Ostermayer S, Billinger K, Sievert H: An endovascular approach to cardioembolic stroke prevention in atrial fibrillation patients. Pacing Clin. Electrophysiol. 26(7 Pt 2), 1604–1606 (2003).
- Ho I, Neuzil P, Mraz T et al.: Use of intracardiac echocardiography to guide implantation of a left atrial appendage occlusion device (PLAATO). Heart Rhythm 4(5), 567–571 (2007).
- Ostermayer SH, Reisman M, Kramer PH et al.: Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J. Am. Coll. Cardiol. 46(1), 9–14 (2005).
- Hanna IR, Kolm P, Martin R, Reisman M, Gray W, Block PC: Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): six-month echocardiographic follow-up. J. Am. Coll. Cardiol. 43(10), 1868–1872 (2004).
- El-Chami MF, Grow P, Eilen D, Lerakis S, Block PC: Clinical outcomes three years after PLAATO implantation. Catheter Cardiovasc. Interv. 69(5), 704–707 (2007).
- Block PC, Burstein S, Casale PN et al.: Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5 year results of the PLAATO study. JACC Cardiovasc. Interv. 2, 594–600 (2009).
- Holmes DR, Reddy VY, Turi ZG et al.: Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374, 534–542 (2009).
- Block P: Watching the Watchman. J. Am. Coll. Cardiol. 49(13), 1496–1497 (2007).
- Maisel WH: Left atrial appendage occlusion – closure or just the beginning? N. Engl. J. Med. 360(25), 2601–2603 (2009).
▪ Indicates problems with anticoagulation with warfarin.
▪ Summarizes difficulties with surgical left atrial appendage (LAA) obliteration.
▪ Summarizes results of the Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) trial.
▪ Summarizes results of PLAATO at 5 years.
▪ Results of the Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) trial.
▪ Outlines concerns of LAA occlusion devices and summarizes PROTECT-AF results.