Perspective - Interventional Cardiology (2013) Volume 5, Issue 4
Is transcatheter aortic valve implantation cost effective in the nonsurgical elderly population?
- Corresponding Author:
- S Chris Malaisrie
Northwestern University, Division of Cardiac Surgery
201 East Huron Street, Suite 11-140, Chicago
IL 60611-2908, USA
Tel: +1 312 695 2517
Fax: +1 312 695 0178
E-mail: cmalaisr@nmh.org
Abstract
Transcatheter heart valves are an emerging technology for the treatment of patients with aortic stenosis (AS). The procedure is widely known as either transcatheter aortic valve implantation (TAVI) or replacement. Unlike surgical aortic valve replacement (AVR), which is the standard treatment for patients with AS who are considered candidates for open heart surgery, TAVI is a less invasive procedure, intended for patients who would otherwise not be offered an operation. In these patients who are considered not suitable for AVR (inoperable), TAVI can both improve survival and relieve symptoms when compared with standard therapy. Results of a randomized clinical trial, PARTNER, have led to the commercial approval of the first transcatheter heart valve (Edwards SAPIEN™, Edwards Lifesciences Corporation, CA, USA) in the USA.
Keywords
aortic stenosis, aortic valve replacement, cost–effectiveness, inoperable, transcatheter heart valve
Transcatheter heart valves are an emerging technology for the treatment of patients with aortic stenosis (AS). The procedure is widely known as either transcatheter aortic valve implantation (TAVI) or replacement. Unlike surgical aortic valve replacement (AVR), which is the standard treatment for patients with AS who are considered candidates for open heart surgery, TAVI is a less invasive procedure, intended for patients who would otherwise not be offered an operation. In these patients who are considered not suitable for AVR (inoperable), TAVI can both improve survival and relieve symptoms when compared with standard therapy. Results of a randomized clinical trial, PARTNER, have led to the commercial approval of the first transcatheter heart valve (Edwards SAPIEN™, Edwards Lifesciences Corporation, CA, USA) in the USA.
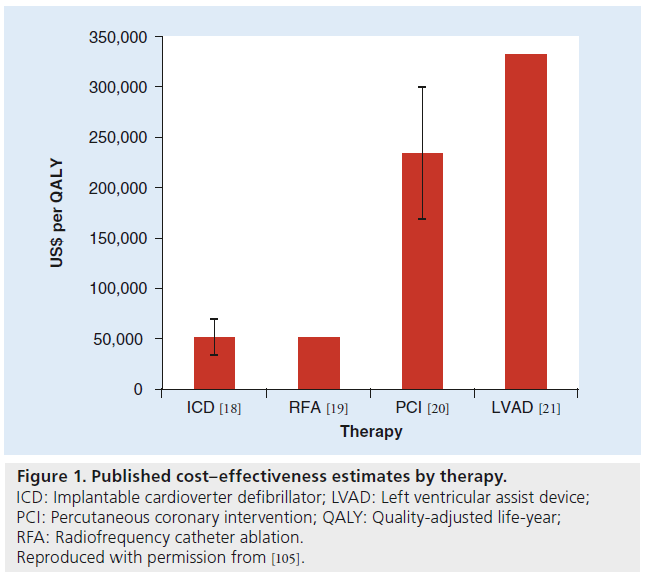
Like most emerging medical technology, the clinical benefit of TAVI in inoperable patients is accompanied by an increased cost of the therapy. Thus, cost–effectiveness analyses are useful to determine the overall value of the new technology. These overall costs include the cost of the procedure (including the cost of the valve) and all postoperative care. The incremental cost–effectiveness ratio (ICER) is a quantifiable measure between two treatment strategies, in this case TAVI versus standard therapy for inoperable patients with AS, and has been demonstrated for several medical interventions (Figure 1). Whether TAVI for the inoperable patient with AS is cost effective compared with standard therapy therefore depends on the relative measures of incremental costs and effectiveness of the procedure.
Figure 1: Published cost–effectiveness estimates by therapy. ICD: Implantable cardioverter defibrillator; LVAD: Left ventricular assist device; PCI: Percutaneous coronary intervention; QALY: Quality-adjusted life-year; RFA: Radiofrequency catheter ablation. Reproduced with permission from [105].
Defining the cohort: inoperable patients
AVR is a life-saving and symptom-relieving treatment for patients with severe, symptomatic AS. However, many patients with severe, symptomatic AS do not undergo AVR, despite class IA guidelines owing to medical conditions perceived as prohibitive of open heart surgery [1]. The definition of inoperability has not been previously well defined and its assessment is often subjective. In the pivotal trial, PARTNER cohort B, that studied the superiority of TAVI compared with standard therapy, inoperability was defined as medical conditions that would result in a combined risk of death or serious irreversible morbidity of 50% after AVR [2]. As a point of reference, AVR was performed in North America in 2006 with an average operative mortality of 2.6% and an observed stroke rate of 1.3% [3]. The trial mandated that inoperability be determined by two cardiac surgeons of the heart team caring for the patient. Therefore, the entry of the truly inoperable patient into the trial required rigorous determination with the basic question for cardiac surgeons treating these patients being: before the advent of TAVI, would this patient be offered an AVR?
The characteristics of the cohort enrolled in the trial provide an invaluable reference point for the inoperable patient (Table 1). Salient features are: the average age of 83 years; New York Heart Association functional class III/IV in greater than 90%; chronic obstructive pulmonary disease in 40%; previous CABG in approximately 40%; cerebral vascular disease in 27%; and peripheral vascular disease in up to 30%. The Society of Thoracic Surgeons predicted risk of mortality for this cohort was 11–12%; and the logistic EuroSCORE was 26–30% [2]. Importantly, neither medical comorbidities nor the risk scores determined inoperability in these patients. Rather, the anatomic and medical characteristics that are not included in the risk models were factors in determining inoperability, which included porcelain aorta, hostile chest (chest irradiation, chest deformity and left internal mammary artery graft adherent to the sternum), oxygen-dependent lung disease, liver disease and frailty (Table 2).
| Characteristic | TAVI (n = 179) | Standard therapy (n = 179) | p-value |
|---|---|---|---|
| Age (years) | 83.1 | 83.2 | 0.95 |
| Male sex (%) | 45.8 | 46.9 | 0.92 |
| STS score | 11.2 | 12.1 | 0.14 |
| Logistic EuroSCORE | 26.4 | 30.4 | 0.04 |
| NYHA class I or II (%) | 7.8 | 6.1 | 0.68 |
| NYHA class III or IV (%) | 92.2 | 93.9 | 0.68 |
| CAD (%) | 67.6 | 74.3 | 0.20 |
| Prior MI (%) | 18.6 | 26.4 | 0.10 |
| Prior CABG (%) | 37.4 | 45.6 | 0.17 |
| Prior PCI (%) | 30.5 | 24.8 | 0.31 |
| Prior BAV (%) | 16.2 | 24.4 | 0.09 |
| CVD (%) | 27.4 | 27.5 | 1.00 |
| PVD (%) | 30.3 | 25.1 | 0.29 |
| COPD: any (%) | 41.3 | 52.5 | 0.04 |
| COPD: O2 dependent (%) | 21.2 | 25.7 | 0.38 |
| Creatinine >2 mg/dl (%) | 5.6 | 9.6 | 0.23 |
| Atrial fibrillation (%) | 32.9 | 48.8 | 0.04 |
| Permanent pacemaker (%) | 22.9 | 19.5 | 0.49 |
| Pulmonary HTN (%) | 42.4 | 43.8 | 0.90 |
disease; COPD: Chronic obstructive pulmonary disease; CVD: Cardiovascular disease;
HTN: Hypertension; MI: Myocardial infarction; NYHA: New York Heart Association;
PCI: Percutaneous coronary intervention; PVD: Peripheral vascular disease; STS: Society of Thoracic
Surgeons; TAVI: Transcatheter aortic valve implantation.
Data taken with permission from [105].
Table 1. Baseline characteristics of inoperable patients randomized to transcatheter aortic valve implantation versus medical treatment (PARTNER cohort B trial).
| Characteristic | TAVI (n = 179) | Standard therapy (n = 179) | p-value |
|---|---|---|---|
| Frailty (%) | 18.1 | 28.0 | 0.09 |
| Porcelain aorta (%) | 19.0 | 11.2 | 0.05 |
| Chest wall radiation (%) | 8.9 | 8.4 | 1.00 |
| Chest wall deformity (%) | 8.4 | 5.0 | 0.29 |
| Liver disease (%) | 3.4 | 3.4 | 1.00 |
Data taken with permission from [105].
Table 2. Anatomic and medical characteristics of inoperability (PARTNER cohort B trial).
In addition, the consensus document from the Society of Thoracic Surgeons and American College of Cardiology has recognized that inoperability has not been well defined, and points out that other terms have also been used for this group of patients, such as ‘extreme risk’ and ‘prohibitive risk’ [4]. Moreover, a patient can be considered inoperable from either anatomic considerations (porcelain aorta or hostile chest) or medical considerations (frailty or end-stage organ dysfunction), as described above. Nevertheless, the society document has stated that the determination of inoperability should be performed by the heart team, which includes both the cardiac surgeon and cardiologist, and this has been reiterated by the US FDA [101] and Centers for Medicare and Medicaid Services [102].
Effectiveness of TAVI in the inoperable patient
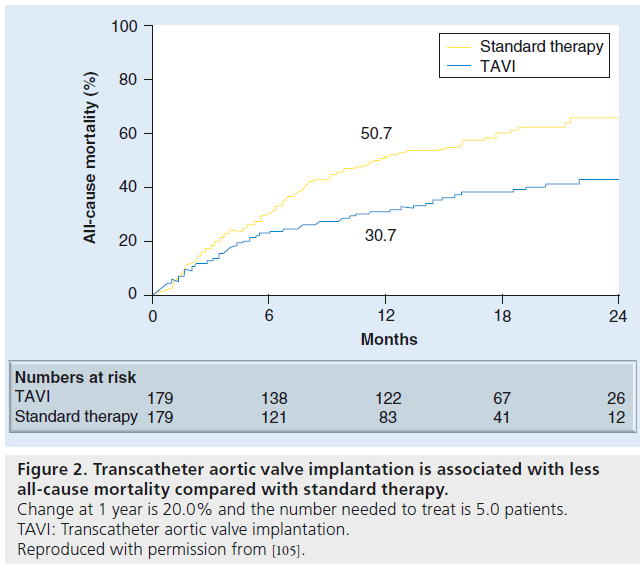
The PARTNER cohort B clinical trial defined the effectiveness of TAVI in the inoperable patient in terms of both improvement in survival and reduction of symptoms [2]. When compared with standard therapy (including balloon aortic valvuloplasty in 84% of patients), TAVI was associated with a statistically significant decrease in all-cause mortality at 1 year (Figure 2) [2]. All-cause mortality in the TAVI group was 30.7% compared with 50.7% in the medical group (hazard ratio: 0.54; p < 0.0001 and number needed to treat: 5). The survival benefit persisted for 2 years during the latest analysis, with all-cause mortality in the TAVI and medical group being 43.4 and 67.7%, respectively (hazard ratio: 0.57; p < 0.0001 and number needed to treat: 4.1) [5]. Moreover, when considering crossovers from the standard therapy to TAVI group, the results are even more convincing that TAVI is associated with improved survival, based on an intent-to-treat analysis. A total of 21 patients (12%) in the standard therapy arm underwent an aortic valve procedure (AVR, apico-aortic conduit or TAVI) resulting in a 38% 1-year mortality. Six patients (3.4%) in the TAVI arm did not receive TAVI owing to death before planned procedure or anatomic preclusions during the procedure.
Figure 2: Transcatheter aortic valve implantation is associated with less all-cause mortality compared with standard therapy. Change at 1 year is 20.0% and the number needed to treat is 5.0 patients. TAVI: Transcatheter aortic valve implantation. Reproduced with permission from [105].
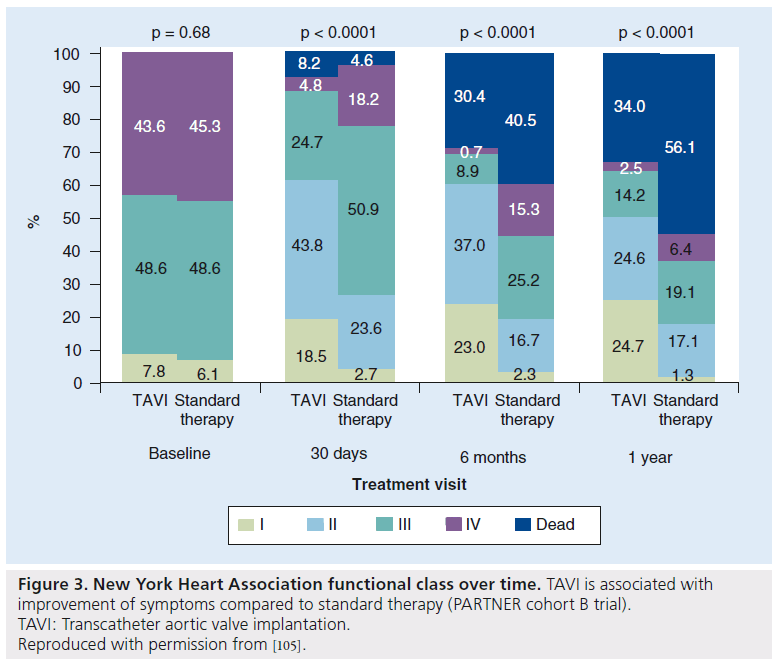
Symptoms in the survivors were also significantly improved compared with standard therapy, with 75% of patients surviving in the TAVI group in the New York Heart Association functional class I or II at 1 year compared with 42% in the medical group (p < 0.0001) [2]. When including deaths in this analysis (Figure 3), the benefit appears less pronounced, but nevertheless statistically significant. Classification of symptoms based on New York Heart Association classification, although a measure of symptom improvement, does not enter into the cost–effectiveness analysis.
Figure 3: New York Heart Association functional class over time. TAVI is associated with improvement of symptoms compared to standard therapy (PARTNER cohort B trial). TAVI: Transcatheter aortic valve implantation. Reproduced with permission from [105].
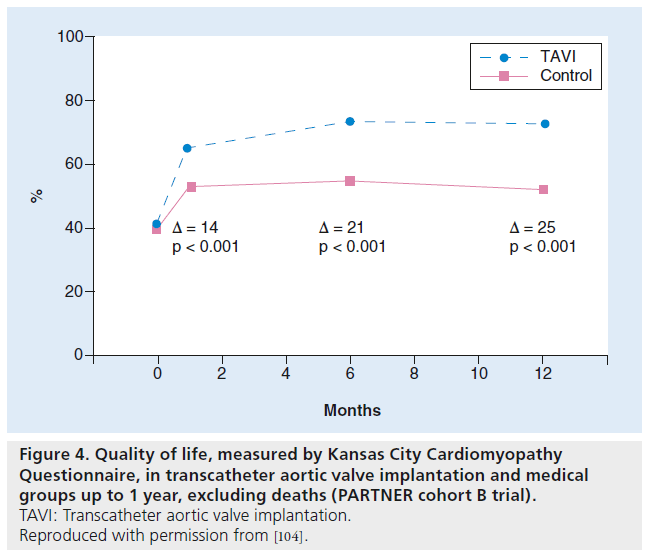
Health-related quality of life was studied in the survivors using the Kansas City Cardiomyopathy Questionnaire and the Medical Outcomes Study Short-Form 12 [6]. Baseline measures of quality of life were similar in the TAVI and medical groups, but were significantly better in the TAVI group at 1 year, as measured by the Kansas City Cardiomyopathy Questionnaire and the Medical Outcomes Study Short- Form 12 (Figure 4). In order to account for deaths and missing data, the proportion of patients with an ‘excellent outcome’, defined as alive and with a greater than 20-point increase from baseline in the Kansas City Cardiomyopathy Questionnaire, was found to be 38% in the TAVI group versus 9.2% in the medical group (p < 0.001) [6]. Quality of life is factored into the utility of an intervention, and the composite is referred to as quality-adjusted life years. Therefore, a patient’s health status can range from 1 (perfect health) to 0 (death). Thus, the overall utility can be measured in terms of life expectancy and health status.
Figure 4: Quality of life, measured by Kansas City Cardiomyopathy Questionnaire, in transcatheter aortic valve implantation and medical groups up to 1 year, excluding deaths (PARTNER cohort B trial). TAVI: Transcatheter aortic valve implantation. Reproduced with permission from [104].
Costs of TAVI
Actual costs, as opposed to hospital charges, can be divided into two broad categories. The first is the costs of the index hospitalization for TAVI, including the cost of the valve, procedural costs, cost of complications, cost of hospital stay (intensive care unit and ward) and medication costs. Only the TAVI group incurred these costs, and the drivers are the cost of the valve and length of hospital stay. Both groups, however, would be subject to follow-up costs, which include rehospitalization, non-inpatient care (rehabilitation, nursing home and home care), outpatient medical care and medication; the main driver of follow- up costs is rehospitilizations.
Costs were directly measured for both groups in the PARTNER cohort B trial for up to 1 year, but all costs after 1 year were estimated [7]. As part of this estimate, life expectancy of the TAVI and medical group was assumed to be 3.1 and 1.2 years, respectively. Several other studies looking at cost–effectiveness have used PARTNER cohort B trial data to formulate their own conclusions.
Cost–effectiveness of TAVI
Typically, the ICER is calculated as incremental costs per incremental quality-adjusted life years. What is considered to be cost effective varies by country, but most countries do not have an explicit threshold for cost–effectiveness (or willingness to pay [WTP]). In the USA, the ICER for hemodialysis is a reasonable, accepted threshold for cost–effectiveness and is estimated to be approximately US$70,000 per quality-adjusted life year [8]. Similarly, in Europe, a threshold of UK£20,000–30,000 (US$30,000–48,000, depending on exchange rate) is proposed by the NIH and NICE [9]. In Canada, a threshold CAD$20,000–100,000 is considered to be cost effective [10], although CAD$50,000 is most commonly used for purposes of sensitivity analyses.
Six studies, using data derived from the PARTNER cohort B trial, have been published comparing the cost–effectiveness of TAVI with standard therapy in inoperable patients (Table 3). Three have shown that the ICER falls within the acceptable range for their respective country’s WTP. The US analysis uses cost data directly collected from the PARTNER cohort B trial, but, nevertheless, an ICER of US$61,889 can be considered cost effective given a WTP threshold of US$70,000 [7]. The Belgian study shows a comparable ICER at €44,900 (US$59,721), but if a lower threshold is used, as prescribed by NICE, then the conclusion is that TAVI may not be cost effective for that country [11]. An important distinction is made in the Belgian study between patients who are inoperable for anatomic reasons and medical reasons. Patients with anatomic reasons for inoperability are more likely to benefit from TAVI compared with patients who have debilitating medical conditions. Therefore, this subgroup of anatomically inoperable patients has a more favorable ICER and may be more cost effective than medically inoperable patients.
| Study (year) | Country | WTP (cost/QALY) | ICER (cost/QALY) | Time horizon | Is TAVR/TAVI cost effective compared with standard therapy? | Ref. |
|---|---|---|---|---|---|---|
| Neyt et al. | Belgium | UK£20,000–30,000 | €44,900 | Lifetime | No | [11] |
| (2012) | (US$31,807–47,710)† | (US$59,721) | ||||
| Doble et al. | Canada | CAD$49,000 | CAD$51,324 | 20 years | No | [13] |
| (2012) | (US$47,825) | (US$50,093) | ||||
| Hancock- | Canada | CAD$50,000 | CAD$32,170 | 36 months | Yes | [12] |
| Howard et al. | (US$50,395) | (US$32,424) | ||||
| (2013) | ||||||
| Murphy et al. | UK | UK£20,000–30,000 | UK£35,956 (95% CI: UK£24,768–65,103) | Lifetime | No | [15] |
| (2013) | (US$32,170–48,256)† | (US$56,963 [95% CI: US$39,238–103,138]) | ||||
| Watt et al. | UK | UK£20,000–30,000 | UK£16,200 | 10 years | Yes | [14] |
| (2012) | (US$30,855–46,283)† | (US$24,993) | ||||
| Reynolds et al. | USA | US$68,537 | US$61,889 (95% CI: US$49,551–78,361) | Lifetime | Yes | [17] |
| (2012) |
†Indicates WTP (cost/QALY) was determined using NICE guidelines.
ICER: Incremental cost–effectiveness ratio; QALY: Quality-adjusted life-year; TAVI: Transcatheter aortic valve implantation; TAVR: Transcatheter aortic valve
replacement; WTP: Willingness to pay.
Data taken with permission from [105].
Table 3. Cost–effectiveness of transcatheter aortic valve implantation compared with standard therapy in inoperable patients per country.
The two Canadian studies differ in their ICER calculation, with the Hancock-Howard et al. study estimating CAD$32,170 (US$32,424) [12] and the Doble et al. study estimating CAD$51,324 (US$50,093). Given the same Canadian threshold for WTP of CAD$49,000 (US$47,825), the Doble study found that TAVI only had a 44% probability of being cost effective. The difference in ICER is related to the difference in time horizon between the two Canadian studies; 20 years for Doble et al. and 3 years for Hancock-Howard et al.
Similarly, the two UK studies showed a difference in ICER calculations, with the Watt et al. study estimating UK£16,200 (US$24,993) [14], and the Murphy et al. study UK£35,956 (US$56,963) [15]. However, when including data outside of the PARTNER cohort B, which showed almost twice the survival benefit, Murphy et al. showed that TAVI could be cost effective with an ICER of UK£19,063 (US$30,854). Interestingly, the only other study to include data other than the PARTNER cohort B data was the Belgian study (mentioned above), which demonstrated an opposite effect. In their analysis, Neyt et al. used data from the continued access arm of the PARTNER cohort B trial. These data have not been published, but did show a 12.7% higher 1-year mortality in the TAVI group as compared with the original PARTNER cohort B study group. This higher 1-year mortality in the TAVI group decreases the survival advantage of the TAVI group as compared with standard therapy [11]. The authors also suggest that results from the PARTNER cohort B trial may not be reproducible, but further analysis of the continued access arm is limited owing to lack of publication of these negative results. Moreover, the authors point out that the randomized PARTNER cohort B groups are, indeed, unbalanced with more chronic obstructive pulmonary disease and atrial fibrillation in the medical group, which biases the results in favor of the TAVI group (Table 1).
Overall, these six studies demonstrate a marginal cost–effectiveness for TAVI in the inoperable patient. In the UK, Murphy et al. showed an ICER of US$56,963 compared with Watt who showed a US$24,993, while in Canada, Doble et al. and Howard-Hancock et al. showed US$50,093 and US$32,424 ICERs, respectively. The wide variation of ICERs in these countries calls into question whether TAVI is cost effective for inoperable patients in those particular countries. WTP thresholds also vary by country and what is cost effective for one country (e.g., USA) may not be so for another (e.g., Belgium).
Cost–effectiveness of TAVI in the real world?
These six studies determining the cost–effectiveness of TAVI in the inoperable patient do not account for real-world results. Variability of outcomes has already been demonstrated in the continued access arm of the same pivotal trial (PARTNER cohort B) using the same inclusion and exclusion criteria. The learning curve for TAVI may be significant (~30 cases [16]) and small centers with incipient TAVI programs may require a significant amount of time to surpass their learning curve [16]. Results may vary in both costs (particularly length of hospital stay) and effectiveness (overall survival). Whether TAVI will be concentrated in high-volume valve centers rather than be scattered among many small centers remains to be determined. Commercial rollout of this new technology in the USA will be met with a mandatory registry (Society of Thoracic Surgeons/American College of Cardiology TVT Registry™ [WA, USA]) for all patients receiving TAVI in the USA [103]. Only from this registry can a credible analysis of real-world results be pursued in the future.
Inappropriate use of TAVI is another variable in the real world. Inappropriate designation of inoperability in a patient who could otherwise have AVR (although high risk) limits the options available to patients and is not pertinent to cost–effectiveness studies comparing TAVI with standard therapy. Indeed, cost–effectiveness studies comparing TAVI with AVR in high-risk patients have been even less convincing [13,17]. On the other hand, offering TAVI to the inoperable patient as a futile measure, by definition, is a disservice to the patient. The PARTNER trial specifically excluded patients with comorbidities that otherwise limit patient survival to less than 1 year, regardless of AS. Society consensus has determined futility as: “lack of medical efficacy, as judged by the patient’s physician; or lack of a meaningful survival, as judged by the personal values of the patient” [4]. Perhaps another practical definition of the futility is suggested by the US FDAlabeled indication for TAVI: “inoperable for open [AVR] and in whom existing comorbidities would not preclude the expected benefit from correction of the [AS]” [101].
Conclusion & future perspective
TAVI is an effective therapy when compared with standard therapy for the patient with AS who is otherwise unsuitable for AVR (inoperable patient). Based on country-specific thresholds of WTP, TAVI for the inoperable patient is marginally cost effective based on varied results from six studies using data primarily from the PARTNER cohort B trial.
In the future, the field of transcatheter heart valves will evolve rapidly. True 5–10-year clinical outcomes (survival) from the PARTNER cohort B trial will become available and the cost of the valves will decrease as more competitors enter the market (current analysis is based on a US$30,000 cost of the transcatheter heart valve) allowing for more accurate cost–effectiveness studies. Moreover, data from the Medtronic Corevalve randomized clinical trial will also become available for study [104]. Although there is optimism that greater experience and improved technology will result in better clinical outcomes, it is more likely that real-world experience will not replicate trial experience in terms of appropriateness of use and tertiary care results. Thus, future studies on cost–effectiveness using updated trial data will appear more favorable, but studies using real-world data may appear less favorable.
Financial & competing interests disclosure
SC Malaisrie has been a speaker for Edwards Lifesciences, LLC and received honorarium. He has also conducted research for Medtronic. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
The inoperable patient with aortic stenosis
▪ Medical conditions that would result in the combined risk of death or serious, irreversible comorbidities of greater than 50%.
▪ The average age and The Society of Thoracic Surgeons score of the PARTNER cohort B patient was 83 years and 11–12%, respectively.
▪ Anatomic and medical factors including porcelain aorta, hostile chest, oxygen-dependent lung disease, liver disease and frailty defined the inoperable cohort.
Effectiveness of transcatheter aortic valve implantation in the inoperable patient
▪ Transcatheter aortic valve implantation (TAVI) improved overall survival and reduced symptoms when compared with standard therapy.
▪ Health-related quality of life was improved in patients who survived after TAVI.
Costs of TAVI in the inoperable patient
▪ The cost of the transcatheter heart valve is measured in multiples (five-times standard surgical heart valves) when compared with surgical heart valves.
▪ Rehospitalizations are the main drivers of postoperative costs.
Cost–effectiveness of TAVI
▪ The incremental cost–effectiveness ratio for TAVI compared with standard therapy for the inoperable patient with aortic stenosis has been calculated in six trials with varying results.
▪ TAVI may not be cost effective, depending on willingness to pay thresholds, which are specific to each country.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Bonow RO, Carabello BA, Kanu C et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114(5), e84–e231 (2006).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med 363(17), 1597–1607 (2010).
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database.Thorac. Cardiovasc. Surg. 137(1), 82–90(2009).
- Holmes DR Jr, Mack MJ, Kaul S et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 59(13), 1200–1254 (2012).
- Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 366(18), 1696–1704 (2012).
- Reynolds MR, Magnuson EA, Lei Y et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 124(18), 1964–1972 (2011).
- Reynolds MR, Magnuson EA, Wang K et al. Cost–effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (cohort B). Circulation 125(9), 1102–1109 (2012).
- Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med. Decis. Making 22(5), 417–430 (2002).
- National Health Service. Guide to the Methods of Technology Appraisal. NICE, London, UK(2008).
- Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 146(4), 473–481 (1992).
- Neyt M, Van Brabandt H, Devriese S, Van De Sande S. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open 2(3), e001032 (2012).
- Hancock-Howard RL, Feindel CM, Rodes-Cabau J, Webb JG, Thompson AK, Banz K. Cost–effectiveness of transcatheter aortic valve replacement compared to medical management in inoperable patients with severe aortic stenosis: Canadian analysis based on the PARTNER trial cohort B findings. J. Med. Econ. 16(4), 566–574 (2013).
- Doble B, Blackhouse G, Goeree R, Xie F. Cost–effectiveness of the Edwards SAPIEN transcatheter heart valve compared with standard management and surgical aortic valve replacement in patients with severe symptomatic aortic stenosis: a Canadian perspective. J. Thorac. Cardiovasc. Surg. 146(1), 52–60 (2012).
- Watt M, Mealing S, Eaton J et al. Cost–effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart 98(5), 370–376 (2012).
- Murphy A, Fenwick E, Toff WD et al. Transcatheter aortic valve implantation for severe aortic stenosis: the cost–effectiveness case for inoperable patients in the United kingdom. Int. J. Technol. Assess. Health Care 29(1), 12–19 (2013).
- Alli OO, Booker JD, Lennon RJ, Greason KL, Rihal CS, Holmes DR Jr. Transcatheter aortic valve implantation: assessing the learning curve. JACC Cardiovasc. Interv. 5(1), 72–79 (2012).
- Reynolds MR, Magnuson EA, Lei Y et al. Cost–effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (cohort A). J. Am. Coll. Cardiol. 60(25), 2683–2692 (2012).
- Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N. Engl. J. Med. 353, 1471–80 (2005).
- Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T, Cohen DJ. Cost– effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm. Electrophysiol. 2, 362–369 (2009).
- Weintraub WS, Boden WE, Zhang Z et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ. Cardiovasc. Qual. Outcomes 1, 12–20 (2008).
- Clegg AJ, Scott DA, Loveman E, Colquitt J, Royle P, Bryant J. Clinical and cost– effectiveness of left ventricular assist devices as destination therapy for people with end-stage heart failure: a systematic review and economic evaluation. Int. J. Technol. Assess. Health Care 23(2), 261–268 (2007).
- Edwards Lifesciences Corporation Edwards SAPIEN: transcatheter heart valve with the RetroFlex 3 Delivery System. www.fda.gov/MedicalDevices/ ProductsandMedicalProcedures/ DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm280840.htm (Accessed 17 April 2013)
- Centers for Medicare and Medicaid Services. Decision memo for transcatheter aortic valve replacement (TAVR) (CAG-00430N). www.cms.gov/medicare-coverage-database/ details/nca-decision-memo.aspx?NCAId=257 &ver=4&NcaName=Transcatheter+Aortic+V alve+Replacement+(TAVR)&bc=ACAAAAA AIAAA& (Accessed 17 April 2013)
- STS/ACC TVT Registry. Tracking real-world outcomes. www.ncdr.com/TVT/ Home/Default.aspx (Accessed 17 April 2013)
- Safety and Efficacy Study of the Medtronic CoreValve® System in the Treatment of Symptomatic Severe Aortic Stenosis in High Risk and Very High Risk Subjects Who Need Aortic Valve Replacement. http://clinicaltrials.gov/show/NCT01240902 (Accessed 5 July 2013)
- Quality of life, measured by Kansas City Cardiomyopathy Questionnaire, in transcatheter aortic valve implantation and medical groups up to 1 year, excluding deaths (PARTNER cohort B trial). www.myamericanheart.org/idc/groups/ ahamah-public/@wcm/@sop/@scon/ documents/downloadable/ucm_426902.pdf (Accessed 17 April 2013)
- OANDA. www.oanda.com (Accessed 17 April 2013)
▪ Websites