Research Article - Neuropsychiatry (2018) Volume 8, Issue 4
Isometric Protocol as a Useful Tool for Evaluating Flexor and Extensor Torques of the Paretic Foot in Post-Stroke Patients
- Corresponding Author:
- Ewa Chlebuś
Clinic for Rehabilitation
Poznań University of Medical Sciences, Poland
Tel: + 48 61 8 334 387
Abstract
Background
Stroke has detrimental effects on locomotors performance of the ankle joint. Reduced force generation abilities of flexor and extensor muscles in the paretic foot resulting in deteriorated ankle stability and motor quality.
Aim
Stroke patients experience weakening of extensor and flexor muscles in the paretic foot. For certain muscles the degree of weakening depends on foot-shank position so different foot positions would result in different extensor-flexor torque ratios. Further the following parameters were measured:
• Peak Torque (PT), Average Torque (AVGT), and AVGT/BW obtained with isometric testing in three foot-shank positions (15° flexion, 0° neutral position, -15° extension) and generated by extensors and flexors of paretic, non-paretic and in healthy volunteers.
• The ratio of AVGT generated by foot extensors to the same values generated by flexors in paretic, non-paretic, and healthy foot in three test positions (15° flexion, 0° neutral position, -15° extension)
Materials and methods
Patients with hemiparesis (No=34) were examined. The control group consisted of 34 healthy volunteers. There were no differences between the groups in terms of age, weight and height. The following parameters were measured: Peak Torque (PT), Average Torque (AVGT), and AVGT/BW. The relationship between extensor and flexor performance was determined by agonist/antagonist ratio, i.e. the ratio of average extensor torque to average flexor torque.
Results
PT, AVGT, and AVGT/BW values of paretic foot extensors differed from those recorded in the control group. As for the non-paretic limb, differences were found only in neutral foot positioning. PT, AVGT, AVGT/BW values of paretic foot flexors differed from the results of healthy subjects. The agonist/antagonist ratio of the paretic foot was different from the results of healthy subjects.
Conclusions
Early after stroke, significant weakening of extensors and flexors occurs in both lower extremities. The paretic limb is marked by abnormal agonist/antagonist ratio.
Keywords
Stroke, Paretic foot, Isometric testing, Rehabilitation
Introduction
Stroke negatively affects ankle mechanics and hinders the realization of ankle joint strategy [1-5]. This type of impairment significantly affects patients’ motor performance due to decreased kinetic abilities and, as a result, increased energy expenditures for gait. The main determinants of ankle mechanical abnormalities in poststroke patients are limited range of motion and decreased force generation ability of foot flexors and extensors, which result in reduced ankle stability as well as abnormal motor dynamics [6,7]. Reduced joint stability impairs the stance stage and consequently affects gait performance [8]. Importantly, recent years have seen extensive research into the causes of limited range of motion of ankle joints in post-stroke patients [9-12]. Studies into the impact of ankle stiffness on the performance of muscles responsible for ankle joint motion have found that ankle stiffness in the affected side in post-stroke patients is primarily the consequence of resistance generated by the gastrocnemius muscle and Achilles tendon [13]. Stretching paretic shank muscles of poststroke patients using the SPM procedure not only enhances range of motion and decreases joint stiffness, it also improves shank muscle force [14].
Most research into paretic lower limb recovery in post-stroke patients has concentrated on foot extensors [15]. Gait performance is directly related to extensor muscle force of the paretic foot [16]. For early stroke rehabilitation programmes, it is crucial to implement exercises aimed at strengthening paretic foot extensors, because they improve functional activity such as gait performance, among others [17]. Even little activation is of great importance for regaining normal gait ability and determining further rehabilitation [18].
Loss of flexor force capabilities in the paretic foot in relation to desired locomotors efficiency has been investigated as a separate research problem [19,20]. When examining the effects of foot flexor weakness on function of post-stroke patient, it has been found that the weakening of foot flexors in the affected side was much more advanced compared with the contralateral limb. Comparisons against values recorded in the control group showed even more significant differences [21]. The shorter the muscle is during measure, the lower its absolute torque value, which has certain implications for gait performance in the commonly occurring flexed position of the paretic foot. Stretching foot flexors improves not only ankle range of motion in the affected limb but also improves those muscles’ torque generating abilities [22-24]. In addition, reduced flexor peak torque brought about by flexed position of the paretic foot impairs gait performance [25].
Professional literature includes reports of studies which applied a combined analysis of the impact of both extensor and flexor force loss in the paretic foot on the function of poststroke patients [26-30]. The majority of studies into the performance of paretic foot extensors and flexors applied the isokinetic protocol [21,25,31], whereas isometric testing has been used extremely rarely [15]. Literature available to us did not include studies that would use the isometric protocol to evaluate extensor and flexor torque of the paretic foot in post-stroke patients in relation to baseline length of studied muscles. There were also no studies examining flexorextensor torque ratios. Moreover, there was no study found that would explore the problem and investigate such parameters in non-paretic limbs and limbs of healthy individuals. In our opinion such study is essential for developing detailed post-stroke recovery programmes that focus on locomotor ability. As demonstrated in our project (see Materials and Methods), it is not always possible to apply the isokinetic protocol to study muscle torque generation in the paretic lower limb. For this reason, we decided to conduct this challenging isometric study, which is a novelty in this area of clinical research.
Hypothesis and Aims
Stroke patients experience weakening of extensor and flexor muscles in the paretic foot.
Hypothesis: For certain muscles the degree of weakening depends on foot-shank position so different foot positions would result in different extensor-flexor torque ratios. Aims of the study were estimation of the following parameters:
• Peak Torque (PT), Average Torque (AVGT), and AVGT/BW obtained with isometric testing in three foot-shank positions (15° flexion, 0° neutral position, -15° extension) and generated by extensors and flexors of paretic, non-paretic and in healthy volunteers
• The ratio of AVGT generated by foot extensors to the same values generated by flexors in paretic, non-paretic, and healthy foot in three test positions (15° flexion, 0° neutral position, -15° extension)
Materials and Methods
▪ Participants
The examined group was formed by patients with paretic lower-extremity hospitalized at the Neurological Rehabilitation Department, Victor Dega Orthopedic Rehabilitation Hospital of Poznan University of Medical Sciences between January 2015 to December 2016. The selection criteria was hemiparesis after first episode of ischemic brain stroke, the score of AMTS>9 indicating the ability to communicate and understand instructions, and no foot clonus and low spasticity (scores of 1.1+ in Ashworth scale) in the examined limb. Furthermore, it was characteristic of patients who met the study criteria that motor impairments in paretic limbs were to an extent selective, so Brumstrom’s Stages of Lower Limb was used, and patients were assigned stages IV, V and VI. Conditions that excluded patients from participation in the study were tetraparesis, multiple strokes, lowerlimb paresis of aetiology different than stroke, cognitive impairment and aphasia, and past ankle injuries of the studied limb.
The control group consisted of healthy individuals recruited among patients’ family members and hospital staff. They showed good overall condition and did not report any past injuries or neurological disorders that could affect ankle structure and function.
The study was approved by the Ethics Commission of Poznan University of Medical Sciences, Poland (project number 539/18). Informed consent was obtained from all subjects prior to the study. It was performed in accordance with the Declaration of Helsinki.
▪ Biomechanical examinations
Subjects were examined 35.32 ± 2.88 mean days after stroke and immediately before the onset of rehabilitation. Biodex System 4 Pro dynamometer was used to perform biomechanical evaluation of muscles and joints and to provide dynamic training in isokinetic, isotonic or isometric conditions [32].
To choose an appropriate study protocol, pilot examination was first conducted with 10 hemiplegic patients 32.65 ± 3.45 mean days after ischemic brain stroke. Each patient was evaluated for force generation capabilities of flexor and extensor muscles of the paretic foot with randomly applied isometric, isokinetic and isotonic protocols with adequate time in between measurements to enable muscle recovery. During the pilot study the isometric protocol turned out to be the only one that patients were able to fully complete without exacerbating pain in the lower paretic limb, which affects the level of active participation in the study. Also, pilot study results indicated that all patients under study will manage to achieve the following foot positions: 15 degrees flexion (Figure 1), 0 degrees neutral (Figure 2), -15 degrees extension (Figure 3).
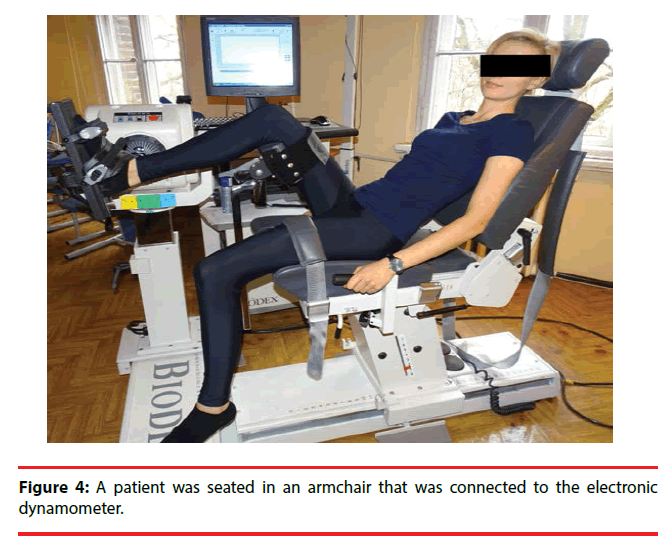
During the examination, a patient was seated in an armchair that was connected to the electronic dynamometer (Figure 4). The torso was stabilized with two belts running across each other and over the chest, whereas the upper limbs were placed on arm supports allowing muscles to loosen. The examined lower limb was positioned on an attachment which offloaded the femoral and knee joint while maintaining the flexion angle of 70 degrees for both joint. The foot was propped up with an attachment allowing maximum loosening of extensor and flexor muscles of the examined foot.
The procedure first focused on the non-paretic limb. Prior to each examination, the researcher established the range of motion in a patient’s ankle. Examination commenced with the dynamometer locking the foot at 15-degree flexion. The patient was asked to do a 5-s maximal contraction of foot flexors. This was followed by a break, and then a 5-s maximal contraction of foot extensors. The patient repeated such a series of alternate contractions 3 times. After completing a series there was a 15-s break to allow the examined muscles to loosen. Then, the foot was locked in the dynamometer in the position of 0 degrees, and the patient performed a series of contractions using the same above-described pattern with another break occurring afterwards. After the break the foot was locked in the dynamometer in the position of 15 degrees extension, and the patient performed a series of contractions using the same above-described pattern. Finally, a report was printed out presenting individual results for the three foot-shank positions. In order to avoid interpretative mistakes, we adopted the following reference terms for the test positions: 15 degrees → flexion, 0 degrees → neutral position, -15 degrees → extension [33].
▪ Data analysis
We decided to evaluate foot extensor and flexor muscles with the Peak Torque parameter, which represents the single highest value recorded during measurement (instantaneous peak torque). Because this parameter does not always fully reflect the actual muscle force generation capabilities, Average Torque was also measured, as it provides the average torque value generated over the course of entire test. Additionally, the ratio of AVGT to subjects’ body mass was calculated, which we assumed would normalize the results. Then, the agonist/antagonist ratio was automatically established; it is a percentage parameter which illustrates torque balance between muscle groups in a given angle position [34].
▪ Statistical analysis
Data were analysed with Statistica 13.1. Demographic data and clinical characteristics are presented as means and standard deviations (SD), medians or frequencies. The Shapiro-Wilk test was used to assess the normality of distributions in the test score. An independent t-test or nonparametric Mann-Whintey U test were used to compare the differences between stroke and control groups (age, height, weight, BMI). The chi-square test was used to compare difference between groups for categorical variables such as risk factors. Nonparametric Kruskal-Wallis test was used to analyze differences between the paretic side, non-paretic side and healthy group. Post hoc comparisons of positions and muscle groups were performed to find out when differences between tested groups are significant. P-values of less than 0.05 were considered statistically significant.
Results
▪ Isometric torque of foot extension
Recorded parameter values representing paretic foot extensor force (PT, AVGT, AVGT/BW) differed considerably from the same values obtained in the control group (p<0.001) (Table 1) as well as from values of the non-paretic foot, yet only in neutral foot positioning in the same patient (PT: p=0.012, AVGT: p=0.011, AVGT/ BW: p=0.027), which is presented in (Table 2). Moreover, it was found that differences between mean PT, AVGT and AVGT/BW values in three test positions (15°, 0°, -15°) were significant.
| Characteristics | Ischemic Stroke Group | Control Group | p value |
|---|---|---|---|
| n=34 | n=34 | ||
| Gender (male/female) | 25/9 | 21/13 | |
| Age (years) | 64.9 ± 9.2 | 61.6 ± 5.6 | p>0.05 |
| Height (cm) | 171.3 ± 9.6 | 171.6 ± 9.7 | p>0.05 |
| Weight (kg) | 81.5 ± 13.9 | 80.2 ± 15.8 | p>0.05 |
| Body mass index (kg/m2) | 27.9 ± 4.9 | 27.2 ± 4.6 | p>0.05 |
| Risk factors | |||
| Smoke | 12 (35.29%) | 10 (29.41%) | p>0.05 |
| Diabetes | 10 (29.41%) | 8 (23.52%) | p>0.05 |
| Arterial hypertension | 30 (88.23%) | 25 (73.52%) | p>0.05 |
| Atrial fibrillation | 15 (44.11%) | 6 (17.64%) | p<0.05 |
| Time since stroke (days) | 35.32 ± 2.88 | ||
| Affected side | |||
| Left | 15 (44.2%) | ||
| Right | 19 (55.8%) | ||
| Localization (Bamford Classification) | |||
| TACI | 4 (11.76%) | ||
| PACI | 16 (47.05%) | ||
| LACI | 12 (35.29%) | ||
| POCI | 2 (5.8%) | ||
| AMTS | 9.67 ± 0.47 | ||
| NIHSS | 10.67 ± 1.5 | ||
| Barthel Index | 67.05 ± 15.52 | ||
| Modified Rankin scale (n) : I/ II/ III | 15/15/4 | ||
| Modified Ashworth scale (n): Grade 1 / 1+ | 18/16 | ||
| Brumstrom Stages of Lower Limb (n) : IV/V/VI | 8/22/4 |
Abbreviations: TACI: Total Anterior Circulation Syndrome; PACI: Partial Circulation Syndrome; LACI: Lacunar Syndrome; POCI: Posterior Circulation Syndrome; AMTS: Abbreviated Mental Test Score; NIHSS: National Institutes Of Health Stroke Scale
Table 1: General characteristics of stroke subjects and control group.
| Isometric Parameter | Paretic Side | Non paretic side | Control Group | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Position | Mean ± SD | median | Mean ± SD | median | Mean ± SD | median | Paretic vs Non Paretic | Paretic vs Control | Non paretic vs Control |
| + 15 0 | 14.6 ± 9.6˟ | 13.2 | 21.6 ± 11.7˟ | 21 | 28.9 ± 14.5˟ | 30 | 0.052 | <0.001 | 0.166 |
| 0 0 | 10.4 ± 6.8* | 9.2 | 17.4 ± 10.4* | 15.1 | 24.1 ± 9.2* | 22.2 | 0.012 | <0.001 | 0.013 |
| - 15 0 | 4.9 ± 2.9*˟ | 5.3 | 8.2 ± 5.5*˟ | 6.5 | 14.8 ± 7.1*˟ | 14.4 | 0.06 | <0.001 | <0.001 |
| AVG PEAK TQ | |||||||||
| + 15 0 | 12.8 ± 9.0˟ | 11.1 | 18.7 ± 11.1˟ | 17.2 | 26.2 ± 14.1˟ | 26.5 | 0.087 | <0.001 | 0.135 |
| 0 0 | 9.3 ± 6.3* | 8.6 | 15.7 ± 10.0* | 12.8 | 21.4 ± 9,2* | 18.1 | 0.011 | <0.001 | 0.026 |
| - 15 0 | 4.6 ± 2.7*˟ | 4.9 | 7.3 ± 5.0*˟ | 5.3 | 13.7 ± 6.9*˟ | 12.5 | 0.098 | <0.001 | <0.001 |
| AVG PT/BW | |||||||||
| + 15 0 | 16.2 ± 11.4˟ | 14.6 | 23.8 ± 15.1˟ | 19 | 33.3 ± 16,9˟ | 33.3 | 0.107 | <0.001 | 0.084 |
| 0 0 | 11.8 ± 8.0* | 10.8 | 20.1 ± 13.6* | 13.9 | 27.8 ± 10.9* | 25.5 | 0.027 | <0.001 | 0.007 |
| - 15 0 | 5.8 ± 3.2*˟ | 6.1 | 9.5 ± 7.0*˟ | 6.9 | 17.6 ± 8.2*˟ | 16.4 | 0.136 | <0.001 | <0.001 |
Table 2: Test parameter values demonstrating foot extensor torque in post-stroke patients on paretic and non-paretic side compared with the control group.
▪ Isometric torque of foot flexion
Recorded values demonstrating paretic foot flexor force (PT, AVGT, AVGT/BW) differed considerably from the same values obtained in the control group (p<0.001), but they did not differ from values recorded in the non-paretic limb. A significant difference between the results of non-paretic limbs and healthy limbs in the control group is an important finding (Table 3).
| Isometric Parameter | Paretic Side | Non paretic side | Control Group | p value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | mean ± SD | median | mean ± SD | median | mean ± SD | median | paretic vs non-paretic | paretic vs control | non-paretic vs control | ||||||||
| + 15 0 | 18.3 ± 14.9˟ | 14.2 | 26.4 ± 18.7˟ | 22.7 | 47.0 ± 20.9ʺ˟ | 42.5 | 0.161 | <0.001 | <0.001 | ||||||||
| 0 0 | 27.3 ± 24.5 | 17.4 | 38.8 ± 28.6 | 36.5 | 78.2 ± 33.2ʺ | 70.8 | 0.412 | <0.001 | <0.001 | ||||||||
| - 15 0 | 33.6 ± 32.2˟ | 22.4 | 47.6 ± 38.7˟ | 34.9 | 107.3 ± 44.7˟ | 104 | 0.388 | <0.001 | <0.001 | ||||||||
| AVG PEAK TQ | |||||||||||||||||
| + 15 0 | 15.4 ± 14.4˟ | 10.1 | 21.7 ± 15.8˟ | 20.1 | 42.5 ± 19.8ʺ˟ | 38.7 | 0.187 | <0.001 | <0.001 | ||||||||
| 0 0 | 23.8 ± 22.0 | 15.2 | 33.7 ± 25.0 | 31.6 | 72.9 ± 32.4ʺ | 66 | 0.385 | <0.001 | <0.001 | ||||||||
| - 15 0 | 31.1 ± 30.5˟ | 19 | 42.0 ± 35.8˟ | 27.8 | 99.9 ± 43.7˟ | 99.9 | 0.545 | <0.001 | <0.001 | ||||||||
| AVG PT/BW | |||||||||||||||||
| + 15 0 | 19.2 ± 17.5˟ | 12.8 | 27.2 ± 19.4˟ | 24.7 | 52.8 ± 20.8ʺ˟ | 52.9 | 0.212 | <0.001 | <0.001 | ||||||||
| 0 0 | 29.7 ± 26.5 | 18.6 | 42.2 ± 31.6 | 36.2 | 90.2 ± 32.5ʺ* | 92.8 | 0.412 | <0.001 | <0.001 | ||||||||
| - 15 0 | 38.5 ± 36.9˟ | 22.8 | 51.7 ± 44.7˟ | 31.5 | 121.3 ± 41.9*˟ | 128.2 | 0.61 | <0.001 | <0.001 | ||||||||
Table 3: Test parameter values demonstrating foot flexor torque in post-stroke patients on paretic and non-paretic side compared with the control group.
Moreover, it was found that differences between mean PT, AVGT and AVGT/BW values were significant in 15° and -15° foot positions.
▪ Isometric extensor-flexor torque ratio
Investigating the ratio of average flexor torque to average extensor torque found that the non-paretic limb shows significant differences between measurements conducted in 15°, 0° and -15° foot positions. This relationship was also found in values of the non-paretic limb as well as limbs of healthy individuals from the control group. Significant differences were also recorded when comparing test values obtained for paretic limbs and limbs of healthy volunteers.
Discussion
Research studies [15,35] have shown that gait performance is determined by ankle joint ability. This ability, on the other hand, depends to a large extent on the performance of foot extensors and flexors [29]. This is important particularly in cases of extensor and flexor dysfunctions caused by brain stroke, which is evidenced by clinical observations and research findings [7,36]. Extensor and flexor weakness in the paretic foot as well as abnormal relationships between muscle forces in post-stroke patients leads to the development of pes equinovarus deformity, which then impairs the stance phase and swing fluency of gait [4,5,37]. Therefore, regaining normal muscle force relationship in foot flexors and extensors reduces the risk of developing pes equinovarus and improves gait fluency and posture stability, which has been proven by other researchers [9-12]. Similar to our study (Tables 2 and 3), Kawakami et al. [8] indicated that post-stroke patients’ function is affected by weakening of both extensors and flexors in the paretic foot compared with equivalent values of non-paretic limb and healthy volunteers. Besides absolute muscle torque values of foot extensors and flexors, it seems that another interesting problem in patients after stroke is a reciprocal relationship found between these forces. Available research literature indicates that foot muscles are characterized by a constant extensor-flexor torque ratio [38]. Invariance of this relationship is a condition for normal ankle function during gait. A similar observation was made in a study into motor performance of the knee joint, and the H/Q rate was unequivocally established as equal to ½, which represents the ratio of knee flexor-extensor force [39-41].
Therefore, the following question arises: do post-stroke patients exhibit abnormal force relationships in antagonistic muscle groups which determine ankle ability? And moreover, may foot positioning in relation to the shank affect these abnormal force relationships? We have not found any research studies devoted to this problem in available professional literature. The Biodex dynamometer and the isometric protocol were applied to investigate foot extensor and flexor torques in paretic and non-paretic limbs in post-stroke patients as well as in healthy limbs in the control group. The choice of this research method was guided by the finding of the pilot study: a few weeks after stroke, patients trying to perform extension or flexion with the paretic foot under protocols different than the isometric protocol reported pain, which resulted in limited muscle contraction force (see Materials and Methods). This is important insofar as the isokinetic protocol is used across the majority of available research studies [28,42-45]. It is worth mentioning, however, that in most studies found in professional literature subjects were examined in a much later period post stroke compared with our study [46,47]. The usefulness of applying the isometric protocol in research into torque forces generated by foot extensors and flexors is also reflected by the fact that studies adopting similar approach are extremely rare [48].
Review of relevant subject literature shows that gait performance in post-stroke patients is to a large extent determined by the force generation capabilities of foot extensor muscles. In our study on paretic and non-paretic limbs as well as healthy limbs (Table 2), it was found that when the foot was in the flexed position, its extensor muscles were capable of generating significantly higher PT, AVGT, and AVGT/ BW forces compared with corresponding measurements of neutral or extended foot position. Independently of this finding, our analysis of PT, AVGT, and AVGT/BW forces generated by paretic foot extensors in different foot positions (Table 2) revealed that these values differ significantly from corresponding values obtained in the control group (p<0.001), which clearly indicates considerable muscle weakness and resulting gait impairments. Cruz and Dhaher [23] demonstrated that functional contraction of paretic foot flexors in post-stroke patients, which can be compared to our extensor torque measurements of flexed foot, results in lower peak torques generated by foot extensors compared with healthy limbs. This can affect swing phase fluency of the paretic limb. Gao et al. [24], on the other hand, found that methods used for stretching foot flexors not only increase extension range of paretic foot ankle in poststroke patients, but they also enhance ankle extensor force. It is worth noting, however, that the mentioned study applied the isokinetic protocol. Also Michielsen et al. [49] used the isokinetic protocol and showed that extending flexor muscles in a paretic foot improves the capability of extensors to generate force.
On the other hand, after comparing PT, AVGT, and AVGT/BW results of the non-paretic foot with corresponding values recorded in the control group, we concluded that they differ substantially but only in the neutral foot position (PT: p=0.013; AVGT: p=0.026; AVGT/BW: p=0.007) and -15° foot extension (PT: p<0.001; AVGPT: p<0.001; AVGPT/BW: p<0.001). For this reason, the following has to be considered: is “hemiparesis”, a term commonly used in clinical practice, an accurate description, since muscle force deficits (notwithstanding the fact that found only in two tested foot positions) were observed also in the unaffected side of patients with paresis? It is possible that observed force deteriorations in non-paretic limbs is the consequence of natural global inactivity of patients immediately after stroke. An additional indication of weakening of non-paretic foot extensors comes from comparing its PT, AVGT, and AVGT/BW values with PT, AVGT, and AVGT/BW values of the paretic foot extensors. The comparison showed no significant differences between results of extended foot (-15 position: PT: p=0.060; AVGT: p=0.098; AVGT/ BW: p=0.136) and flexed foot (position 15: PT: p=0.052; AVGT: p=0.087; AVGT/BW: p=0.107). Muscle weakening in the non-paretic side was also confirmed by Thajchayapong et al. [50], Novak and Novak and Brouwer [43] and Hsu et al. [51].
Relatively few biomechanical studies concentrate on evaluating the weakening of paretic foot flexors and its potential impact on motor performance of post-stroke patients. Analysis of PT, AVGT, and AVGT/BW values of paretic foot flexors (Table 3) showed that they differ significantly from corresponding values obtained in the control group for all three test positions (-15° foot extension p<0.001, 0° neutral foot position p<0.001, 15° foot flexion p<0.001). Weakening of flexor muscles in paretic foot was also proved by Freirei et al. [21] by using the isokinetic protocol and comparisons with nonparetic side and a control group.
Similar to the identified relationship between extensor muscle force value and different footshank positions, this case also demonstrated that the extended foot-shank position influenced the results obtained for the paretic, non-paretic and healthy limb. The highest PT, AVGT, and AVGT/BW values of foot flexors were recorded in the extended foot position, whereas the lowest ones in the flexed position (Table 3). This is consistent with study results by Kemertzis et al. [22] and Gao et al. [24,52], who proved that peak torque of foot flexors correlates with an increase in their length, which is consistent with our measurements of the extended position. Furthermore, after comparing PT, AVGT, and AVGT/BW of non-paretic foot flexors with corresponding values measured in the control group we concluded that they differ substantially in all three test measurement positions (Table 3).
Similar to foot extensor evaluation, study results also confirm that the non-paretic limb of post-stroke patients experiences significant weakening of foot extensor muscles. Again, it is worth noting that in light of research findings the term hemiparesis is marked by terminological imprecision. Bilateral weakening of foot flexor muscles in post-stroke patients is further evidenced by the lack of any significant differences between PT, AVGT, and AVGT/ BW of the paretic and non-paretic foot in all three measurement positions.
Differences between agonist/antagonist ratios were significant in the paretic, non-paretic and healthy limb and in all three measurement positions (Table 4). This indicates that the extensor-flexor torque ratio is variable and changes with different foot positions and may be very important in examining impaired gait performance in post-stroke patients. Importantly, we found significant differences between extensor-flexor torque ratios only when comparing the non-paretic foot and the healthy foot and only in 0° neutral foot position p=0.002 and 15º flexed position p=0.019. The finding is important with regard to the problem of paretic foot “bumping” against ground during swing phase, which is commonly observed in clinical practice. It is our opinion that this inadequate force generation in the paretic foot combined with abnormal relationship between forces generated by foot extensors and flexors accounts for impairments in ankle function during gate. Lin et al. [29] proved that reducing the strength of extensors and flexors of the paretic foot affects equally the correctness of walking but in various aspects concerning mainly speed (extensors) and spatial coordination (flexors). Therefore, we believe that the separate assessment of extensors or flexors of the paretic foot does not give a full view of the degree of dysfunctionality of the patient in terms of the ability to control the movement of the ankle and possible gait quality. Hence, in our study we proposed to evaluate the relationship of the AVGPT flexors values to the AVGPT extensors of the foot. Kesar et al. [27] also drew attention to the principle of joint improvement of antagonistic muscles in their operation, which is important in relation to the rehabilitation program. The rationales for this concept are, according to these researchers, the sequential activation of these muscles in a walk.
| AGON vs ANTAGON | % | Paretic Side | Non-paretic side | Control Group | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | median | Mean ± SD | median | Mean ± SD | median | Paretic vs Non Paretic | Paretic vs Control | Non paretic vs Control | ||
| + 15 0 | 101.8 ± 55.2ʺ˟ | 87.2 | 92.0 ± 49.0ʺ˟ | 92.3 | 70.0. ± 43.0ʺ˟ | 62.9 | 1 | 0.019 | 0.079 | |
| 0 0 | 58.4 ± 32.3ʺ* | 55.2 | 55.5 ± 51.3ʺ* | 39.9 | 36.0 ± 16.0ʺ* | 29 | 0.42 | 0.002 | 0.182 | |
| - 15 0 | 26.6 ± 22.9*˟ | 21.9 | 26.6 ± 27.7*˟ | 19.9 | 16.0 ± 8.0*˟ | 14 | 1 | 0.050 | 0.225 | |
Table 4: Agonist/antagonist ratios for three conditions of isometric test.
▪ Indications for physiotherapists and patients
To assess the function of the ankle joint in patients in the early period after the stroke, measurements of the strength of both the flexors and the extensors of the foot should be used. In our research, we showed significant weakness of these muscles in both limbs, therefore in the initial period of rehabilitation we should use exercises strengthening both groups of muscles. The muscle strength of the extensor and flexor of the feet depends on the length of the muscle at the time of generating the contraction, therefore taking into account the results obtained by us, the optimal method of strengthening should include exercises at different angles of the foot relative to the shank.
Study Limitations
The most important limitation concerns the methodology of the study itself. The -15°, 0° and 15° test positions were imposed from above and resulted from a “rigid” test protocol that made it impossible to assess the PT, AVGPT and AVGPT/BW values for other foot settings relative to the shank. We believe that much valuable information would be provided by studies at other angular values. In addition, the methodology of the test in the isometric protocol assumes that the patient tightens the test muscles to the test command, which does not mean that he actually does it. Another limitation was a relatively small group of respondents.
References
- Kunkel D, Potter J, Mamode L. A cross-sectional observational study comparing foot and ankle characteristics in people with stroke and healthy controls. Distabil. Rehab 39(12), 1149-1154 (2017).
- Jeon SN, Choi JH. The effects of ankle joint strategy exercises with and without visual feedback on the dynamic balance of stroke patients. J. Phys. Ther. Sci 27(8), 2515-2518 (2015).
- Yalcin E, Akyuz M, Onder B, et al. Position sense of the hemiparetic and non-hemiparetic ankle after stroke: is the non-hemiparetic ankle also affected? Eur. Neurol 68(5), 294-299 (2012).
- Kim K, Lee S, Kim D, et al. The effects of ankle joint muscle strengthening and proprioceptive exercise programs accompanied by functional electrical stimulation on stroke patients' balance. J. Phys. Ther. Sci 27(9), 2971-2975 (2015).
- Rogind H, Christensen J, Danneskiold-Samsøe B, et al. Posturographic description of the regaining of postural stability following stroke. Clin. Physiol. Funct. Imaging 25(1), 1-9 (2005).
- Abe H, Michimata A, Sugawara K, et al. Improving gait stability in stroke hemiplegic patients with a plastic ankle-foot orthosis. Tohoku. J. Exp. Med 218(3), 193-199 (2009).
- Wu CL, Huang MH, Lee CL, et al. Effect on spasticity after performance of dynamic-repeated-passive ankle joint motion exercise in chronic stroke patients. Kaohsiung. J. Med. Sci 22(12), 610-617 (2006).
- Kawakami K, Miyasaka H, Nonoyama S, et al. Randomized controlled comparative study on effect of training to improve lower limb motor paralysis in convalescent patients with post-stroke hemiplegia. J. Phys. Ther. Sci 27(29), 2947-2950 (2015).
- Boffeli TJ, Collier RC. Minimally invasive soft tissue release of foot and ankle contracture secondary to stroke. J. Foot. Ankle. Surg 53(3), 369-375 (2014).
- Diong J, Herbert RD. Is ankle contracture after stroke due to abnormal intermuscular force transmission? J. Appl. Biomech 31(1) 13-18 (2015).
- Yeh CY, Chen JJ, Tsai KH. Quantitative analysis of ankle hypertonia after prolonged stretch in subjects with stroke. J. Neurosci. Meth 137(2), 305-314 (2014).
- Kwah LK, Harvey LA, Diong JH, et al. Half of the adults who present to hospital with stroke develop at least one contracture within six months: an observational study. J. Physiother 58(1), 41-47 (2012).
- Kawakami Y, Kanehisa H, Fukunaga T. The relationship between passive ankle plantar flexion joint torque and gastrocnemius muscle and achilles tendon stiffness: implications for flexibility. J. Orthop. Sports. Phys. Ther 38(5), 269-276 (2008).
- Selles RW, Li X, Lin F, et al. Feedback-controlled and programmed stretching of the ankle plantarflexors and dorsiflexors in stroke: effects of a 4-week intervention program. Arch. Phys. Med. Rehabil 86(12), 2330-2336 (2005).
- Dorsch S, Ada L, Canning CG, et al. The strength of the ankle dorsiflexors has a significant contribution to walking speed in people who can walk independently after stroke: an observational study. Arch. Phys. Med. Rehabil 93(6),1072-1076 (2012).
- Ng SS, Hui-Chan CW. Contribution of ankle dorsiflexor strength to walking endurance in people with spastic hemiplegia after stroke. Arch. Phys. Med. Rehabil 93(6), 1046-1051 (2012).
- Andrews AW, Bohannon RW. Short-term recovery of limb muscle strength after acute stroke. Arch. Phys. Med .Rehabil. 84(1), 125-130 (2003).
- Blanchette AK, Noël M, Richards CL, et al. Modifications in ankle dorsiflexor activation by applying a torque perturbation during walking in persons post-stroke: a case series. J. Neuroeng. Rehabil. 11, 98 (2014).
- Nadeau S, Gravel D, Arsenault AB, et al. Dynamometric assessment of the plantarflexors in hemiparetic subjects: relations between muscular, gait and clinical parameters. Scand. J. Rehabil. Med 29(3), 137-146 (1997).
- Palmer JA, Hsiao H, Awad LN, et al. Symmetry of corticomotor input to plantarflexors influences the propulsive strategy used to increase walking speed post-stroke. Clin. Neurophysiol 127(3), 1837-1844 (2016).
- Freire B, Dias CP, Goulart NB, et al. Achilles tendon morphology, plantar flexors torque and passive ankle stiffness in spastic hemiparetic stroke survivors. Clin. Biomech 41(1), 72-76 (2017).
- Kemertzis MA, Lythgo ND, Morgan DL, et al. Ankle flexors produce peak torque at longer muscle lengths after whole-body vibration. Med. Sci. Sports. Exerc 40(11), 1977-1983 (2008).
- Cruz TH, Dhaher YY. Impact of ankle-foot-orthosis on frontal plane behaviors post-stroke. Gait. Posture 30(3), 312-316 (2009)
- Gao F, Ren Y, Roth EJ, et al. Effects of repeated ankle stretching on calf muscle-tendon and ankle biomechanical properties in stroke survivors. Clin. Biomech 26(5), 516-522 (2011).
- Kobayashi T, Leung AK, Akazawa Y, et al. Quantitative measurement of spastic ankle joint stiffness using a manual device: a preliminary study. J. Biomech. 43(9):1831-1834 (2010).
- Eng JJ, Lomaglio MJ, Macintyre DL. Muscle torque preservation and physical activity in individuals with stroke. Med. Sci. Sports. Exerc 41(7),1353-1360 (2009).
- Kesar TM, Perumal R, Reisman DS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke 40(12), 3821-3827 (2009).
- Sekiguchi Y, Muraki T, Tanaka N, et al. Relationship between activation of ankle muscles and quasi-joint stiffness in early and middle stances during gait in patients with hemiparesis. Gait. Posture 42(3), 348-353 (2015).
- Lin PY, Yang YR, Cheng SJ, et al. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch. Phys. Med. Rehabil. 87(4), 562-568 (2006).
- Kitatani R, Ohata K, Sato S, et al. Ankle muscle coactivation and its relationship with ankle joint kinematics and kinetics during gait in hemiplegic patients after stroke. Somatosens. Mot. Res 33(2), 79-85 (2016).
- Kwah LK, Herbert RD, Harvey LA, et al. Passive mechanical properties of gastrocnemius muscles of people with ankle contracture after stroke. Arch Phys Med Rehabil. 93(7), 1185-1190 (2012).
- Biodex Multi-Joint System. Isokinetic Source Book. Biodex Medical Systems, Inc.
- Hislop HJ PJ. The isokinetic concept of exercise. Phys. Ther 47(2), 114–117 (1967).
- Malerba JL, Adam ML, Harris B, et al. Reliability of dynamic and isometric testing of shoulder external and internal rotators. J. Orthop. Sports. Phys. Ther 18(4), 543–552 (1993).
- Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys. Ther. 83(1), 49-57 (2003).
- Ohata K, Yasui T, Tsuboyama T, et al. Effects of an ankle-foot orthosis with oil damper on muscle activity in adults after stroke. Gait. Posture 33(1), 102-107 (2011).
- Jeon SN, Choc JH. The effects of ankle joint strategy exercises with and without visual feedback on the dynamic balance of stroke patients. J. Phys. Ther. Sci 27(1), 2515-18 (2015).
- Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur. J. Appl. Physiol 112(1), 267-275 (2012).
- Baratta R, Solomonow M, Zhou BH, et al. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am. J. Sports. Med 16(2), 113–122 (1988).
- Deli CK, Paschalis V, Theodorou AA, et al. Isokinetic knee joint evaluation in track and field events. J. Strenght. Cond. Res 25(9), 2528–2536 (2011).
- Lanshammar K, Ribom EL. Differences in muscle strength in dominant and non-dominant leg in females aged 20–39 years—a population-based study. Phys. Ther. Sport 12(2), 76–79 (2011).
- .Meeteren Jv, Roebroeck ME, Stam HJ. Test-retest reliability in isokinetic muscle strength measurements of the shoulder. J. Rehabil. Med 34(2), 91-95 (2002).
- Novak AC, Brouwer B. Kinematic and kinetic evaluation of the stance phase of stair ambulation in persons with stroke and healthy adults: a pilot study. J. Appl. Biomech 29(4), 443-452 (2013).
- Maynard V, Bakheit AM, Shaw S. Comparison of the impact of a single session of isokinetic or isotonic muscle stretch on gait in patients with spastic hemiparesis. Clin. Rehabil 19(2), 146-154 (2005).
- Wist S, Clivaz J, Sattelmayer M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Arch. Phys. Med. Rehabil 59(2), 114–124 (2016)
- Burpee JL, Lewek MD. Biomechanical gait characteristics of naturally occurring unsuccessful foot clearance during swing in individuals with chronic stroke. Clin. Biomech 30(10),1102-1107 (2015).
- Teixeira-Salmela LF, Olney SJ, Nadeau S, et al. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch. Phys. Med. Rehabil 80(10), 1211-1218 (1999).
- Soo-Kyung, Tae Heon L, Sang Sook L. The Effects of Changes of Ankle Strength and Range of Motion According to Aging on Balance. Rehabil. Med. 37(1), 10–16 (2013).
- Michielsen ME, Selles RW, van der Geest JN, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil. Neural. Repair 25(3), 223-233 (2011).
- Thajchayapong M, Alibiglou L, Lilaonitkul T, et al. Mechanical abnormalities of the spastic ankle in chronic stroke subjects. Conf. Proc. IEEE. Eng. Med .Biol. Soc 1(1), 3688-3691 (2006).
- Hsu AL, Tang PF, Jan MH. Test-retest reliability of isokinetic muscle strength of the lower extremities in patients with stroke. Arch. Phys. Med. Rehabil 83(8):1130-1137 (2002).
- Gao F, Grant TH, Roth EJ. Changes in passive mechanical properties of the gastrocnemius muscule at the muscle fascicle and joint levels in stroke. Arch. Phys. Med. Rehabil 90(5), 819 -926 (2009).