Research Article - Interventional Cardiology (2020) Volume 12, Issue 7
Lambda-like waveform: A new risk predictor of impending malignant arrhythmias in patients with coronary artery disease
- Corresponding Author:
- Guangqiang Wang
Division of Cardiology,
Yantai Yuhuangding Hospital of Qingdao University,
Yantai, Shandong 264000,
People’s Republic of China,
E-mail: wgq198632@126.com
Received date: October 19, 2020; Accepted date: November 09, 2020; Published date: November 16, 2020
Abstract
Background: The association between the appearance of a lambda-like waveform and occurrence of malignant arrhythmias during acute myocardial ischemia is unclear in patients with Coronary Artery Disease (CAD).
Hypothesis: Lambda-like waveform is a new risk predictor of impending malignant arrhythmias in patients with CAD.
Methods: We evaluated electrocardiograms (ECGs) from 8 patients with transient ischemia. The lambda-like pattern was defined as a distinctively notched, giant J wave with a descending ST-segment elevation followed by an alternately negative T wave. The typical pattern was followed by ventricular tachyarrhythmias and/or severe bradyarrhythmias in the CAD group.
Results: Lambda-wave patientshad a higher incidence of syncope, CAD, arrhythmia, cardiopulmonary resuscitation, obvious coronary artery stenosis, slower heart rate, and longer PR and QT intervals than the control patients. In addition, more lambda-wave patients took medicine for coronary artery dilatation and had recurrent malignant arrhythmias than the control patients. A difference with respect to the distribution and morphology of ischemic ECG waveforms between the 2 groups was observed. Furthermore, the incidence of severe bradyarrhythmias and/or ventricular tachyarrhythmias was significantly higher in the CAD group than that in the control group.
Conclusion: An inferior and lateral lambda-like pattern may predict malignant arrhythmias in patients with CAD. A notched giant J wave may also be a risk factor for impending arrhythmias in inferior and lateral leads. The relevance of this pattern to acute ischemia may be important; however, no risk stratification or management strategies for this pattern have been established.
Keywords
Coronary artery disease • Electrocardiogram • Lambda-like waveform • Malignant arrhythmia
Introduction
Sudden Cardiac Death (SCD) is the most common cause of all cardiac deaths [1]. SCD is a heterogeneous condition that may be caused by acute ischemia, structural defects, myocardial scars, and/or genetic mutations. Among adults, SCD is associated with Coronary Artery Disease (CAD). CAD is further classified as atherosclerotic and non-atherosclerotic. Atherosclerosis is the main pathological basis of CAD, which can result in coronary artery stenosis, occlusion and/or spasm leading to myocardial ischemia, hypoxia, and even myocardial necrosis. CAD causes >75% of SCDs in patients aged over 50 years [2]. The most common cause of CAD-induced SCD is life-threatening arrhythmias, including severe bradyarrhythmias (sinus bradycardia, sinus arrest, and atrioventricular block) and ventricular tachyarrhythmias (ventricular tachycardia and ventricular fibrillation).
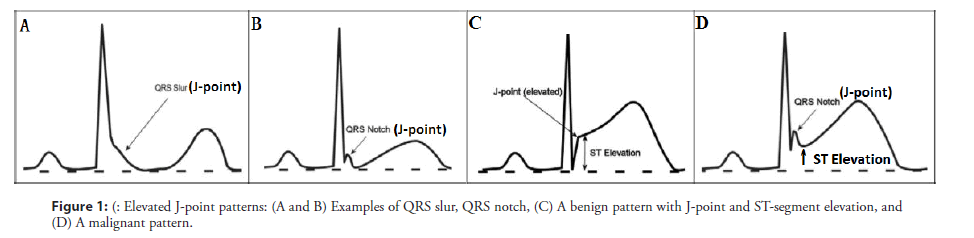
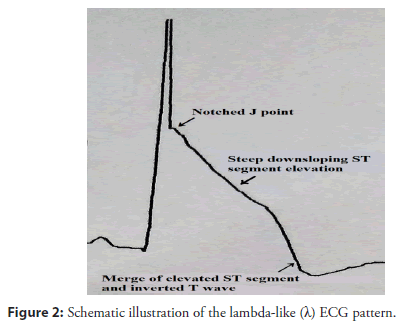
Asystole and pulseless electrical activity are also important causes of CAD-induced SCD. In patients with CAD, uncertainty exists as to whether electrocardiogram (ECG) territory, degree of J-point elevation, ST-segment morphology, and T-wave alterations are associated with a risk of impending malignant arrhythmias. Gussak et al. proposed that a lambda wave could represent a new clinical entity/arrhythmogenic syndrome and a new ECG marker for SCD [3,4]. A recent report described this waveform as suggestive of idiopathic ventricular fibrillation (VF) with early repolarization (ER) abnormalities, and might be a high-risk marker for SCD [5]. According to a consensus report, the typical J wave is characterized by an elevation of ≥ 0.2 mv of the QRS-ST junction (J-point) in the inferior (II, III, and aVF) and/or lateral (I, aVL, and V4 to V6) leads with either a slurring or notching morphology, excluding leads V1 to V3, with a QRS duration <120 ms (Figures 1A and 1B) [6,7]. A notched, giant J-wave accompanied by a steeply down-sloping ST-T segment elevation and negative T wave is defined as a lambdalike waveform, which was labeled by Gussak et al [3]. based on similarity with the lower case lambda (λ) (Figure 2). The lambda-like waveform is associated with life-threatening arrhythmias in patients with CAD and can occasionally develop during acute myocardial ischemia due to induced coronary spasms, although the mechanism has not been fully elucidated [8,9]. No study has evaluated the clinical features or impact on clinical outcomes of this distinctive ECG manifestation. Our study aims to identify the uncommon ECG features that indicate an elevated risk of life-threatening arrhythmias in patients with CAD. We present the lambda-like pattern from Holter monitoring in patients with acute ischemia-induced malignant arrhythmias.
Methods
Study design and definition of the lambda-like waveform
We performed a retrospective, case-control study that included 8 patients with CAD and 16 age-matched, sex-matched control patients without CAD. ECGs were evaluated in random order by 2 physicians who were blinded to patient grouping. The ECGs were checked for the presence of an elevated J-point, and lambda-like patterns were identified. Cipriani et al. characterized a triangular QRS-ST-T waveform, which they defined as the extreme form of a lambda-like waveform [10]. We analyzed the ECGs of patients with CAD and normal patients, and compared the lambda-like patterns proximate to the malignant arrhythmia episodes and remote from syncope to a benign variation in inferior and/or lateral leads (Figure 1C). The benign variation was a twisting J-point elevation accompanied by a rapidly ascending or obliquely straight STsegment elevation in the absence of a personal or family history of SCD; the benign variation was associated with a short QT interval and attenuation of J-point elevation during increases in heart rate [11]. Patients with inherited arrhythmia syndromes, such as long/ short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia, were excluded because of known genetic variations in ion channels [12]. Holter monitoring was performed during fatal arrhythmia episodes, and ECGs were recorded whenever dynamic repolarization changes were observed in the rhythm strip. As a result, transient ECG changes that occurred before the onset of malignant arrhythmia would be recorded. Reversible factors causing J waves or ST elevation were aggressively sought and ruled out.
Study population, inclusion and exclusion criteria
Holter monitors of the selected patients who visited our hospital between March 1, 2018 and April 30, 2019 were reviewed. The CAD group included (1) coronary stenosis ≥ 50% and/or coronary spasm by coronary angiography but without type 1 myocardial infarction or other heart diseases, and (2) a lambda waveform followed by malignant arrhythmia in ECG. Patients were qualified for inclusion in the control group if they had (1) no history or evidence of heart disease or no unexplained syncope, and (2) their ECG was reported as a benign variation.
Follow up and study endpoint
All selected patients were followed up clinically by telephone contact or office visit. The study endpoints were in-hospital and 30-day follow-up major adverse cardiovascular events, which were defined as the composite of unstable angina, acute myocardial infarction, recurrent malignant arrhythmia, and SCD.
ECG classification
An elevated J-point was defined as an elevation of at least 1 mm (0.1 mV) above the baseline in at least 2 continuous inferior leads (II, III, and aVF), lateral leads (I, aVL, and V4 to V6), or both leads, manifesting as QRS slurring (a smooth transition from the QRS end to the ST segment) or notching (a positive J-deflection inscribed on the S wave) [13]. The anterior precordial leads (V1 to V3) were excluded from the analysis of the elevated J-point to avoid the inclusion of patients with right ventricular dysplasia or Brugada syndrome [14]. The J-point amplitude (the height from the isoelectric line) was measured at the QRS-ST junction in the case of slurred J waves or the peak J point in the case of notched J waves and relative to the QRS onset to minimize any baseline-wandering effect. A typical J-point elevation was defined as an amplitude >2 mm (0.2 mV). A J-wave width of >80 ms was regarded as a giant J-wave during the peri-event period in patients with a J-point elevation. The morphology of the ST-segment elevation after the J point was divided into 3 types: ascending, horizontal, and descending. The amplitude of the ST-segment elevation was measured at the end of the J wave (J terminal or Jt), and the classification of horizontal (<0.1 mV), ascending (>0.1 mV), or descending (>0.1 mV) was done 100 ms after the Jt [15]. J/T was the ratio of J-point elevation magnitude and T-wave amplitude on the same ECG lead. The descending ST-segment elevation was defined as a J/T ratio of >1. The QT interval was calculated on the inferior lead with the highest magnitude of J-point elevation.
Ethics
Discrepancies were resolved by consensus. Our study complied with the Declaration of Helsinki, and our institution’s ethics review board approved the study’s protocol. Written informed consent was obtained from the patients in relation to the publication of this study.
Statistical analysis
The continuous variables were expressed as means+standard deviations. The categorical variables were expressed as numbers and percentages, and were compared using Fisher’s exact test, as appropriate. A two-tailed p-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM®SPSS® statistical software, version 24.0 (IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics of selected patients
A total of 24 consecutive patients were selected by Holter monitoring on admission and allocated to either the CAD group (n=8) or control group (n=16) according to a paired ratio of 1:2. Table 1 shows the baseline clinical characteristics of the study population. The selected patients were male with a mean age of 57.5 ± 9.1 years. No significant differences between the groups with respect to risk factors, chest tightness and pain, hypersensitivity troponin I, B-type natriuretic peptide, left ventricular ejection fraction, coronary angiography, and percutaneous coronary intervention were observed. None of the patients had echocardiographic or angiographic evidence of myocardial infarction. However, the incidence of syncope, CAD, arrhythmia, cardiopulmonary resuscitation, and obvious coronary artery stenosis was significantly higher in the CAD group than that in the control group (75% vs. 6.25%, p=.001; 62.5% vs. 0%, p=.001; 100% vs. 18.75%, p<.001; 37.5% vs. 0%, p=.028; 50% vs. 0%, p=.007). Heart rate was significantly slower, and the PR and QT intervals of the ECG were significantly longer in the CAD group than those in the control group (55 ± 6 vs. 67 ± 8 bpm, p=.001; 213.75 ± 26.14 vs. 166.69 ± 24.36 ms, p<.001; 512.63 ± 43.92 vs. 398.38 ± 21.74 ms, p<.001). In addition, the levels of low-density lipoprotein cholesterol and total cholesterol were significantly lower in the CAD group than those in the control group (2.01 ± 0.37 vs. 2.81 ± 0.77 mmol/L, p=.012; 3.7 ± 0.54 vs. 4.79 ± 1.26 mmol/L, p=.028). Moreover, the CAD group had a significantly higher incidence of oral therapy for coronary artery dilatation and recurrent malignant arrhythmia than the control group (100% vs. 31.25%, p= .002; 37.5% vs. 0%, p=.028).
| Baseline characteristics of selected patients | |||
|---|---|---|---|
| Variable | CAD group (n=8) | Control group (n=16) | p Value |
| Male, n(%) | 8(100) | 16(100) | 1 |
| Age (y, mean ± SD) | 59 ± 11.78 | 56.75 ± 7.71 | 0.578 |
| Hypertension, n(%) | 3(37.5) | 10(62.5) | 0.39 |
| Smoking, n(%) | 5(62.5) | 9(56.25) | 1 |
| Diabetes, n(%) | 0(0) | 3(18.75) | 0.526 |
| Symptoms | |||
| Chest tightness, n(%) | 6(75) | 10(62.5) | 0.667 |
| Chest pain, n(%) | 4(50) | 11(68.75) | 0.412 |
| Syncope, n(%) | 6(75) | 1(6.25) | 0.001 |
| History of CAD, n(%) | 5(62.5) | 0(0) | 0.001 |
| LDL-C (mmol/L, mean ± SD) | 2.01 ± 0.37 | 2.81 ± 0.77 | 0.012 |
| CHOL(mmol/L, mean ± SD) | 3.7 ± 0.54 | 4.79 ± 1.26 | 0.028 |
| Cardiopulmonary resuscitation before admission, n(%) | 3(37.5) | 0(0) | 0.028 |
| Onset of arrhythmia, n(%) | 8(100) | 3(18.75) | <.001 |
| Electrocardiogram at the onset of symptoms | |||
| Heart rate (bpm, mean ± SD) | 55 ± 6 | 67 ± 8 | 0.001 |
| PR interval (ms, mean ± SD) | 213.75 ± 26.14 | 166.69 ± 24.36 | <.001 |
| QT interval (ms, mean ± SD) | 512.63 ± 43.92 | 398.38 ± 21.74 | <.001 |
| hsTnI (pg/ml, mean ± SD) | 290.35 ± 667.51 | 3.09 ± 2.47 | 0.263 |
| BNP (pg/ml, mean ± SD) | 74.5 ± 101.43 | 17.96 ± 11.6 | 0.159 |
| LVEF (%, mean ± SD) | 65.75 ± 4.1 | 67.31 ± 4.14 | 0.392 |
| CAG in the proximal or middle of coronary arteries Stenosis | |||
| ≥50%, n(%) | 4(50) | 0(0) | 0.007 |
| <50%, n(%) | 2(25) | 5(31.25) | 1 |
| Coronary myocardial bridge, n(%) | 2(25) | 2(12.5) | 0.578 |
| Localized plaque, n(%) | 4(50) | 5(31.25) | 0.412 |
| Coronary slow flow, n(%) | 0(0) | 5(31.25) | 0.13 |
| Right coronary artery, n(%) | 6(75) | 6(37.5) | 0.193 |
| Left circumflex artery, n(%) | 2(25) | 4(25) | 1 |
| Left anterior descending, n(%) | 7(87.5) | 11(68.75) | 0.621 |
| Oral therapy for coronary artery dilatation, n(%) | 8(100) | 5(31.25) | 0.002 |
| PCI, n(%) | 1(12.5) | 0(0) | 0.333 |
| In-hospital and 30-day follow-up MACE, n(%) | 3(37.5) | 0(0) | 0.028 |
Abbreviations: CAD: Coronary Artery Disease; SCD: Sudden Cardiac Death; LDL-C: Low Density Lipoprotein Cholesterol; CHOL: Total Cholesterol; bpm: Beat per Minute; hsTnI: Hypersensitivity Troponin I; BNP: B-type Natriuretic Peptide; LVEF: Left Ventricular Ejection Fraction; CAG: Coronary Angiography; PCI: Percutaneous Coronary Intervention; MACE: Major Adverse Cardiovascular Events.
Table 1: Baseline characteristics of selected patients.
Distribution and morphology of ECG findings
Holter ECG monitoring at 25 mm/sec and 10 mm/mV revealed that the 8 patients in the CAD group had electrocardiographic lambdalike patterns on their ECGs that were followed by ventricular tachyarrhythmias (Figure S1, Supporting Information) and/or severe bradyarrhythmias (Figure S2, Supporting Information). Table 2 presents the ECG characteristics of the patients with lambda-like patterns in the CAD group.A significant difference in the distribution and morphology of ischemic waveforms between the groups (p<.05) was observed. The lambda-like waveforms of the CAD group were mainly located in the inferior and lateral ECG leads compared to the ER pattern of the control group (75% vs. 6.25%, p=.001). The morphology of the lambda-like waveform in the CAD group was different from that of the ER pattern in the control group (Figure 1C). In addition, the CAD group had a higher incidence of T-wave alternans than the control group (87.5% vs. 6.25%, p<.001) (Figure S3, Supporting Information). The incidence of severe bradyarrhythmias and/or ventricular tachyarrhythmias was significantly higher in the patients with a lambda- like waveform than that in the patients with an ER pattern (50% vs. 6.25%, p=.028; 62.5% vs. 12.5%, p=.021; 87.5% vs. 6.25%, p<.001). The lambda-like waveform is described as a distinctively notching and giant J-wave (amplitude 1.33 ± 0.41 mV; width 105.71 ± 3.09 ms) with descending ST-segment elevation followed by an alternately negative T-wave in the inferior and lateral leads; this is closely associated with the occurrence of severe bradyarrhythmias and/or ventricular tachyarrhythmias that trigger syncope in patients with CAD.
| Comparison of ECG parameters in selected patients with lambda-like pattern at a time remote from syncope and in control subjects | |||
|---|---|---|---|
| Variables | CAD group(n=8) | Control group(n=16) | p Value |
| Distribution of abnormal J-point and ST segment elevation | |||
| Inferior, n(%) | 7(87.5) | 3(18.75) | 0.002 |
| Lateral, n(%) | 7(87.5) | 2(12.5) | 0.001 |
| Both, n(%) | 6(75) | 1(6.25) | 0.001 |
| Shape of elevated J-point | |||
| Notching, n(%) | 7(87.5) | 0(0) | < .001 |
| Slurring, n(%) | 1(12.5) | 4(25) | 0.631 |
| J-wave width of >80 ms | |||
| Notching (ms, mean ± SD) | 105.71 ± 3.09 | 0 | <.001 |
| Slurring (ms, mean ± SD) | 89 ± 0 | 72.75 ± 11.3 | 0.288 |
| J-point elevation of >0.2 mV | |||
| Notching (mV, mean ± SD) | 1.33 ± 0.41 | 0 | <.001 |
| Slurring (mV, mean ± SD) | 1.10 ± 0 | 0.32 ± 0.06 | 0.002 |
| Morphology of ST segment elevation | |||
| Ascending, n(%) | 0(0) | 9(56.25) | 0.009 |
| Horizontal, n(%) | 0(0) | 7(43.75) | 0.054 |
| Descending, n(%) | 8(100) | 0(0) | <.001 |
| Amplitude of ST segment elevation | |||
| Ascending (mV, mean ± SD) | 0 | 0.12 ± 0.02 | <.001 |
| Horizontal (mV, mean ± SD) | 0 | 0.12 ± 0.03 | <.001 |
| Descending (mV, mean ± SD) | 0.8 ± 0.46 | 0 | 0.002 |
| T wave | |||
| Positive, n(%) | 2(25) | 14(87.5) | 0.005 |
| Negative, n(%) | 6(75) | 2(12.5) | 0.005 |
| Alteration, n(%) | 7(87.5) | 1(6.25) | <.001 |
| Arrhythmia | |||
| Severe bradyarrhythmia, n(%) | 5(62.5) | 2(12.5) | 0.021 |
| Ventricular tachyarrhythmia, n(%) | 7(87.5) | 1(6.25) | <.001 |
| Both, n(%) | 4(50) | 1(6.25) | 0.028 |
Table 2: Comparison of ECG parameters in selected patients with lambda-like pattern at a time remote from syncope and in control subjects.
Discussion
Our study focused on the lambda-like ECG pattern in patients with CAD. We are the first to find an association between this pattern and malignant arrhythmias. In addition, we innovatively explained its underlying mechanism.
Incidence of the lambda-like pattern
Cipriani et al. found that the triangular waveform is a rare ECG pattern that has an annual incidence of 0.7% among consecutive patients with ST elevation myocardial infarction (STEMI) [10]. However, an evaluation of the clinical frequency of the lambdalike pattern or its relationship with the occurrence of malignant arrhythmias was not possible in the present study. The incidence of the lambda-like pattern could not be estimated from our results because too few data were available from high-risk patients; these patients often die as a consequence of sudden arrhythmic death before they present. The typical pattern might be missed in patients who present late because Holter ECG monitoring results suggest that the patternis transitional, and ECGs may indicate early and transient ischemia; however, the symptoms of myocardial ischemia might be silent. Therefore, acquiring early ECG recordings is especially important for patients with lambda-like patterns.
Clinical implications
The typical lambda-like pattern may be a distinctive characteristic associated with malignant arrhythmias. In our study, this pattern in the corresponding ECG leads and the ST- segment elevation of the premature ventricular contractions or paroxysmal ventricular tachycardia were associated with the culprit coronary arteries (Figure S1, Supporting Information). Therefore, this pattern might have indicated lesions in the proximal or middle regions of coronary arteries. Myerburg et al. found that a spasm that occurred in the proximal region of a culprit artery showed lambda-like STsegment elevations in the corresponding ECG leads [16].
This lambda-like pattern, which is closely related to malignant arrhythmias, should be distinguished from other specific STsegment elevation ECG patterns described previously. Sato et al. reported that typical J waves, which had the characteristic STelevation patterns caused by acute ischemia, were associated with VF during coronary spasms, especially the lambda-like pattern [17]. Prominent J waves with this pattern may predispose patients to phase 2 reentry that, in turn, may cause short-coupled premature ventricular complexes, polymorphic VT, and VF [18]. Kukla et al. showed that this pattern was associated with the development of VF in 3 patients with STEMI [19]. Aizawa et al. reported that this pattern was common among patients with typical STEMI complicated by VF (58%) and strongly associated with VF (specificity, 95.9%) [20]. Cipriani et al. found that, of 5 patients with triangular waveforms who experienced VF, 4 had cardiogenic shock, and 2 died during STEMI [10]. The sole slurring J-wave without elevation, which is a feature of the lambda-like pattern, may be useless to identify patients with both CAD and malignant arrhythmias. Our findings suggest that the notched J-wave already lies in the inferior and lateral leads without acute ischemia; however, sometimes the notched J wave only appeared with STsegment elevation. Notched J waves always exist, regardless of the morphology of the ST-segment elevation, and become obvious with the aggravation of ischemia. Horizontal ST-segment elevation may be a transitional form of the steeply descending elevation (Figure S4, Supporting Information).
We also found that T-wave alternans associated with the lambdalike waveform may be a strong marker for predicting malignant arrhythmias. Electrocardiographic markers distinguishing this malignant from normal variants are of prime clinical importance. Establishing the risk stratification of the lambda- like pattern depends on common definitions, methodologies for the measurement of this pattern, and collaborative epidemiological and mechanistic research [11,15]. Current treatments may be insufficient to prevent recurrent arrhythmias in patients whose malignant arrhythmias are induced by the lambda-like pattern.
Possible pathophysiological basis and gene mutation analysis
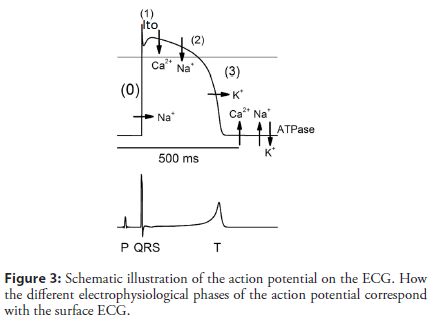
A QRS complex, a J-point, ST segments, and T waves in ECG represent rapid depolarization (phase 0), ER (phase 1), plateau phase (phase 2), and repolarization (phase 3) of action potential (AP), respectively (Figure 3) [7,21]. The J-ST-T configuration is thought to be sensitive to acute ischemia in the myocardium, as is the case for the lambda-like pattern [22]. The J point is defined as a transition from the end of a QRS complex to the start of an ST segment, and its elevation can be induced or accentuated by myocardial ischemia. The ischemic J wave can be caused by any culprit lesion of either the right or left coronary arteries, which are more often located in the inferior and lateral leads. Numerous ionic currents, calcium cycling, signal transduction, and others can become altered during acute ischemia; however, both channel opathies and ischemia may share common mechanisms that result in a lambda-like pattern, which may be a risk factor for the development of ventricular arrhythmias due to phase 2 reentry [23]. Recent basic electrophysiologic research has also linked the arrhythmogenesis to notch-related AP, increased dispersion of repolarization, and phase 2 reentry [24]. The exact ionic basis that leads to the lambda-like pattern in this clinical entity remains unclear. This pattern may be largely mediated by a 4-aminopyridine sensitive transient outward current (Ito). Depending on the Ito density and amplitude of the Ito- mediated spike and dome, an alteration of L-type calcium channel kinetics, which is secondary to accentuation of the spike and dome, results in partial or complete loss of the epicardial dome and is associated with rapidly up sloping or steeply down-sloping ST- segment elevation in ECG, respectively [25]. A much greater predominance of Ito in the right ventricular epicardium than the left ventricular epicardium may explain the higher prevalence of malignant arrhythmias in the inferior and lateral leads. Genetic studies have focused on candidate genes that might influence the Ito current. The transmural gradient in the Ito current in dog myocardium has been attributed to 3 major genes: (1) KCND3 encoding the α subunit of the Ito channel, (2) KChIP2 encoding the β-subunit of the Ito channel, and (3) IRX5 as a transcriptional factor that regulates KCND3. One could speculate that an interaction between polymorphisms of these 3 genes and myocardial ischemia may provide the necessary conditions for the generation of life- threatening arrhythmias [14]. Hu et al. suggested that an SCN5A gene mutation might predispose individuals to ischemia-induced VT or VF [26]. Potet et al. reported a unique SCN5A mutation that led to typical STsegment elevations in the inferior or right precordial leads [27]. Riera et al. showed that this identical lambda-like pattern was present in inferior leads and was not related to ischemia [28,29]. The molecular basis of the transmural distribution of Ito is still debated, and further research is required to assess its genetic basis in humans. The random nature of malignant arrhythmias in patients with similar clinical variables remains enigmatic, despite several studies examining the occurrence of malignant arrhythmias during CAD [29]. This initial response to ischemia most likely occurs in only genetically susceptible individuals, and patients who have latent ion-channel dysfunction may overcome the elevated risk. Patients may be inherently predisposed to developing malignant arrhythmias during acute ischemia episodes and have an elevated risk of early out-of-hospital malignant arrhythmias during the next serious ischemic episode. In addition, sympathetic and parasympathetic activities influence ST-segment elevation via their active effects on inward and outward currents in phase 1 and 2 of AP.
Study limitations
Our study has several limitations. The number of patients and follow-up times were small and limited, respectively. A possibility exists that the control patients may experience major adverse cardiovascular events in the future. Serial ECGs revealed that the notched J wave might be a stable (maintaining the same pattern over several years) rather than transient phenomenon, which suggests a permanent electrophysiological disorder. Large, long, and prospective population-based studies are needed to confirm the risks associated with the notched J-wave. In addition, the provocation to induce coronary spasm could not be performed in the CAD group because of the inverse risk-benefit balance. Our understanding of the underlying mechanisms is incomplete, information regarding genetic determinants and therapeutic responses is inadequate, and relationship between ER and other conditions involving accelerated repolarization and sudden arrhythmic death is unclear. Finally, randomized, controlled trials are needed to test the hypothesis that the lambda-like pattern is closely associated with lethal arrhythmias.
Conclusion
In conclusion, the inferior and lateral lambda-like pattern may be an independent predictor of the occurrence of malignant arrhythmias in patients with CAD. The notched and giant (tall and wide) J-wave may also be a risk marker for impending arrhythmias in the inferior and lateral leads. The relevance of a lambda-like pattern to acute myocardial ischemia may be important in patients with CAD; however, no established risk stratification or management strategies for this pattern are currently available.
References
- Jabbari R, Risgaard B, Holst AG, et al. Cardiac symptoms before sudden cardiac death caused by coronary artery disease: A nationwide study among young Danish people. Heart. 99(13): 938–943 (2013).
- Israel CW. Mechanisms of sudden cardiac death. Indian Heart J. 66: S10–S17 (2014).
- Gussak I, Bjerregaard P, Kostis J. Electrocardiographic ‘Lambda’ wave and primary idiopathic cardiac asystole: A new clinical syndrome? J Electrocardiol.37(2): 105–107 (2004).
- Tarantino N, Santoro F, Guastafierro F, et al. "Lambda-wave" ST-elevation is associated with severe prognosis in stress (takotsubo) cardiomyopathy. Ann Noninvasive Electrocardiol.23(6): e12581 (2018).
- Yagi S, Ueno R, Sutou K, et al. Lambda-like J wave due to acute myocardial infarction of the diagonal branch. J Med Invest. 66: 185–187 (2019).
- Patton KK, Ellinor PT, Ezekowitz M, et al. Electrocardiographic early repolarization: A scientific statement from the AmericanHeartAssociation. Circulation. 133: 1520-1529 (2016).
- de Bliek EC. ST elevation: Differential diagnosis and caveats. A comprehensive review to help distinguish ST elevation myocardial infarction from nonischemic etiologies of ST elevation. Turk J Emerg Med.18: 1–10 (2018).
- Wang GQ, Zhao N, Zhang CH, et al. Lambda-like ST-segment elevation in acute myocardial infarction triggered by coronary spasm may be a new risk predictor for lethal ventricular arrhythmia: A case report. Medicine. 97(49): e13561 (2018).
- Yu ML, Zhang Q, Huang XM. Acute coronary syndrome due to right coronary spasm and documented lambda-like J waves. Clin Res Cardiol. 107: 729–732 (2018).
- Cipriani A, D’Amico G, Brunello G, et al. The electrocardiographic “triangular QRS- ST-T waveform” pattern in patients with ST-segment elevation myocardial infarction: Incidence, pathophysiology and clinical implications. J Electrocardiol. 51(1): 8–14 (2018).
- Bastiaenen R, Behr ER. Early repolarisation: Controversies and clinical implications. Heart. 98(11): 841–847 (2012).
- Walsh R, Peters NS, Cook SA, et al. Paralogue annotation identifies novel pathogenic variants in patients with Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia. J Med Genet.51(1): 35–44 (2014).
- Naruse Y, Tada H, Harimura Y, et al. Early repolarization is an independent predictor of occurrences of ventricular fibrillation in the very early phase of acute myocardial infarction. Circ Arrhythm Electrophysiol.5(3): 506–513 (2012).
- Patel RB, Ng J, Reddy V, et al. Early repolarization associated with ventricular arrhythmias in patients with chronic coronary artery disease. Circ Arrhythm Electrophysiol.3: 489–495 (2010).
- Choi HO, Nam GB, Jin ES, et al. Temporal variation and morphologic characteristics of J- waves in patients with early repolarisation syndrome. Heart.99(24): 1818– 1824 (2013).
- Myerburg RJ, Kessler KM, Mallon SM, et al. Life-threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary artery spasm. N Engl J Med.326(22): 1451–1455 (1992).
- Sato A, Tanabe Y, Chinushi M, et al. Analysis of J waves during myocardial ischaemia. Europace.14: 715–723 (2012).
- Yan GX, Joshi A, Guo D, et al. Phase 2 reentry as a trigger to initiate ventricular fibrillation during early acute myocardial ischemia. Circulation.110(9): 1036–1041 (2004).
- Kukla P, Jastrzebski M, Sacha J, et al. Lambda-like ST segment elevation in acute myocardial infarction-a new risk marker for ventricular fibrillation? Three case reports. Kardiol Pol.66(8): 873–877 (2008).
- Aizawa Y, Jastrzebski M, Ozawa T, et al. Characteristics of electrocardiographic repolarization in acute myocardial infarction complicated by ventricular fibrillation. J Electrocardiol.45(3): 252–259 (2012).
- Timour Q, Frassati D, Descotes J, et al. Sudden death of cardiac origin and psychotropic drugs. Front Pharmacol.3: 76 (2012).
- Brady WJ, Syverud SA, Beagle C, et al. Electrocardiographic ST-segment elevation the diagnosis of acute myocardial infarction by morphologic analysis of the ST segment. Acad Emerg Med.8(10): 961–967 (2001).
- Naruse Y, Tada H, Harimura Y, et al. Early repolarization increases the occurrence of sustained ventricular tachyarrhythmias and sudden death in the chronic phase of an acute myocardial infarction. Circ Arrhythm Electrophysiol.7(4): 626–632 (2014).
- Rezus C, Floria M, Moga VD, et al. Early repolarization syndrome: Electrocardiographic signs and clinical implications. Ann Noninvasive Electrocardiol. 19(1): 15–22 (2014).
- Shu J, Zhu T, Yang L, et al. ST-segment elevation in the early repolarization syndrome, idiopathic ventricular fibrillation, and the Brugada syndrome: Cellular and clinical linkage. J Electrocardiol.38(4-supp- S): 26–32 (2005).
- Hu D, Viskin S, Oliva A, et al. Novel mutation in the SCN5A gene associated with arrhythmic storm development during acute myocardial infarction. Heart Rhythm. 4(8): 1072–1080 (2007).
- Potet F, Mabo P, Le Coq G, et al. Novel Brugada SCN5A mutation leading to ST segment elevation in the inferior or the right precordial leads. J Cardiovasc Electrophysiol.14(2): 200–203 (2003).
- Riera AR, Ferreira C, Schapachnik E, et al. Brugada syndrome with atypical ECG: Downsloping ST-segment elevation in inferior leads. J Electrocardiol.37(2): 101–104 (2004).
- Jastrzebski M, Kukla P. Ischemic J wave: Novel risk marker for ventricular fibrillation? Heart Rhythm.6(6): 829–835 (2009).