Short Report - Interventional Cardiology (2009) Volume 1, Issue 2
Latest on the pathology of drug-eluting stents
- Corresponding Author:
- Renu Virmani
CVPath Institute, Inc., 19 Firstfield Road, Gaithersburg, MD 20878, USA
Tel: +1 301 208 3570
Fax: +1 301 208 3745
E-mail: rvirmani@cvpath.org
Abstract
Keywords
hypersensitivity, pathology, restenosis, stent thrombosis, vascular remodeling
Drug-eluting stents (DES) were designed with the primary purpose of inhibiting neointimal proliferation after percutaneous coronary intervention [1,2]. A better understanding of the mechanisms of neointimal growth (restenosis) within bare-metal stents (BMS) led to the development of first-generation DES (the polymer-based sirolimus-eluting stent [SES], Cypher™ [Cordis Corp., FL, USA]; and the polymer-based paclitaxel-eluting stent, Taxus™ [Boston Scientific, Natik, MA, USA]). Prior to approval of these stents they had been tested for safety and efficacy in animal models [3,4] followed by large clinical trials with subsequent approval by the US FDA for use in humans. Both used an already approved pre-existing BMS platform (Bx vel ocity™ [Cordis Corp.] and Express™ stent [Boston Scientific]) coated with a polymer that allowed controlled release of anti-proliferative drugs (sirolimus [Cordis Corp.] or paclitaxel [Boston Scientific]). While DES have significantly reduced the rates of restenosis compared with BMS, they have not completely eradicated it. Moreover, late stent thrombosis (LST) has emerged as a major safety concern, especially in patients with ‘off-label’ use such as bifurcation lesions, long lesions (>30 mm), acute myocardial infarction (AMI) [5–7], saphenous vein bypass grafts, left main disease, chronic total occlusion and renal failure. Pathologic studies of patients dying from late DES thrombosis demonstrates delayed arterial healing, characterized by persistent fibrin deposition, mild smooth muscle growth and poor endothelialization as the primary substrate [8]. In this article we will consider the current status of DES in clinical practice and discuss the strengths and limitations of recent advances in this new technology of DES.
Pathology (first-generation DES)
Our understanding of the pathophysiology of late DES thrombosis is derived from human autopsy pathologic samples from patients who received these devices and died from stent-related causes. Since 2004, when the first autopsy case report (Figure 1) appeared describing the pathologic findings in a patient with late DES thrombosis [9], our laboratory has acquired over 150 cases in our stent registry and therefore our understanding of the pathology underlying LST has evolved. Most recently, we have expanded our studies to include living patients, in whom we reported correlative pathologic findings in thrombectomy specimens aspirated from living patients with late DES thrombosis who had undergone intravascular ultrasound (IV US) (Figure 2) and optical coherence tomography (OCT) at the time of clinical presentation of LST [10–13].
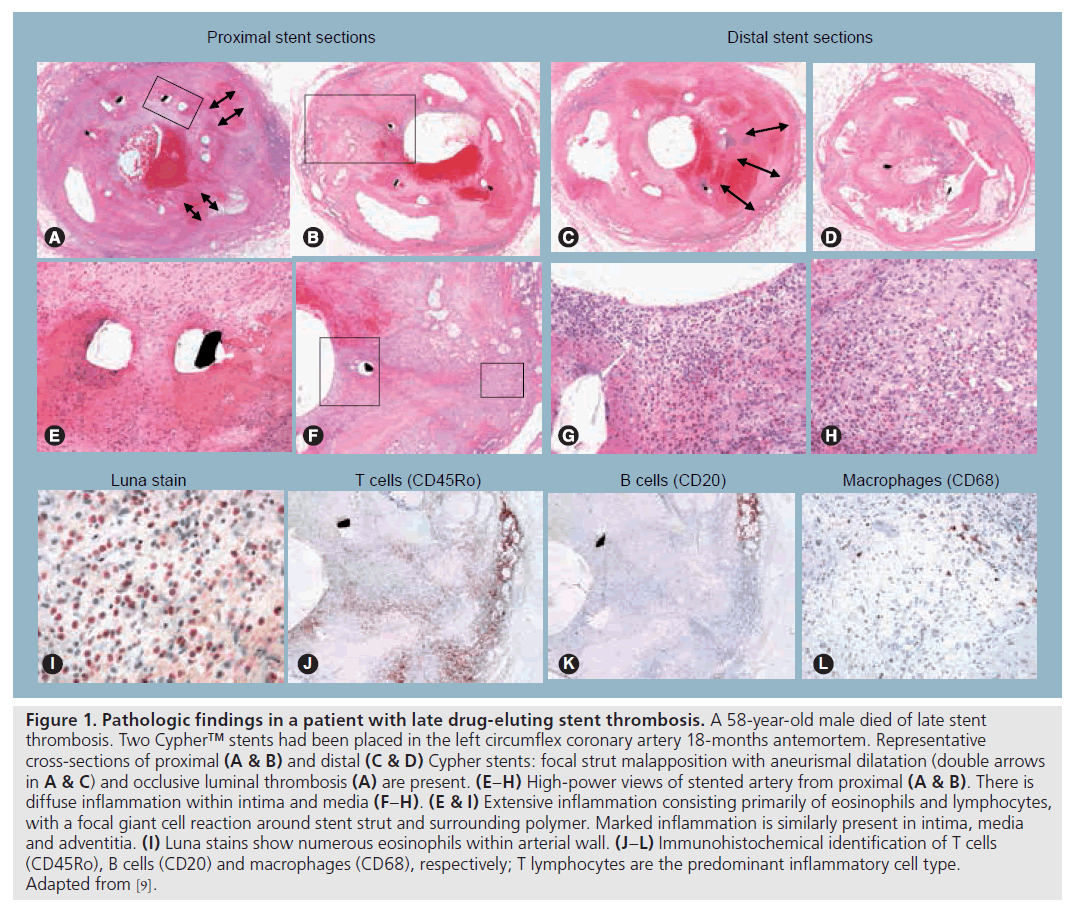
Figure 1: Pathologic findings in a patient with late drug-eluting stent thrombosis. A 58‑year-old male died of late stent thrombosis. Two Cypher™ stents had been placed in the left circumflex coronary artery 18‑months antemortem. Representative cross-sections of proximal (A & B) and distal (C & D) Cypher stents: focal strut malapposition with aneurismal dilatation (double arrows in A & C) and occlusive luminal thrombosis (A) are present. (E–H) High-power views of stented artery from proximal (A & B). There is diffuse inflammation within intima and media (F–H). (E & I) Extensive inflammation consisting primarily of eosinophils and lymphocytes, with a focal giant cell reaction around stent strut and surrounding polymer. Marked inflammation is similarly present in intima, media and adventitia. (I) Luna stains show numerous eosinophils within arterial wall. (J–L) Immunohistochemical identification of T cells (CD45Ro), B cells (CD20) and macrophages (CD68), respectively; T lymphocytes are the predominant inflammatory cell type. Adapted from [9].
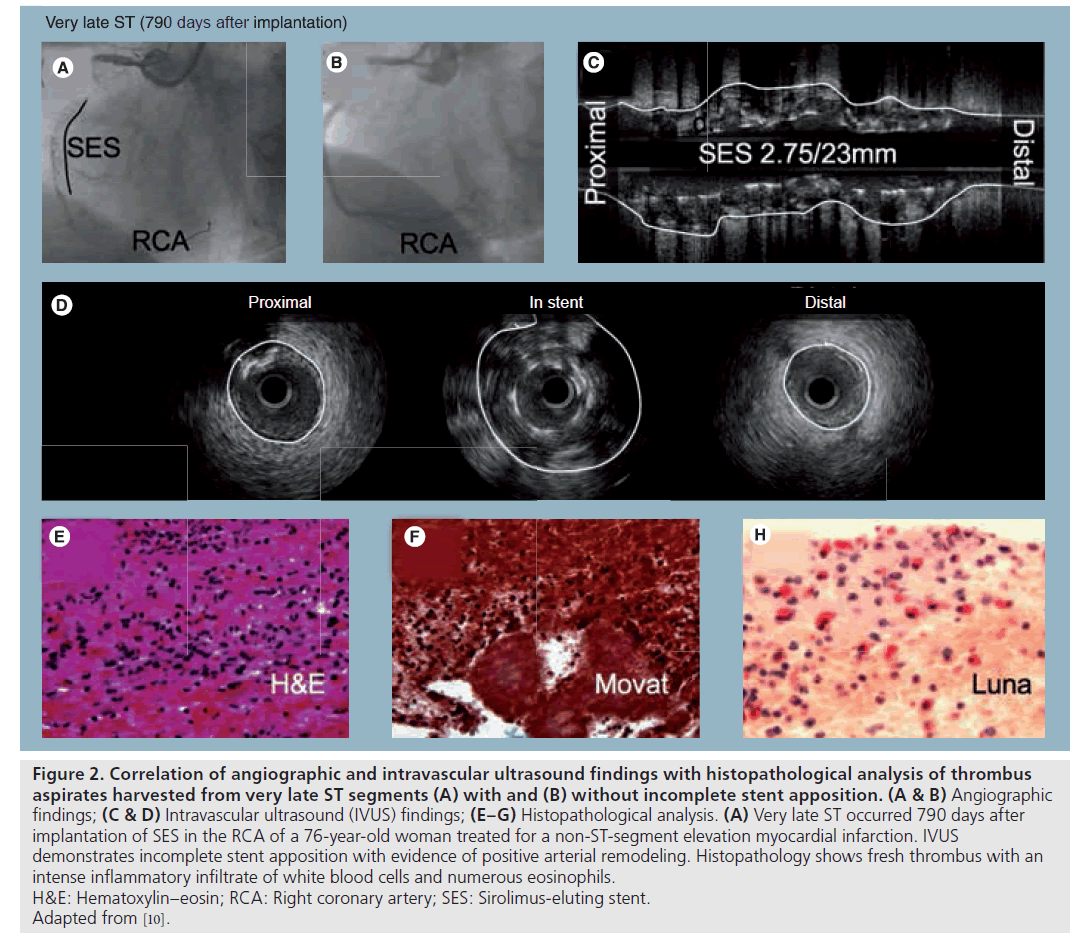
Figure 2: Correlation of angiographic and intravascular ultrasound findings with histopathological analysis of thrombus
aspirates harvested from very late ST segments (A) with and (B) without incomplete stent apposition. (A & B) Angiographic
findings; (C & D) Intravascular ultrasound (IVUS) findings; (E–G) Histopathological analysis. (A) Very late ST occurred 790 days after
implantation of SES in the RCA of a 76‑year-old woman treated for a non-ST-segment elevation myocardial infarction. IVUS
demonstrates incomplete stent apposition with evidence of positive arterial remodeling. Histopathology shows fresh thrombus with an
intense inflammatory infiltrate of white blood cells and numerous eosinophils.
H&E: Hematoxylin–eosin; RCA: Right coronary artery; SES: Sirolimus-eluting stent.
Adapted from [10].
The pathologic mechanisms underlying LST were first reported by our laboratory and served to underscore the importance of stent design. In 2003, we reported incomplete endothelial coverage with persistent fibrin within the necrotic core at 16 months following Cypher stent implantation [14] in the absence of luminal thrombosis. At 1 year later, we reported a new pathologic entity, localized hypersensitivity reaction, associated with late DES thrombosis in a patient with a Cypher stent that was characterized histologically by an intense peristrut and diffuse inflammatory cell infiltrate rich in eosinophils and T lymphocytes [9]. In 2006, we demonstrated delayed healing in both Cypher and Taxus DES compared with BMS at autopsy. Pathologically, there was persistence of fibrin around the stent struts, incomplete endothelialization and sparse smooth muscle coverage compared with BMS implanted for similar durations [15]. Subsequently, it was shown that a lack of endothelial strut coverage was the single best correlate of LST, using data from a larger number of autopsy cases [16]. Joner et al. found other pathologic features associated with LST, including stent malapposition, stent struts penetrating the necrotic core, local hypersensitivity, restenosis and bifurcations. The arterial healing in DES lesions was heterogeneous, especially in thrombosed cases, suggesting that the underlying lesion morphologies also contribute to delayed healing and LST. For example, bifurcation is one of the more susceptible sites for stent thrombosis. The incidence of stent thrombosis at bifurcation with DES is significantly greater than BMS at autopsy. Flow disturbances at bifurcations have been well described, but with the use of DES, there is delayed arterial healing from drug effects and a continued risk of thrombosis. Another underlying plaque morphology associated with a greater incidence of LST is observed in patients presenting with AMI from plaque ruptures. We have observed a significantly higher incidence of LST in patients treated with DES for plaque rupture compared with those with stable lesions (fibrous cap >250 μm) [17]. Furthermore, we have observed heterogeneity of healing in AMI patients at culprit sites (site of plaque rupture) with greater delayed healing compared with nonculprit sites within the same section, emphasizing the importance of underlying plaque pathology.
In addition, there have been recent improvements in imaging modalities such as OCT and angioscopy that have allowed for in vivo assessment of neointimal strut coverage following stent implantation. Greater stent tissue coverage was confirmed in BMS compared with DES, using both angioscopy and OCT [18]. These observations of focal coverage are significantly less in Cypher at 6 months versus BMS, and have also been observed to occur in the Taxus.
As mentioned above, localized hypersensitivity is one of the complications that we have reported following DES implantation [9]. Clinical manifestations of hypersensitivity include rash, itching, hives or dyspnea; however, these are not always present when there is localized hypersensitivity at the site of stent placement. The most important complication of hypersensitivity reaction is thrombosis, as most cases with this reaction have had LST documented at autopsy. The inflammatory infiltrate is characterized by a diffuse eosinophilic and lymphocytic infiltrate, with focal areas of granulomatous reaction consisting of macrophages and multinucleated giant cells localized in areas of the polymer resulting in a positive remodeling of the vessel, malapposition and subsequent LST. Furthermore, our observations revealed that this phenomenon is limited to the Cypher stent, and that most of our cases of hypersensitivity reactions are observed beyond 4 months. Therefore, it has been proposed that persistence of the nonerodable polymer contributes to the hypersensitivity reaction, since the drug should be completely eluted by this time, as documented in animal release kinetic studies. Cypher nonerodable polymers consists of poly(etheyleneco- vinyl acetate [PEVA]) and poly(n-butyl methacryalte [PBMA]), and both have been associated with hypersensitivity reactions in humans and animals when used in nonvascular locations [19,20].
Recently, Cook and colleagues performed an in vivo IV US study in which thrombi were aspirated from patients with very late DES thrombosis and correlated with IV US findings and histopathology of the thrombus [10]. The study concluded that very late DES thrombosis is associated with histopathological and serological signs of inflammation. In particular, eosinophilic infiltrates were associated with IV US evidence of vessel remodeling leading presumably to secondary stent malapposition. The findings suggested eosinophilic coronary arteritis due to delayed-type hypersensitivity reaction as one of the causes of very late DES thrombosis. Compared with other causes of myocardial infarction, eosinophilic infiltrates were more common in thrombi harvested from very late DES thrombosis, particularly in Cypher stents, and correlated with the extent of stent malapposition. As previous autopsy studies of patients with very LST showed hypersensitivity as one of the pathologic substrates of underlying DES LST, the current data in living patients presenting with LST – and if the thrombus aspirate showed a large number of inflammatory cells – there was an association with incomplete stent apposition and vessel enlargement in 70% of cases. However, if the aspirate had a high eosinophilic count, it was mostly associated with the SES [10].
Recently, we showed that the incidence of stent fractures in the first generation of DES is a common phenomenon and is observed in 29% of stented lesions at autopsy, which is much higher than clinically reported (Figure 3). A high rate of adverse pathologic findings was observed in lesions with grade V stent fractures (i.e., lesion with multiple strut fractures with acquired transection with gap in the stent body), while grade I –IV fractures (I: involving single strut fracture; II : two or more strut fractures without deformation; III: two or more strut fractures with deformation; and IV : multiple strut fractures with acquired transection but without gap) did not have a significant impact on the pathological outcome. Stent length, duration of implant and usage of SES were independent predictors of stent fracture [21].
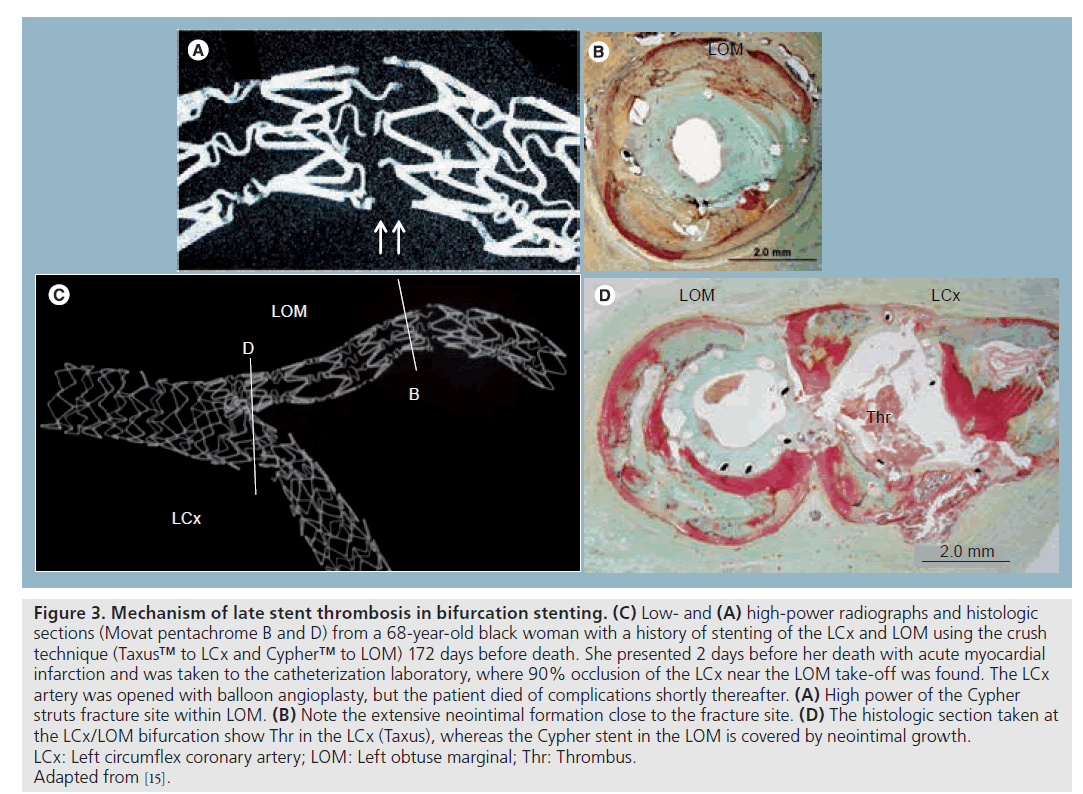
Figure 3: Mechanism of late stent thrombosis in bifurcation stenting. (C) Low- and (A) high-power radiographs and histologic sections (Movat pentachrome B and D) from a 68‑year-old black woman with a history of stenting of the LCx and LOM using the crush technique (Taxus™ to LCx and Cypher™ to LOM) 172 days before death. She presented 2 days before her death with acute myocardial infarction and was taken to the catheterization laboratory, where 90% occlusion of the LCx near the LOM take-off was found. The LCx artery was opened with balloon angioplasty, but the patient died of complications shortly thereafter. (A) High power of the Cypher struts fracture site within LOM. (B) Note the extensive neointimal formation close to the fracture site. (D) The histologic section taken at the LCx/LOM bifurcation show Thr in the LCx (Taxus), whereas the Cypher stent in the LOM is covered by neointimal growth. LCx: Left circumflex coronary artery; LOM: Left obtuse marginal; Thr: Thrombus. Adapted from [15].
Second-generation DES (clinical findings)
The second generation of DES with zotarolimus- (Endeavor® [Medtronic Vascular, CA, USA]) and everolimus- (XienceTM V [Abbott Vascular, CA, USA]) eluting stents were approved by the US FDA in 2008. These DES have shown significant reduction of target vessel revascularization compared with the respective BMS in each of the initial small, randomized studies [22,23]. However, a larger randomized clinical trial, SPIRIT III , demonstrated that Xience V stents had a significantly reduced angiographic late loss, without an increase of stent thrombosis over the Taxus stents [24]. On the other hand, the ENDEAVOR III trial showed that Endeavor stents were associated with a higher late loss compared with Cypher stents [25]. Nevertheless, a later trial comparing Endeavor (ENDEAVO R IV ) with Taxus stents showed an equivalent target lesion revascularization rate. Furthermore, no cases of very LST over 4 years from earlier ENDEAVO R trials have been reported, thus indicating that the long-term safety of the Endeavor stent may be superior to the first generation of DES [26].
Components of DES
Drug-eluting stents consist of three major components: the stent platform to scaffold the vessel; the polymer to deliver the drug; and drugs to inhibit neointimal growth [27]. A number of strategies are being employed to improve stent design including novel stent platforms, biodegradable polymers as well as polymer-free drug delivery and pharmacological agents that not only inhibit neointimal proliferation, but also promote endothelialization.
▪ Stent platform
The first generation of DES (Taxus and Cypher) had thick struts made from stainless steel (316 L stainless steel) mounted on a balloon-expandable system (Express and Bx Vel ocity) and the strut thickness ranged from 130 to 140 μm. The radial strength of 316 L stainless steel is dependent upon the thickness of the stent strut (316 L is a Society of Automotive Engineers steel grade. The 316 L stainless steel has high molybdenum and extra-low carbon content leading to a high resistance to corrosion). It was reported that stents with thicker struts have higher rates of restenosis, probably associated with greater vessel wall injuries [28]. In the second generation of DES (e.g., Endeavor and Xience V ) the stent platform is constructed from cobalt chromium with thin struts (80– 90 μm), which result in decreased neointimal response. Preclinical data demonstrated that stents with thinner struts have greater endothelialization compared with those with thicker struts [29]. Despite thinner struts, cobalt–chromium alloys maintain radiopacity, allowing both flexibility and trackability.
▪ Polymer
In the first-generation DES, nonerodable polymers were used on both Cypher and Taxus stents (in Cypher: PEVA and PBMA; and in Taxus: poly[styrene-b-isobutylene-b-styrene]). These polymer-based coatings were selected based on durability, release kinetics and drug miscibility. However, doubts have been raised about the long-term safety of durable polymers, particularly in relation to the risk of persistent inflammation and LST. The inflammatory reaction may result from poor integrity of the polymer, uneven application, webbing of the polymers or surface debris [30]. In preclinical studies, we showed that the luminal inflammation was high in both Cypher and Taxus at 28 days and that it persisted at 90 days in the rabbit model. Whereas in the pig model a granulomatous inf lammation has been observed in 30–60% of Cypher stents at 90 and 180 days, the inflammation may persist for longer periods, even up to 1 year [31,32].
Biodegradable polymer
There is general consensus that the continued presence of polymer is a key factor in LST. Therefore, in the development of the second-generation stents, there is a drive to utilize biodegradable polymers for drug delivery. There are many different types of biodegradable polymers; the earliest and most commonly used type of biodegradable polymers in the medical industry belongs to the polyester family, which includes poly(lactide), poly(glycolide) and poly(glycolic-co-lactic acid).
In our preclinical studies using the porcine coronary artery model, some biodegradable polymers have shown promising results, with minimal inflammation and sustained efficacy over time [Virami R, Unpublished Data]. The degradation speed is controlled by many factors and may be accelerated by low or high pH, reactive hydrolytic groups in the backbone, less crystallinity and smaller polymer size [33]. In a vessel with inflammation, the pH is expected to be low, which will accelerate polymer degradation.
The most important property, at least from a pathologist’s point of view, is that they should not evoke an inflammatory/toxic response and should metabolize in the body once it has fulfilled its purpose. Of course, it should have a long shelf life and be sterilizable. The biodegradable polymer-coated polylactic acid biolimus A9 NaboriTM (Terumo, Belgium) and BioMatrix® (Biosensors International, Singapore) have been recently approved in Europe and demonstrated a significant reduction of angiographic restenosis in the first-in-man STEALTH trial [34] and an all-comers LEADERS trial [35]. The animal studies in pigs and rabbits also showed significant reduction in neointimal growth and better endothelialization than the nonerodable Cypher and Taxus stents. Similarly, significantly less inflammation was observed for the Nabori stent in the rabbit model and better neointimal coverage than historic first-generation DES. Cypher and Taxus have been associated with impaired local endothelium-dependent vasomotion at adjacent stent segments [36]. Similarly, Hamilos et al. recently showed preservation of endothelial function of adjacent biolimus A9-eluting Nabori stents in humans [37], thus suggesting that it is likely the characteristic of bioerodable polymer that is different in the two stents and not the drug, since sirolimus and biolimus A9 bind to the FK binding protein 12 and subsequently inhibit the mTOR. This prevents the degradation of p27kip1, a cyclin-dependent kinase inhibitor that plays an important role in regulating vascular smooth muscle cell migration and proliferation. In conclusion, we believe that biodegradable polymers are safer than nonerodable polymers because polymer degradation and the resulting inflammatory response is a transient phenomenon if it does occur and thereafter leaves a BMS behind.
Drug
In the first-generation DES, the use of antiproliferative agents such as sirolimus and paclitaxel showed a dramatic reduction of restenosis rates over BMS [7,38]. This led to the generalized use of DES in patients with coronary artery disease. While the two drugs operate through different mechanisms of action, they both inhibit proliferation of smooth muscle cells and impair re-endothelialization. The drug is completely eluted by 90 days in normal healthy animal models but in atherosclerotic arteries it is possible that the drugs are released over a longer period and therefore remain effective in suppressing neointimal formation long term. Yet there is some doubt that DES can be effective permanently, as long-term follow-up for 5 years in clinical trials have demonstrated a gradual but significant increase of target revascularization rates from 1 to 5 years, especially in the first generation of DES [39,40].
It is likely that newer analogs of rapamycin such as zotarolimus and everolimus, used in second- generation DES (Endeavor and Xience V ) have similar biological effects. The Xience V stent uses a lower concentration of everolimus than first-generation DES, with similar release kinetics as Cypher. Although Endeavor has a higher drug dose (zotarolimus) than the other first- and second-generation DES, it has the fastest release kinetics among the currently available DES; therefore, the tissue concentration in the arterial wall is likely to be less at 30 days, and the late loss is also greater than the other DES.
Other drugs being tested in DES include biolimus A9, tacrolimus and pimecrolimus. Biolimus A9, as stated above, is also a rapamycin analog with high lipophilicity but similar suppression of smooth muscle cells to sirolimus and its other analogues. Pimecrolimus and tacrolimus act through the Ca/calmodulin- dependent protein phosphatase calcineurin pathway. Both the drugs failed to show clinical efficacy; in fact, pimecrolimus has been associated with greater late loss as compared with BMS with the use of permanent and bioerodable polymers, suggesting that calcineurin blockage may even induce greater smooth muscle cell proliferation when locally delivered on the stent platform [41].
Next-generation DES
Next-generation DES are being developed to further refine current device technology and respond to clinical needs within the cardiovascular stent arena. The optimal drug– polymer–device iteration continues to be sought, with novel designs focused on platform (biodegradable stents), polymer (polymer-free delivery) and drug (prohealing technology), and are briefly discussed below.
▪ Polymer-free technology
In the first-generation stents, polymeric coatings were incriminated as a potential cause of localized hypersensitivity reaction and LST; therefore, the second-generation designs focused on biocompatible and bioerodable polymers to reduce hypersensitivity and late thrombosis. An alternative approach, referred to as polymer-free technology, avoids the use of any polymer at all, utilizing the stent structure itself as a drug carrier. The JanusTM stent (Sorin Biomedica Cardio., Italy) has grooves on the abluminal side of stent struts that serve as ‘reservoirs’ for the drugs loaded onto the stent. However, the Janus stent with tacrolimus failed to significantly reduce late loss as compared with bare stent in clinical studies [42]. The Yukon® stent (Translumina, Germany) is another stent specifically designed for drug elution. It uses ‘microporous’ technology consisting of pores with a diameter of less than 2 nm, which therefore allows the drugs to be eluted slowly. Recently, the Yukon stent with sirolimus on a microporous structure has shown noninferiority in restenosis rates compared with the Taxus stent in preclinical and clinical studies [43]. A third design, the BioMatrix® Freedom™ stent (Biosensors International) has microporous structures on the abluminal surface, enabling the loading of 225 μm of biolimus A9. The BioMatrix Freedom stent with biolimus A9 delivered abluminally has been tested and showed sustained efficacy in preclinical studies in the porcine coronary artery model without the induction of significant inflammation and is currently undergoing clinical trials.
▪ Prohealing technology (proendothelial approaches)
Thus far, the primary role of drugs eluted from stent platforms has been one of inhibition (i.e., antiproliferative drugs aimed at reducing inflammation and smooth muscle cell proliferation). Unfortunately, these cell cycle-modifying agents exhibit concomitant endothelial toxicity, resulting in a precarious balancing act between adequate endothelialization (healing) while suppressing smooth muscle cell proliferation. Prohealing technology, designed to enhance re-endothelialization, is used in the Genous™ stent (OrbusNeich, FL, USA), which has an anti-CD34 antibody coating on the stent that attracts endothelial progenitor cells. In the first clinical study (Healthy Endothelial Accelerated Lining Inhibits Neoinitimal Growth – Firstin- Man [HEALING]), no thrombotic events were observed with moderate late lumen loss of 0.66 ± 0.52 mm in Genous stents [44]. A preclinical study showed improved endothelialization of Cypher stents when it was coated with the anti-CD 34 antibody compared with Cypher noncoated stent [45]. These results suggest that the combination of endothelial progenitor cell-capture technology and antiproliferative drug could be modified for safety while maintaining efficacy.
▪ Biodegradable stent
The permanently placed stent platform has a potential for adverse effects such as inflammatory reactions, restenosis, blockage of side branches and thrombotic risk. The idea of a completely biodegradable stent that remains in situ only as long as necessary and disappears once the job is done is very attractive. There are several polymeric degradable stents in development. One of the earliest was described by Tamai et al. (Igaki-Tamai® stent); the stent was made of poly(L-lactic acid) (PLLA) and did not have a drug coating [46]. A small trial showed satisfactory 6‑month follow-up performance with hyperplasia comparable to BMS and complete degradation at 4 years. Recently, the bioabsorable everolimus-eluting coronary stent (Abbott Vascular, IL, USA), which also consists of a PLLA polymer with an everolimus coating, was tested in a first-in-man trial, and showed feasibility and safety with no restenosis, although mild stent recoil was observed [47]. In order to preserve the radial force, thick struts (150 μm) were applied, which increased the profile of the catheter and therefore led to reduced deliverability. In vivo imaging modalities, such as IV US and OCT, were used to survey the degradation of this stent in humans. Although some stents appeared to be completely dissolved at the 2‑year follow-up [48], the degradation rates may vary and may depend on lesion/ patient characteristics. In preclinical studies, we have observed almost complete degradation of the polymers with no inflammation, with positive remodeling at 2 years following bio-absorabable everolimus-eluting coronary stent implantation in the porcine model [Virmani R, Unpublished Data].
Conclusion
The need for safer and more efficacious intravascular devices continues to drive advances in stent technology. The evolution from first- to second-generation DES has resulted in greater safety and less target-vessel revascularization. The third generation of DES with biodegradable polymers in preclinical trials and at least 2 years in randomized clinical trials show equivalent late loss, less LST, inflammation and better vascular response to exercise-induced vasoconstriction. However, regarding totally bioabsorbable DES stents, although showing promise in preclinical and small first-in-man clinical studies, we need to wait for the final verdict from larger all-comer trials to accurately assess their value.
Executive summary
Lessons from autopsy pathology studies of drug-eluting stents
▪ Autopsy studies have revealed delayed healing following implantation of drug-eluting stents (DES) as compared with bare-metal stents (BMS). The delayed healing consists of poor endothelialization, persistence of fibrin and a marked decrease in smooth muscle cell presence in the neointima. Although DES significantly reduce restenosis, they have been associated with late stent thrombosis (LST). LST is multifactorial, but the most important predictor is presence of uncovered stent struts (>30%), a marker for poor endothelialization. The frequency of LST is highest in patients receiving DES for ‘off-label’ indications. We have shown that the main causes of LST are placement of DES for acute myocardial infarction, bifurcation lesions, long and overlapped stenting, malapposition and hypersensitivity reactions.
Second-generation DES
▪ The second-generation DES reduced strut thickness, allowing for more rapid endothelialization. In addition, they used less polymer and utilized different polymer types, including more hydrophilic polymers, which resulted in less inflammation. Finally, either through the reduction of drug dose and/or faster release kinetics, the second-generation DES also demonstrated less fibrin with more rapid healing as compared with the first-generation DES.
Future perspective
▪ The first- and second-generation DES all use nonerodable polymers, which remain permanently in the body and with time, continue to induce inflammation. Biodegradable polymers should result in less toxicity to the vessel wall while reducing neointimal formation. In animal studies, some biodegradable polymers have shown promise with minimal inflammation observed during the degradation phases. Further improvements are emerging, such as polymer-free technology for drug delivery and prohealing technology to enhance re-endothelializiation. Finally, the use of permanent stent platforms may continue to induce inflammation and, therefore, the concept of a totally biodegradable stent is appealing.
Financial & competing interests disclosure
Renu Virmani receives company sponsored research support from: 3F Therapeutics/ATS Medical; Abbott Vascular; Ablation Frontiers; Abraxis Bioscience, Inc.; AccelLab, Inc.; Affinergy, Inc.; AGA Medical Corp.; AK International Co., Ltd; AlchiMedics; Alvimedica Medical Technologies; Amaranth Medical, Inc.; AngioDynamics, Inc.; AngioScore, Inc.; Angiomed GmbH & Co.; Angioslide Ltd; Angel Medical Systems, Inc.; Angioblast Systems, Inc.; Apnex Medical, Inc.; Arbor Surgical, Inc.; Ardian, Inc.; Atritech, Inc.; Atrium Medical Corp.; Avantec Vascular; Bard Peripheral Vascular, Inc.; B-Balloon Ltd; Biotronik AG; Biogen IDEC; Biotegra, Inc.; Biomerix; BioPAL, Inc.; Biosensors International; Biomer Technology Ltd; Boston Scientific Corp.; ByPass Medical Technologies, Ltd; CardioDex, Ltd; Cardica, Inc.; CardioKinetix, Inc.; CardioFocus, Inc.; Cardiovascular Research Foundation- Korea; CardioMind, Inc.; Cardiovascular Research Foundation; Cierra, Inc.; CoAptus Medical Corp.; Coherex Medical, Inc.; Concentric Medical; Conor Med Systems; CorAssist Cardiovascular Ltd; Cordis Corporation; CoRepair, Inc.; Correx, Inc.; Corindus, Inc.; CorNova Inc.; CVRx, Inc.; CyberHeart Inc.; Devax, Inc.; Edwards Lifesciences, LLC; Elixir Medical Corp.; Elutex, Inc.; ev3, Inc.; Evalve, Inc.; Gardia Medical Ltd; Gem Biosystems; GlaxoSmithKline; HemCon; InfraReDx, Inc.; Invatec Technology Center GmbH; Jerini AG; Kaneka Corp; Laax, Inc.; Lumen Biomedical, Inc.; Lutonix, Inc.; Maquet Cardiovascular; Medtronic AVE; Medtronic Heart Valves; Meril Life Sciences Pvt Ltd; Microvention, Inc.; Minnow Medical, LLC; Miravant Medical, LLC; Neovasc Medical Ltd; Novartis Pharmaceuticals Corporation; NovoStent Corp.; OrbusNeich Medical, Inc.; Oregon Medical Laser Center; Paragon Intellectual Properties, LLC; Prescient Medical, Inc.; Probiodrug AG; ReLeaf Medical; Relisys Medical Devices Ltd; ReValve Vascular Ltd; Revascular Therapeutics; Sahajanand Medical Technologies Pvt. Ltd; Sorin Biomedica Cardio S.r.l; Surmodics, Inc.; Takeda Pharmaceuticals North America; Terumo Corp.; Theregen, Inc.; TissueGen, Inc.; Top Spin; Toray Industries, Inc.; Transluminal Technologies; Vascular Therapies, LLC; VIA Pharmaceuticals, Inc.; Volcano Therapetutics, Inc.; X-Cell Medical, Inc.; and Xtent, Inc.
Renu Virmani is a consultant for: Medtronic AVE; Abbott Vascular; WL Gore; Volcano Therapeutics, Inc.; Prescient Medical; CardioMind, Inc.; Direct Flow; and Atrium Medical Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Farb A, Sangiorgi G, Carter AJ et al.: Pathology of acute and chronic coronary stenting in humans. Circulation 99(1), 44–52 (1999).
- Rogers C, Welt FG, Karnovsky MJ, Edelman ER: Monocyte recruitment and neointimal hyperplasia in rabbits. Coupled inhibitory effects of heparin. Arterioscler. Thromb. Vasc. Biol. 16(10), 1312–1318 (1996).
- Suzuki T, Kopia G, Hayashi S et al.: Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 104(10), 1188–1193 (2001).
- Heldman AW, Cheng L, Jenkins GM et al.: Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 103(18), 2289–2295 (2001).
- Morice MC, Serruys PW, Sousa JE et al.: A randomized comparison of a sirolimuseluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773–1780 (2002).
- Stone GW, Moses JW, Ellis SG et al.: Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Moses JW, Leon MB, Popma JJ et al.: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Daemen J, Wenaweser P, Tsuchida K et al.: Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Virmani R, Guagliumi G, Farb A et al.: Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004).
- Cook S, Ladich E, Nakazawa G et al.: Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120(5), 391–399 (2009).
- McFadden EP, Stabile E, Regar E et al.: Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364(9444), 1519–1521 (2004).
- Matsumoto D, Shite J, Shinke T et al.: Neointimal coverage of sirolimus-eluting stents at 6‑month follow-up: evaluated by optical coherence tomography. Eur. Heart J. 28(8), 961–967 (2007).
- Higo T, Ueda Y, Oyabu J et al.: Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent – angioscopic study. J. Am. Coll. Cardiol. Img. 2(5), 616–624 (2008).
- Guagliumi G, Farb A, Musumeci G et al.: Images in cardiovascular medicine. Sirolimus-eluting stent implanted in human coronary artery for 16 months: pathological findings. Circulation 107(9), 1340–1341 (2003).
- Joner M, Finn AV, Farb A et al.: Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Finn AV, Joner M, Nakazawa G et al.: Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435–2441 (2007).
- Nakazawa G, Joner M, Ladich E et al.: Drug-eluting stents implantation in acute myocardial infarction significantly delays healing and increases stent thrombosis (Abst.). Circulation 116(16), II 628 (2007).
- Gonzalo N, Barlis P, Serruys PW et al.: Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc. Interv. 2(5), 445–452 (2009).
- Ahmed DD, Sobczak SC, Yunginger JW: Occupational allergies caused by latex. Immunol. Allergy Clin. North Am. 23(2), 205–219 (2003).
- Leggat PA, Kedjarune U: Toxicity of methyl methacrylate in dentistry. Int. Dent. J. 53(3), 126–131 (2003).
- Nakazawa G, Finn AV, Vorpahl M et al.: Incidence and predictors of drug-eluting stent fracture in human coronary artery – pathologic analysis. J. Am. Coll. Cardiol. (2009) (In press).
- Fajadet J, Wijns W, Laarman GJ et al.: Randomized, double-blind, multicenter study of the Endea vor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVO R II trial. Circulation 114(8), 798–806 (2006).
- Beijk MA, Neumann FJ, Wiemer M et al.: One‑year results of a durable polymer everolimus-eluting stent in de novo coronary narrowing (The SPIRIT FIRST trial). EuroIntervention 1, 266–272 (2005).
- Stone GW, Midei M, Newman W et al.: Randomized comparison of everolimuseluting and paclitaxel-eluting stents: two‑year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation 119(5), 680–686 (2009).
- Kandzari DE, Leon MB, Popma JJ et al.: Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J. Am. Coll. Cardiol. 48(12), 2440–2447 (2006).
- Leon M: The three‑year results of ENDEAVO R IV . Presented at: TCT Conference 2009. San Francisco, CA, USA, 21–25 September 2009.
- Nakazawa G, Finn AV, Kolodgie FD, Virmani R: A review of current devices and a look at new technology: drug-eluting stents. Expert Rev. Med. Devices 6(1), 33–42 (2009).
- Kastrati A, Mehilli J, Dirschinger et al.: Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 103(23), 2816–2821 (2001).
- Joner M, Nakazawa G, Finn AV et al.: Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52(5), 333–342 (2008).
- Finn AV, Kolodgie FD, Harnek J et al.: Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 112(2), 270–278 (2005).
- Wilson GJ, Nakazawa G, Schwartz RS et al.: Comparison of inflammatory response after implantation of sirolimus- and paclitaxeleluting stents in porcine coronary arteries. Circulation 120(2), 141–149 (2009).
- Nakazawa G, Finn AV, Ladich E et al.: Drug-eluting stent safety: findings from preclinical studies. Expert Rev. Cardiovasc. Ther. 6(10), 1379–1391 (2008).
- Middleton JC, Tipton AJ: Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21(23), 2335–2346 (2000).
- Grube E, Hauptmann K, Buellesfeld L, Lim V, Abizaid A: Six‑month results of a randomized study to evaluate safety and efficacy of a biolimus A9 eluting stent with a biogradable polymer coating. EuroIntervention 1, 53–57 (2005).
- Windecker S, Serruys PW, Wandel S et al.: Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 372(9644), 1163–1173 (2008).
- Togni M, Windecker S, Cocchia R et al.: Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 46(2), 231–236 (2005).
- Hamilos MI, Ostojic M, Beleslin B et al.: Differential effects of drug-eluting stents on local endothelium-dependent coronary vasomotion. J. Am. Coll. Cardiol. 51(22), 2123–2129 (2008).
- Stone GW, Ellis SG, Cox DA et al.: A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Morice MC, Serruys PW, Barragan P et al.: Long-term clinical outcomes with sirolimuseluting coronary stents: five‑year results of the RAVEL trial. J. Am. Coll. Cardiol. 50(14), 1299–1304 (2007).
- Aoki J, Abizaid AC, Serruys PW et al.: Evaluation of four‑year coronary artery response after sirolimus-eluting stent implantation using serial quantitative intravascular ultrasound and computerassisted grayscale value analysis for plaque composition in event-free patients. J. Am. Coll. Cardiol. 46(9), 1670–1676 (2005).
- Verheye S, Agostoni P, Dawkins KD et al.: The GENESIS (randomized, multicenter study of the pimecrolimus-eluting and pimecrolimus/paclitaxel-eluting coronary stent system in patients with de novo lesions of the native coronary arteries) trial. JACC Cardiovasc. Interv. 2(3), 205–214 (2009).
- Tamburino C, Di Salvo ME, Capodanno D et al.: Real world safety and efficacy of the Janus tacrolimus-eluting stent: long-term clinical outcome and angiographic findings from the Tacrolimus-Eluting STent (TEST) registry. Catheter Cardiovasc. Interv. 73(2), 243–248 (2009).
- Byrne RA, Kastrati A, Kufner S et al.: Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) trial. Eur. Heart J. 30(20), 2441–2449 (2009).
- Aoki J, Serruys PW, van Beusekom H et al.: Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) registry. J. Am. Coll. Cardiol. 45(10), 1574–1579 (2005).
- Nakazawa G, Granada JF, Alviar C et al.: Anti-CD34 antibodies immobilized on the surface of sirolimus eluting stents enhance stent endothelialization. J. Am. Coll. Cardiol. Intervent. Accepted for publication (2009).
- Tamai H, Igaki K, Kyo E et al.: Initial and 6‑month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 102(4), 399–404 (2000).
- Ormiston JA, Serruys PW, Regar E et al.: A bioabsorbable everolimus-eluting coronary stent system for patients with single de novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371(9616), 899–907 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al.: A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2‑year outcomes and results from multiple imaging methods. Lancet 373(9667), 897–910 (2009).