Review Article - Interventional Cardiology (2009) Volume 1, Issue 1
Left main coronary artery disease
- Corresponding Author:
- M Faisal Khan
Cardiac Catheterization Laboratories
Caritas St, Elizabeth’s Medical Center and Tuft’s University School of Medicine
736 Cambridge Street, Boston, MA 02135, USA
E-mail: faisal.khan@caritaschristi.org
Abstract
Keywords
atherosclerosis, bare-metal stent, coronary artery bypass grafting , coronary artery disease, drug-eluting stent, fractional flow reserve, intravascular ultrasound, left main, percutaneous coronary intervention
Once solely in the surgical realm, the management of left main (LM) coronary artery (LMCA) disease has migrated progressively towards interventional cardiology with each advance in percutaneous technique and device therapy. There is now considerable debate regarding the optimal management of this high-risk area of disease. This review will provide an overview of LMCA disease, its diagnosis and how the management of this complex area of disease has progressed, as well as some of the controversies surrounding its diagnosis and optimal therapy.
Anatomy
The LMCA most often arises from the superior portion of the left aortic sinus just below the sinotubular ridge of the aorta. It courses forward for a short but variable distance between the pulmonary artery and the left auricle before bifurcating into its two principal branches, the left anterior descending (LAD) and left circumflex (LCx) arteries. In 30% of individuals it also gives rise to a Ramus Intermedius vessel. In the native circulation it subtends at least 75% of the left ventricular blood flow, underlying its importance.
Like the rest of the coronary circulation it can also vary in size, between 3 and 6 mm in diameter and 1–30 mm in length (mean length of 10 mm) [1]. In 0.4% of individuals it may be absent with separate ostia of the LAD and LCx [2]. Angiographically, it is best visualized in angiographic projection or with slight left anterior oblique (0–10°) and with slight cranial angulation (0–10°), but it ought to be viewed in several projections to exclude eccentric stenosis. The distal LM is usually best seen with minimal right anterior oblique projection and caudal angulation (20°/20°).
Diagnosis
▪ Clinically
The clinical indicators of LMCA stenosis are easily recognized and include a crescendo pattern of angina pectoris, an ECG with ST-segment depression with pain or with simultaneous anterior and inferior ST segment changes, and fluoroscopic calcification of the LMCA [3]. These indicators, however, have a low sensitivity and positive predictive value and are hence of limited diagnostic value.
▪ Exercise stress testing
Certain findings on exercise testing or nuclear imaging may be suggestive of LM disease but also lack sensitivity. They are however of importance in the overall management since potential cardiac catheterization to confirm the diagnosis requires added caution. With treadmill exercise stress testing, pronounced ischemic findings (especially ST elevation or ST elevation in unusual leads such as avR associated with widespread ST depression) at relatively low heart rates or early on a stress testing protocol are suggestive of LMCA disease [4]. Exercise-induced hypotension is also an uncommon but important finding in this context.
▪ Nuclear scintigraphy
A LM pattern on exercise nuclear testing is characterized by perfusion defects in the LAD and LCx territories (i.e., reduced nuclear tracer uptake in the septal, anterior and lateral walls). It may also be associated with a picture of ‘balanced’ ischemia where there is uniform diminution of tracer uptake with stress, often indicative of LM with three-vessel disease. This may be accompanied by transient ischemic dilation (TID), which is considered present when the image of the left ventricular cavity appears to be significantly greater after stress as compared with that at rest. TID is a way to detect balanced ischemia in patients with apparently normal myocardial perfusion. Shiba et al. showed that TID was more frequently observed in patients with LMCA disease than in those without (31 vs 13%; p = 0.003) [5]. In this study TID was identified as a significant predictor for detecting LM disease in the univariate analysis but not on multiple logistic regression analysis.
Other patterns with a high correlation to LM disease include an increase in lung uptake of the tracer immediately after exercise. In the same study as above Shiba et al. found lung uptake of radiotracers to be the best single nonperfusion marker of LM disease [5].
Low sensitivities of a LM-pattern defect are reported with planner thallium imaging (13–24%), and myocardial single photon emission computed tomography (SPECT) (7–21%), partly because a similar pattern may be found in patients with triple vessel disease and in patients with proximal LAD and LCx disease without LM arterial involvement [5] (LM equivalent, which is discussed later).
▪ Angiography
The gold standard for diagnosis of LM disease remains coronary angiography, although the advent of cardiac imaging including computed tomography (CT) and MRI increases the number of options now available for ascertaining the diagnosis. Whereas 70% angiographic luminal narrowing is most often used as the threshold for hemodynamic significance of native coronary arteries, in the LM this threshold is taken to be 50% or greater luminal narrowing. The incidence of such disease is 5% of all patients undergoing coronary arteriography [6]. Isolated LM disease (as opposed to LM with disease in the other epicardial arteries) is found in less than 0.5–1% of patients [7–10].
The Veterans Administration (VA) Cooperative Study [11] and the Coronary Artery Surgery Study (CASS) registry [12] established the 50% luminal narrowing as the threshold of significance more then 25 years ago. In the VA study a trend towards benefit was seen in patients with 50–75% stenosis with preserved left ventricular function, although the greatest benefit was seen in the higher risk patients with more than 75% stenosis and/or left ventricular dysfunction. These results were also reflected in the CASS registry, where patients in the worst prognostic group had the greatest impact on survival with surgery after 3 years (82 vs 34%). At 15 years, those with mild LMCA stenosis, mildly reduced left ventricular function and a nonstenotic, dominant right coronary artery (rca ) did not have a survival benefit [13].
The correct diagnosis of the severity of LM stenosis remains the critical component in ensuring the correct management strategy is followed. As it is accepted that coronary angiography is a technique vulnerable to error in estimating stenosis, particularly by visual estimation, use of additional modalities may be required to ensure accurate determination of the state of LMCA. Two such strategies are intravascular ultrasound (IVUS) and fractional flow reserve (FFR).
▪ IVUS & LM
Indications for IVUS can be considered by the limitations of angiography. In the presence of LMCA stenosis, quantitative coronary angiography is the least reproducible of any coronary arterial segment with significant intra- and inter-observer variability [14–17]. Autopsy studies that compare IVUS and angiography have demonstrated missreporting of the significance of LMCA lesions with angiography. The reasons for this may be secondary to: first, the diffuse nature of atherosclerotic process, which in the LM artery and the bifurcation may especially affect the appreciation of disease because of the lack of a normal reference segment [18]; second, a short LMCA may make identification of a normal reference segment difficult and ostial disease may require careful catheter placement for a full appreciation of the extent of the disease [18]; third, there is compensatory enlargement (positive remodeling) of the vessel as plaque burden increases to preserve lumen size [19]; and fourth, there may be unique geometric issues in LMCA disease because the correlation between angiography and necropsy or IVUS appears to be somewhat better in non-LMCA stenosis [20,21].
Intravascular ultrasound confers the ability to examine accurately the coronary artery architecture, the extent of atherosclerotic plaque and changes in vessel dimensions as a result of the atherosclerotic process. Hence, IVUS is a very useful complimentary method to assess the severity of LMCA disease and should always be considered in angiographically borderline LMCA lesions.
Intravascular ultrasound is also a very useful adjunct after percutaneous coronary intervention (PCI) to ensure good stent apposition and no evidence of dissection or other issues that may play a significant role in the short- or long‑term outcomes for patients.
▪ IVUS criteria for LMCA disease
Fassa et al. investigated the lower range of the minimum luminal area (MLA) of the LMCA in 121 consecutive patients and found a potential cut-point of less than 7.5 mm2 [22]. Of these patients, 86% underwent coronary artery bypass grafting (CABG) and it was deferred in those with a MLA greater than 7.5 mm2. After a mean follow-up of 3.3 years there was no significant difference in major adverse cardiac events (MACE) for the two groups.
Of note, this study was not designed to address the question of whether all LM lumen areas of less than 7.5 mm2 require revascularization. The cut-off is based on two standard deviations below a threshold obtained from a receiver-operating characteristic rather then a more physiologic or clinical parameter.
In attempting to add a physiologic parameter, Jasti et al. compared FFR to IVUS in patients with angiographically ambiguous LMCA stenosis [23]. Strong correlations between FFR and MLA or minimum lumen diameter (MLD) were found. Benchmarked against FFR, an IVUS MLD cut point of 2.8 mm had the highest sensitivity and specificity (93 and 98%, respectively) to determine the significance of the LMCA stenosis, followed by an MLA of 5.9 mm2 (93 and 95%, respectively).
When angiographic assessment of LMCA was compared with IVUS, Sano and associates in a retrospective analysis of 115 patients, reported that fewer than half of the patients with intermediate LMCA stenosis (as determined on angiography) had significant stenosis by IVUS evaluation [24], which emphasizes the need for interventional cardiologists to avoid the ‘oculostenostic’ reflex [25,26] and to consider IVUS or FFR before proceeding to revascularization in patients whose angiograms are inconclusive. Of note, the concept of areas of vulnerable plaque [27] within the coronary tree was stressed by a small study where 30 patients underwent both single-vessel coronary intervention and an IVUS of the LMCA [28]. A total of 21 patients appeared to have normal LMCA by angiography, but all showed some plaque on IVUS. Eight of the nine patients who had a cardiovascular event during the subsequent 38 months (range: 27 to 47) had a LMCA area stenosis of 20%.
Beyond the assessment of coronary stenosis, IVUS may have an important role to play in PCI of unprotected LM disease. Park and colleagues presented IVUS data from the Revascularization for Unprotected LMCA Stenosis: Comparison of Percutaneous Coronary Angioplasty versus Surgical Revascularization (MAIN-COMPARE) registry, in which patients with unprotected LM disease underwent elective PCI with stenting with bare-metal stents (BMS) or drug-eluting stents (DES) that was either guided by IVUS (756 patients) or conventional angiography (219 patients) [201]. After performing propensity-score matching, a total of 201 matched pairs of patients were created. In these patients, there was a trend for lower mortality at 3 years with IVUS guidance compared with angiography guidance, but the difference was not statistically significant (6.0 vs 13.6%; p = 0.063). However, in 145 matched pairs of patients receiving DES only, the 3‑year incidence of mortality was significantly lower with IVUS guidance compared with angiography only. By contrast, the use of IVUS guidance did not reduce the risk of mortality in 47 matched pairs of patients receiving only BMS.
▪ Fractional flow reserve
An inherent limitation of both angiography and IVUS is their inability to predict whether a stenosis is potentially ischemia-inducing. As a consequence, especially in patients with intermediate LMCA disease, FFR measurements have successfully been applied to assist decision-making with regards to revascularization. If the FFR measurement is greater than 0.75, revascularization is not needed and a treatment approach of using optimal medical therapy can be pursued instead.
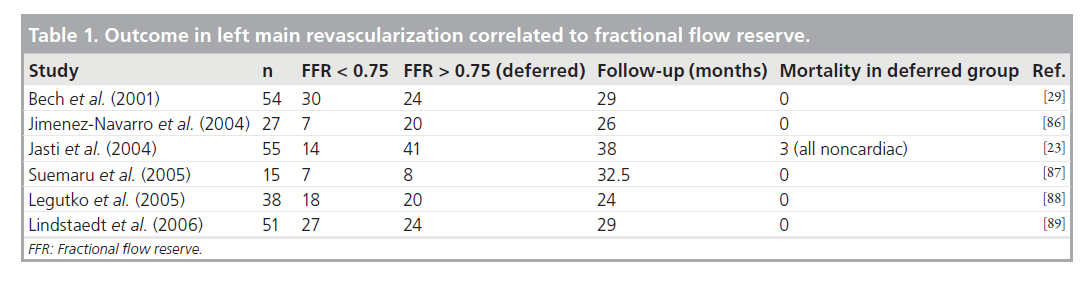
Bech et al. demonstrated, in patients with equivocal disease, that FFR is a lesion-specific index able to quantify ischemia secondary to the LMCA and that deferral of surgical treatment is safe if the FFR value is greater than 0.75 [29]. In the 54 patients they studied, medical instead of surgical treatment was used in 24 patients with FFR values greater than 0.75, while coronary bypass surgery was performed in the rest of the patients. Mean follow-up was 29 months. The survival rates of the patients in the medical treatment and surgical groups were 100 and 97%, respectively. The event-free survival was 76% in the medical treatment group and 83% in the surgical group. No death or acute myocardial infarction (MI) occurred in any of the deferred patients. Other studies that have correlated FFR with the extent of LMCA disease or outcomes after revascularization are summarized in Table 1.
▪ Cardiac imaging
More recently cardiac CT and MRI have been shown to have a high correlation with angiography for the diagnosis of LM disease. This may be particularly useful in surveillance imaging after revascularization of the LM often performed after stenting.
▪ Multislice computed tomography
Multislice computed tomography (MSCT), also called multidetector coronary angiography, has rapidly gained in popularity and applicability. The first attempts to image the heart were in the very early days of CT in the 1970s [202]. However, due to the rapid motion of the heart and relatively long acquisition times (more than 10 s per slice) of early equipment, only large pathological lesions such as tumors along the surface of the heart could be detected. Rapid advancements in detector, x-ray tube generators, circuitry and computers in the 1990s allowed the development of multirow CT scanners. The advent of these types of scanners has enabled a significant increase in spatial and temporal resolution and reduced scan times. For example, with a modern 64-slice MSCT scanner one achieves an in-plane resolution of 0.4 mm, a slice thickness of 0.6 mm and a temporal resolution of 165 ms [30]. The simultaneous acquisition of 64 parallel cross-sections enables the imaging of the entire coronary artery tree in a single breath hold for 10 s [31]. With 256-slice MSCT the acquisition can be performed in as little as two beats and pulsing of the beam by prospective cardiac triggering also allows for reduced exposure to radiation.
On a per patient basis, MSCT has good diagnostic accuracy for detecting more than 50% luminal stenosis with a sensitivity of 97% (CI: 94–98%) and specificity of 86% (CI: 78–90%) compared with quantitative conventional coronary angiography [32,33]. In a recent review, the sensitivity for detection of significant stenosis in the LMCA, based on data from 13 studies, was 62 of 62 (100%) and specificity was 815 of 821 (99%) [34]. This is based on the fact that this area and proximal portions of the LAD experience the least motion and run approximately parallel to the acquired transverse plane, hence allowing reliable visualization [35].
Not surprisingly then, in a number of studies sensitivity in the LM did not significantly differ from the LAD although there was a significant difference from imaging in the LCx (p < 0.01) and right coronary arteries (p < 0.02), especially for more significant stenosis [34]. Specificity, however, was higher in the LM than in the LAD (p < 0.0001), LCx (p < 0.0001) and RCA (p < 0.0001). Positive predictive value for significant stenosis in the LMCA was 91%, and negative predictive value was 100%.
In addition to the delineation of the native coronary artery lumen, cardiac MSCT also permits visualization of a deployed coronary stent. This is especially so in the LMCA as the lumen diameter here is the largest of the coronary tree and often has the least amount of motion artifact, reducing some of the most troublesome variables leading to error [36]. Gilard et al., using a 16-slice scanner showed a sensitivity of 100%, a specificity of 92% and positive and negative predictive values of 100 and 92%, respectively, for LM instent restenosis (ISR) [37]. Van Mieghem et al. assessed 74 patients and found that 64-slice MSCT correctly identified all patients with ISR (ten out of 70), but misclassified five patients without ISR (false-positives) [38]. Overall, the accuracy of MSCT for detection of angiographic ISR was 93%. The sensitivity, specificity, and positive and negative predictive values were 100, 91, 67 and 100%, respectively. When the analysis was restricted to patients with stenting of the LMCA with or without extension into a single major side branch (SB), accuracy was 98%. When both branches of the LMCA bifurcation were stented, accuracy was 83%. For the assessment of stent diameter and area, MSCT showed correlation with IVUS of 0.78 and 0.73, respectively. Of note, if intermediate stenosis is detected within the LM, then like with angiography, its significance would need to be further assessed by FFR or IVUS.
▪ Cardiovascular MRI
Cardiovascular magnetic resonance imaging (CMRI) has some advantages and limitations compared with cardiac CT imaging. Advantages of CMRI include the absence of ionizing radiation and contrast media as well as no requirement for heart rate control with b-blockers [39]. These features are advantageous in certain patients such as those with renal dysfunction or younger patients for whom the avoidance of radiation is particularly important [40].
In addition, coronary artery calcification, which lowers specificity with coronary CT angiography [41–43], is not prominent on CMRI images because of its low proton content. As a result, detection of coronary lesions in heavily calcified coronary segments by CMRI can be more reliable than by cardiac CT [43].
However, the procedure with CMRI requires a skilled center with skilled operators and technicians since high-quality, artifact-free acquisition is time-consuming and the machines remain vendor-specific. More importantly, although stents are not a contraindication for CMRI [44], the stent may interfere with local image quality and this reduces the potential of CMRI for post- PCI surveillance of ISR. As a whole, as compared with coronary CT, CMRI for the native coronary arteries is limited to a more select patient population [40,44].
LM equivalent disease
Significant (>70%) proximal LAD and LCx disease is so called because it appears to behave similarly to true LM disease [45], although its prognosis may be better [46]. The CASS registry is again the largest experience with this group of patients, with CABG associated with a significant increase in mean survival at more then 16 years of follow-up [47], as compared with medical therapy.
Treatment options
The VA Co-operative Surgery Study suggested an early survival advantage with surgery as compared with medical therapy but diminishing returns by the time of 18‑year follow-up [11,48]. In the CASS registry, however, the survival advantage appeared to persist with the 15-year cumulative estimates revealing survival of 37% of 1153 patients in the surgical arm versus 27% of 331 patients in the medical group (p < 0.0001) [49]. Surgical revascularization improved prognosis in most clinical and angiographic subgroups.
Against these results, the early experience with balloon angioplasty was relatively poor, with up to 30% 1-year mortality in some series [50]. This was secondary to abrupt closure, high rates of restenosis as well as a selection bias introduced by the fact that many of these patients had comorbidities that made them poor candidates for surgery in the first place (and hence a likely higher 1-year mortality). However, this early experience did serve to allay concerns regarding acute periprocedural risk including early unfounded concerns of hemodynamic collapse even during temporary balloon inflation [50].
What the guidelines say currently
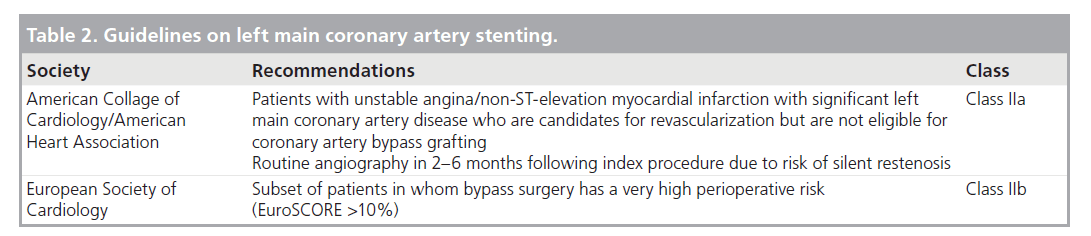
It is hardly surprising given the large area of myocardium at risk, the protection that CABG provided compared with medical therapy, and the experience with early balloon angioplasty, that this area of disease remained in the surgical realm, which is reflected in the practice guidelines. The American College of Cardiology/American Heart Association (ACC/AHA), Society for Cardiac Angiography and Interventions [51] and European Society of Cardiology [52] guidelines indicate the preferred revascularization option for LM CAD as CABG (Class I). PCI with stenting for elective cases is categorized as a Class III (not recommended) indication unless the patient is not a bypass surgery candidate (Table 2).
Table 2. Guidelines on left main coronary artery stenting.
Protected LM PCI
The distinction between protected and unprotected LMCA disease was made once CABG was established as the gold standard of therapy and was made based on at least one patent graft to the LAD or LCx arteries [53].
The outcome of protected LM intervention is more favorable than when no patent graft to the LAD or LCx exists [54–56]. The acute risks of the procedure are mitigated by the fact that essentially one is treating the equivalent of an ostial lesion of a single vessel [55].
Improving percutaneous outcomes
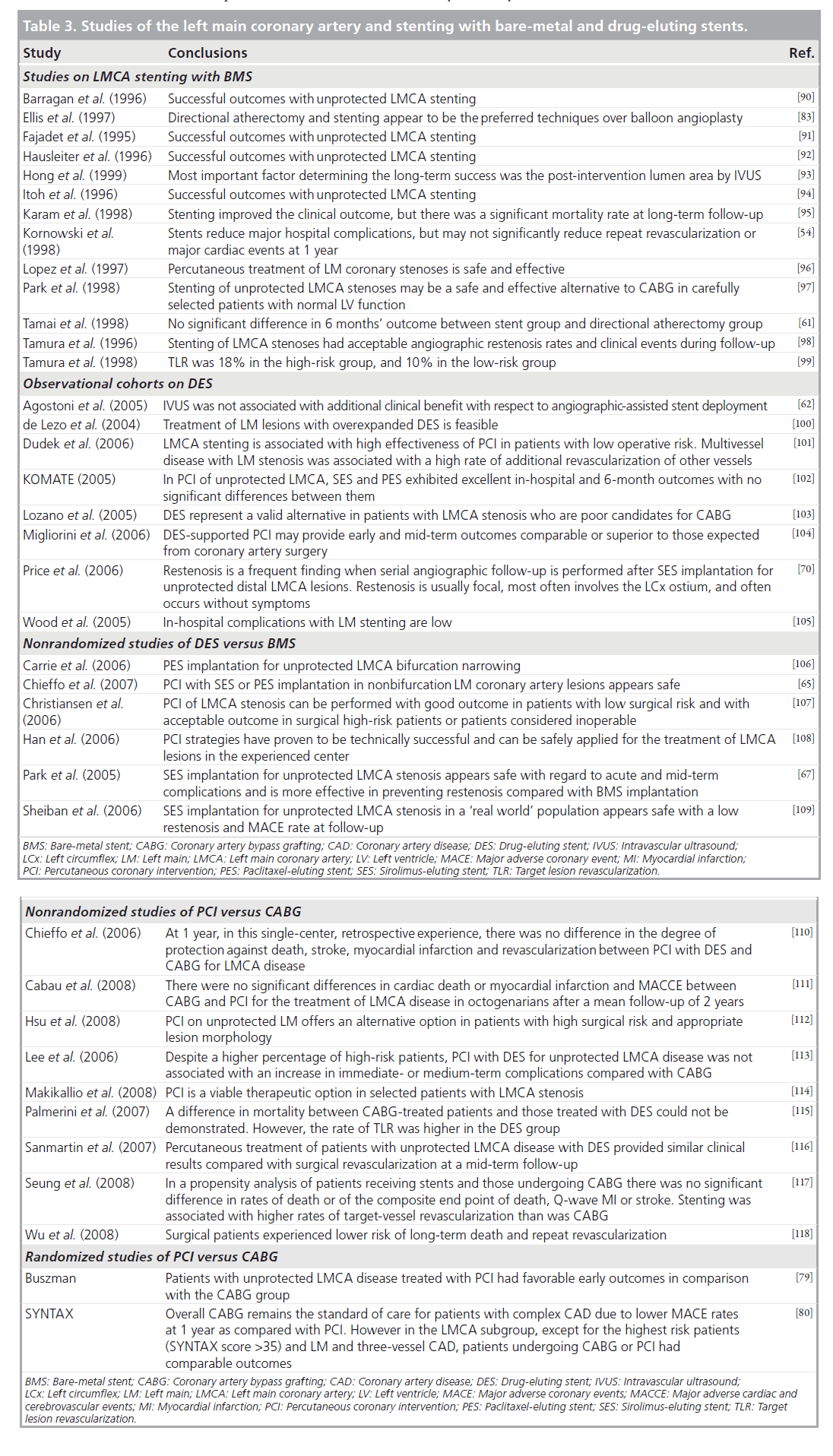
The benefit of stenting is well recognized and includes optimizing eventual coronary lumen geometry, reducing periprocedural complications and reducing restenosis. In terms of the LMCA, the advent of BMS did indeed decrease the rates of peri-procedural complications as well as restenosis [57–59] but repeat revascularization rates remained high [60]. Table 3 summarizes some of the LMCA BMS trials.
Table 3. Studies of the left main coronary artery and stenting with bare-metal and drug-eluting stents.
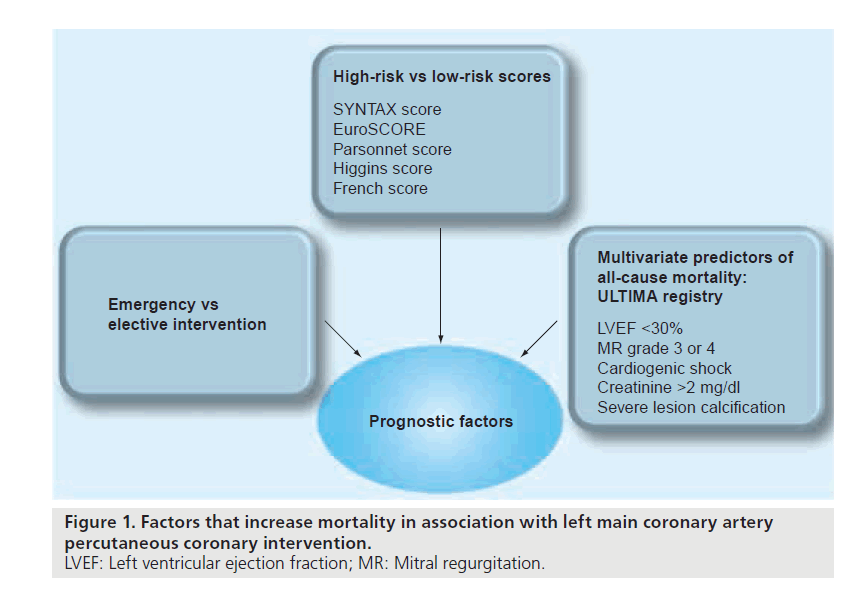
Further refinement in the prognosis occurred after it became clear that in-hospital and 1-year mortality was dependant on several patient and anatomic characteristics. Figure 1 summarizes patients at the highest risk for mortality after LM stenting. In comparison to patients with acute MI who were found to have a mortality rate of 35.7% or those who underwent bailout PCI who had a mortality rate of 40%, Kosuga et al. found that those undergoing elective LM PCI carried a significantly lower in-hospital mortality rate of 3.6% [56].
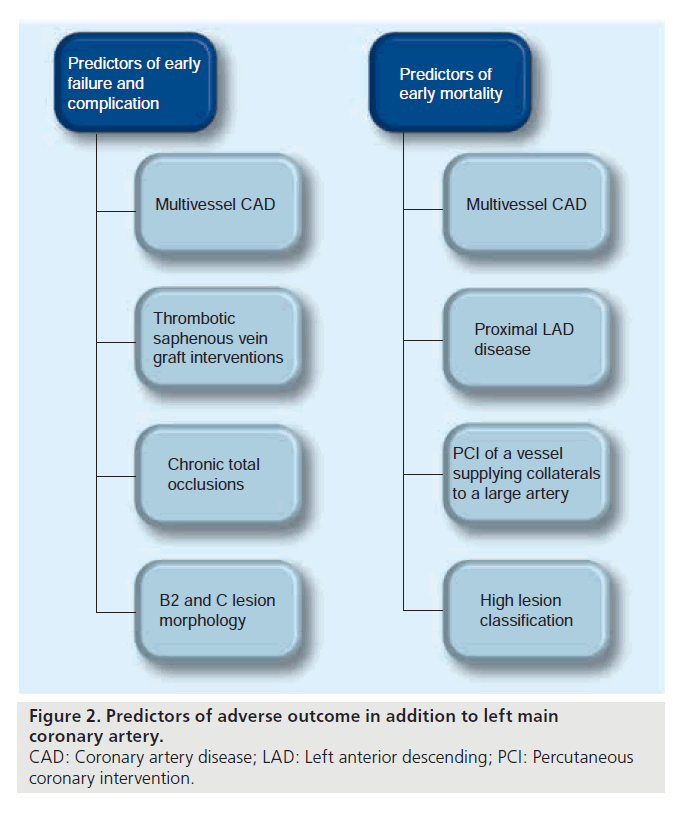
Figure 1. Factors that increase mortality in association with left main coronary artery percutaneous coronary intervention. LVEF: Left ventricular ejection fraction; MR: Mitral regurgitation.
The Ultima registry [58,61] suggested a number of predictors of all-cause mortality in patients undergoing LM intervention, which are also summarized in Figure 1. Other predictors of mortality and early failure are summarized in Figure 2 and include multivessel coronary artery disease and lesion morphology.
▪ Lesion location
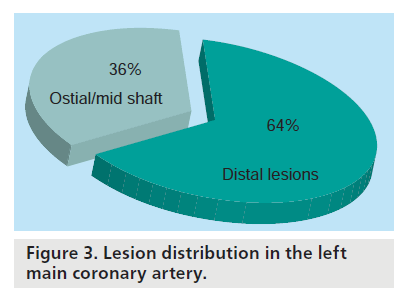
In addition to the importance of the predictors discussed above it became clear that lesion location played a significant role in the acute and longer-term outcomes of the procedure. Figure 3 summarizes the incidence of lesions in the LM with approximately two-thirds occurring at the bifurcation and the other third at the ostium or mid-shaft. Ostial or mid-shaft (nonbifurcation) lesions are the least prone to restenosis whereas distal lesions involving the bifurcation are at higher risk [60,62–65]. Valgimigli et al. found significantly higher MACE rates in patients with distal LMCA disease versus those with ostial or mid-shaft lesions (30 vs 11%) with a hazard ratio of 3.42 [64].
The registry of Chieffo et al. of 147 patients undergoing PCI with DES of ostial or mid-shaft lesions found a 0% in-hospital and 2.7% mortality in follow-up of greater than 2 years [65]. Target vessel revascularization (TVR) in this time was 4.7%. To aid understanding of these outcomes, although these are not matched cohorts, a report from the Cleveland Clinic in the lowest risk patients undergoing CABG reported a 3‑year mortality of 4.5% [66] and two British studies reported an overall 2‑year mortality of 5 and 6% in patients undergoing off-pump and on-pump CABG for LMCA disease [67,68]. This suggests excellent outcomes in unprotected LMCA stenting of ostial or mid‑shaft lesions.
▪ Advent of DES
Evidence following the introduction of DES in 2003 demonstrated improved outcomes in LM PCI as compared with BMS. In addition to a higher frequency of procedural success, improved late outcomes including lower rates of restenosis were reported [64,69,70]. Table 3 summarizes some of the trials using DES.
A number of nonrandomized studies comparing BMS to DES were subsequently published (Table 3). In a pooled analysis, Biondi-Zoccai et al. revealed the superiority of DES in MACE and TVR with an OR of 0.34 (95% CI: 0.16–0.71; p < 0.05) [63].
They also performed an exploratory subgroup analysis of DES for nonbifurcational lesions and for low- and high-risk unprotected LMCA stenting [63].
In nonbifurcational lesions, their analysis revealed an in-hospital death rate of 0.9% (95% CI: of 0–2.1%) and MI of 3.2% (CI: 0–5.6%). After a median follow-up of 10 months, MACE was 14.7% (CI: 6.2–23.2%), death 4.1% and TVR 6.7%.
In low-risk patients (defined by a EuroScore of <6 or Parsonnet score of <15), in-hospital death was found to be 3.0% and MI also 3.0%. In higher risk patients (EuroScore >6, Parsonnet Score >15) in-hospital death was 6.6%, MI 1.3% and death at 8 months 12%. Again for some perspective of these numbers, in a cohort of patients with LMCA disease undergoing CABG, the Cleveland Clinic reported 2.3% in-hospital mortality and 15.6% 3‑year mortality [66]. For low-risk patients from the Cleveland cohort 3‑year mortality was 4.5 and 6.5% in the intermediate-risk quartile. It was 20 and 39.8% in the two highest risk quartiles, respectively [66]. Additionally, a pooled weighted average of almost 11,000 patients undergoing CABG reported over the last decade reveals an in-hospital mortality of 2.8% and a 30‑day mortality of 3–4.2% [71].
After 8–10 months follow-up, the meta-analysis suggests that in selected patients there were favorable outcomes with PCI of the LMCA especially with DES, although clinical follow-up is currently at the mid-term threshold. In selecting between DES, an analysis from RESEARCH and T-SEARCH revealed no significant difference between sirolimus- (Cypher®) and paclitaxel- (Taxus®)eluting stents [72], and the randomized ISAR-Left-Main study provided further data on the safety and efficacy of LMCA stenting as well as revealing comparable clinical and angiographic outcomes for the Cypher and Taxus stents [73]. In real-world clinical practice, however, this choice may be superseded by the increasing worldwide usage of the everolimus-eluting stent (Xience™/Promus™), although no data have been published as of yet with the exclusive use of this stent in the LMCA setting.
▪ Provisional single-stent versus multiple-stent approach
As discussed above, although DES appears to be superior to BMS in LMCA trials, the complexities of polymer and drug delivery as well as mechanical stent coverage especially at the site of bifurcation lesions remains a challenge [74,75].
Inadequate coverage at the ostium of the SB makes this site the most frequent location for restenosis after conventional stenting of branch vessel stenosis. Attempts to provide better stent coverage of the SB origin by using culotte, crush or kissing stent techniques creates multiple layers of metal [76] and permanent polymer (four to six layers) and is associated with an increased incidence of complication as compared with nonbifurcation lesions. In addition, nonuniform stent strut distribution is associated with variable drug delivery and eventually an increase in restenosis. This is especially true of the LM bifurcation where restenosis is seen most frequently at the ostium of the LCX artery [53,60].
The practice of provisional single versus upfront multiple stent techniques has evolved to an understanding that ‘less is often more’ [77,203]. Park revealed that in comparison to a single stent the kissing stent or crush technique had increased risk of restenosis [67]. When possible the use of a single stent with rescue of the SB as necessary has been shown to significantly improve outcome [53]. Of note, patients with restenosis in this area can present with sudden cardiac death [57].
Of note, several novel technologies in development for branch vessel treatment are being specifically designed for branch vessel application and thus provide more uniform coverage with less metal and polymer. These technologies may hold the key to improving outcomes at the LMCA bifurcation.
▪ Strategy for LMCA interventions
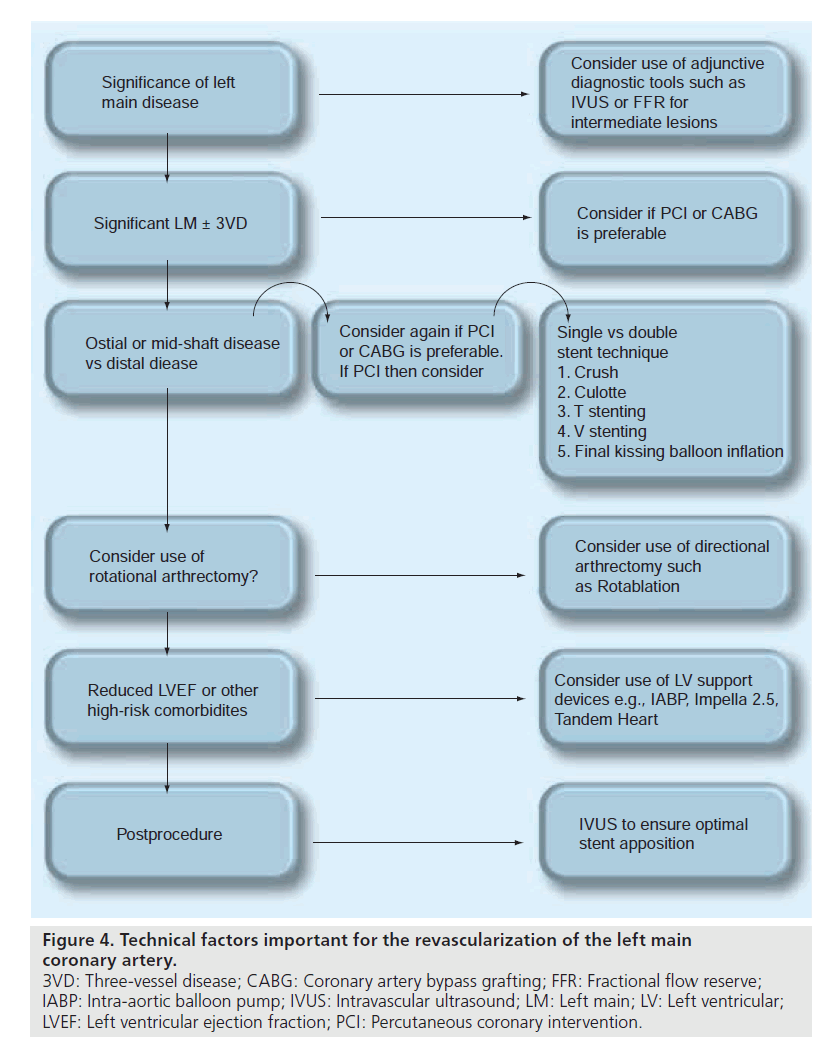
For successful revascularization of the LMCA several factors ought to be taken into account prior to determining the management of the patient. Figure 4 summarizes several potential scenarios that ought to be considered prior to undertaking any LMCA revascularization.
Figure 4. Technical factors important for the revascularization of the left main coronary artery. 3VD: Three-vessel disease; CABG: Coronary artery bypass grafting; FFR: Fractional flow reserve; IABP: Intra-aortic balloon pump; IVUS: Intravascular ultrasound; LM: Left main; LV: Left ventricular; LVEF: Left ventricular ejection fraction; PCI: Percutaneous coronary intervention.
▪ PCI with DES versus CABG
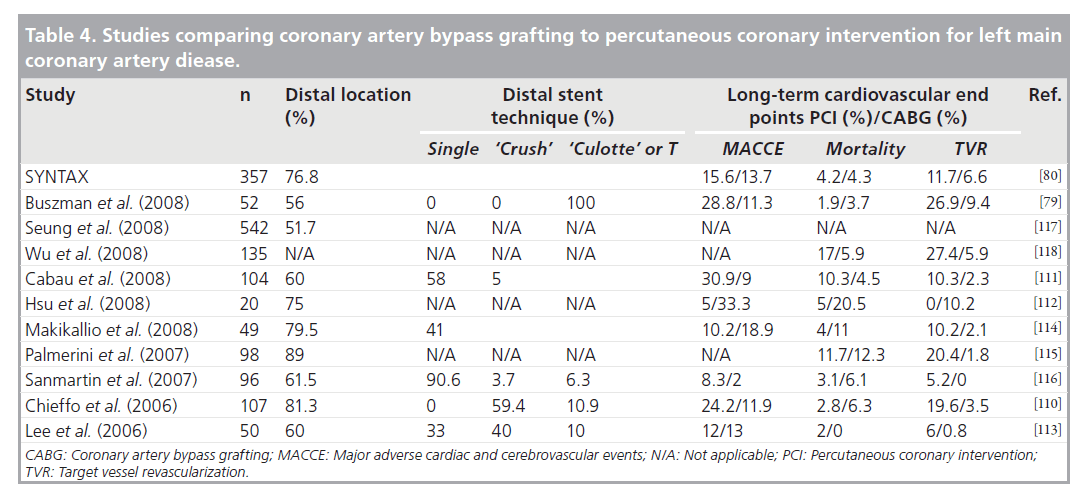
The ultimate question in the progression of improved outcomes with percutaneous intervention is how PCI would perform in this era as compared with CABG. To help answer this, we assembled data as part of a recent systematic review of studies that compared outcomes of DES to CABG [78]. Studies with the majority of patients undergoing emergent revascularization or with severely reduced left ventricular ejection fraction (LVEF) were excluded. A total of 11 studies comprising 3781 patients met the inclusion criteria. A total of 1287 patients underwent PCI of whom 80% had DES. The studies found are detailed in Table 4. Of note, these data include two randomized controlled trials [79,80] including the recently published SYNTAX trial [80].
Table 4. Studies comparing coronary artery bypass grafting to percutaneous coronary intervention for left main coronary artery diease.
The characteristics of these patients included 25% with diabetes, 62% with hypertension and 55% with dyslipidemia. Anatomic characteristics, type of stenting procedure and outcomes are also summarized in Table 4.
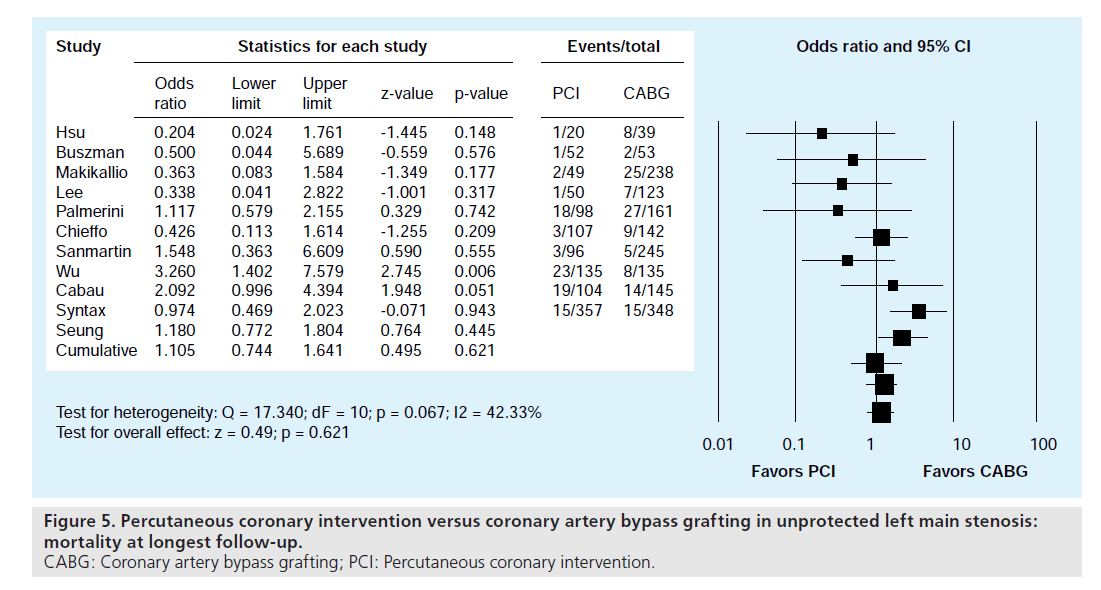
We found no significant difference in the incidence of all-cause mortality between patients who underwent PCI as compared with those patients who underwent CABG (OR: 1.11; 95% CI: 0.74–1.64; Figure 5). Although TVR at longest follow up occurred more frequently in patients undergoing PCI (OR: 4.63; 95% CI: 2.09–7.37), long-term major adverse cardiac and cerebrovascular events (MACCE) were similar in the two groups. Of note, MACCE at 30 days was significantly reduced in PCI patients (OR: 0.28; 95% CI: 0.15–0.50).
Figure 5. Percutaneous coronary intervention versus coronary artery bypass grafting in unprotected left main stenosis: mortality at longest follow-up. CABG: Coronary artery bypass grafting; PCI: Percutaneous coronary intervention.
These results suggest that patients undergoing CABG suffer from increased acute MACCE, likely early stroke, but TVR increases over the follow-up period so that MACCE rates eventually are not significantly different. Similar results were reported in the LM subgroup of the randomized SYNTAX trial [80] and in a metaanalysis comparing PCI to CABG of native coronaries [81]. Of note, the nonsignificance of the longer term MACCE also suggests that in the current era, the increased rate of TVR is not costing the patient undergoing PCI in terms of MI, stroke or death as compared with CABG.
As part of these findings, it is worth emphasizing the LM subgroup analysis of the SYNTAX trial (which although prespecified remains observational in nature after the overall trial did not meet its noninferiority end point). Overall, the 12‑month MACCE rates were similar in the CABG and PCI groups (13.7 vs 15.8%; p = 0.44). However, following the earlier arguments of anatomic location and patient selection, patients with LM disease and low SYNTAX scores (<22) had MACCE rates that trended toward favoring PCI over CABG (7.7 vs 13%; p = 0.19). As may be expected, as the SYNTAX score rose, MACCE rates trended instead to favoring CABG so that the comparable rates with a Syntax score of 23–32 were 15.5 vs 12.6% (p = 0.54) and in patients with a score greater than 33 the rates were further in favor of CABG (>12.9 vs 25.3%; p = 0.008). In a further analysis of the LM subgroup, patients with LM disease only, and LM and single-vessel disease had nonsignificantly reduced MACCE rates at 12 months with PCI as compared with CABG, further emphasizing the fact that with careful patient selection PCI now appears to be comparable to CABG.
It is worth noting that most of the follow-up in the trials above including SYNTAX ranged from 12 to 24 months, a time period in which much of the benefit from CABG may not have accrued. However, in all of the trials, most of which are driven by TVR rates, it is only the PCI arm that specified routine angiographic surveillance. In the initial Scripps experience – with routine surveillance angiography – the TLR rate was 38% [70]. However, in a cohort at the same center but instead using an ischemia-driven TLR definition, this rate was observed in 14% of patients. This underscores the confounding impact of routine surveillance and the oculostenostic reflex [25,53]. Routine surveillance may, however, play an important role especially since restenosis can be asymptomatic and abrupt [57,70]; however, Chieffo’s study (with very low MACE rates for ostial and midshaft lesions undergoing PCI) suggests that it can be reserved for distal LM disease especially if treated with a multiple stent technique. This is an area that as MSCT evolves, it may be able to play an increasing role in decreasing the need for repeat invasive procedures.
It is also worth noting that the CABG arm of these studies had a repeat revascularization rate as low as 0.82% and as high as 10.2%. Although this discrepancy cannot be explained solely by the rate of arterial revascularization it undoubtedly plays an important role. The importance of the left and right internal mammary conduits is wellrecognized [53,82]. However, just as the application of rigorous interventional technique (see ‘Strategy for LMCA Interventions’ above) is critically important to the acute and long-term durability of every PCI, the techniques surrounding arterial revascularization and the consequent proportion of right and especially left internal mammary grafts anastamozed are of long-term consequence to the durability of surgical revascularization and should likely enter into the discussion of the procedure of choice for each patient, in other words, in a patient eligible for both procedures if revascularization with an arterial graft is not possible consideration ought to be given more strongly to pursuing PCI. It is worth noting that despite a single arterial revascularization rate of 97.3%, SYNTAX still had a residual revascularization rate close to 6.0%. Surgeons ought to consider that in order to reduce this rate further one of the options likely lies in increasing the proportion of patients receiving dual arterial grafts whenever possible.
Until head-to-head randomized data is available (trials underway, see under ‘Future Perspective’), these data would indicate that PCI today may be comparable to CABG in selected patients undergoing elective revascularization of the LMCA. It would appear then that the next advance ought to be in the refinement of how best to triage those patients who fall into the ‘select’ category so that they have a choice of revascularization options. This triage should be made so as to optimize their individual outcomes.
LMCA PCI for acute MI
In-hospital mortality rates are expectedly high, 35% in primary PCI of the LM presenting as an acute MI [56,83]. In the SHOCK Trial registry [84], 16% of patients with cardiogenic shock complicating MI had significant LM disease. These patients had a higher mortality rate (79%) versus 42% in those with LAD disease and 37% in those with LCx disease. The expected efficacy of PCI with stent as compared with balloon angioplasty alone was illustrated in an observational registry where patients presenting with an acute MI and unprotected LM stenosis had lower rates of in-hospital death and bypass surgery with stenting [85].
Conclusion
Although one of the shortest segments of the coronary tree, the LMCA remains a challenge in its accurate diagnosis and optimal management. Although CABG remains the standard of care, in the current era with DES and improved medical therapy, emerging studies suggest excellent shortand medium-term results with PCI so that the revascularization of choice ought to be tailored to each patient. A consensus decision through consultation with surgery, the patient and their family should be sought. However, it can certainly be argued that in selected patients, updates in practice guidelines as related to the role of PCI should be considered. In higher risk patients such as those with low LVEF, bifurcational disease, complex three-vessel disease or renal failure, CABG will likely remain the revascularization procedure of choice.
Future perspective
Impacts in the future that will likely affect decision-making regarding the management of LMCA disease include the outcome of currently enrolling randomized controlled trials, emerging technologies and the rising cost of heathcare delivery. SYNTAX was not powered to answer the PCI versus CABG question but COMBAT, a trial comparing DES with a sirolimus-eluting stent (Cypher) to CABG in LMCA PCI, is currently enrolling and has been powered to answer this question [204]. Emerging technologies, especially the dedicated bifurcation stents may improve outcomes of distal LM stenting and increase the number of patients who would be suitable candidates for PCI. Finally, the rapidly increasing emphasis on the cost of healthcare delivery will also likely favor PCI over CABG, especially since the length of procedural hospitalization is significantly shorter. Unless the randomized trials show otherwise, the future of LMCA revascularization, with these factors working in tandem, appears to be one in which the patients will increasingly be managed by PCI with only the higher risk patients, who are suitable for surgery, going on to CABG. In addition to the randomized trials, to ensure that outcomes for our patients continue to improve into the future, further study is required on how best to dichotomize this choice.
Acknowledgement
The authors would like to acknowledge JP Carrozza, MD, for help with assembling Table 1. The image of the 3D cardiac CT was provided courtesy of Michael Blake, MD.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Fox C, Davies MJ, Webb Peploe MM: Length of the LM coronary artery. Br. Heart J. 35, 796–798 (1973).
- Danias PG, Stuber M, McConnell MV, Manning WJ: The diagnosis of congenital coronary anomalies with magnetic resonance imaging. Coron. Artery Dis. 12, 621–626 (2001).
- Plotnick GD, Greene HL, Carliner NH et al.: Clinical indicators of LM coronary artery disease in unstable angina. Ann. Intern. Med. 91, 149–153 (1979).
- Salem BI, Terasawa M, Mathur VS et al.: Exercise testing and LM coronary artery disease: experience with 57 patients. Cardiovasc. Dis. 5, 384–390 (1978).
- Shiba C, Chikamori T, Hida S et al.: Important parameters in the detection of LM trunk disease using stress myocardial perfusion imaging. J. Cardiol. 53, 43–52 (2009).
- Giannoglou GD, Antoniadis AP, Chatzizisis YS et al.: Prevalence of narrowing N or = 50% of the LM coronary artery among 17,300 patients having coronary angiography. Am. J. Cardiol. 98, 1202–1205 (2006).
- Miller GA, Honey M, el-Sayed H: Isolated coronary ostial stenosis. Cathet. Cardiovasc. Diagn. 12, 30–34 (1986).
- Tommaso CL, Applefeld MM, Scherlist L et al.: Incidence and etiology of isolated LM coronary artery stenosis (abstract). Chest 86, 284 (1984).
- Welch CC, Proudfit WL, Sheldon WC: Coronary arteriographic findings in 1,000 women under age 50. Am. J. Cardiol. 35, 211–215 (1975).
- Yamanaka O, Hobbs RE: Solitary ostial coronary artery stenosis. Jpn. Circ. J. 57, 404–410 (1993).
- Detre KM, Murphy ML, Hultgren HN: Effect of coronary bypass surgery on longevity in high and low risk patients: report from the VA Cooperative Coronary Surgery Study. Lancet 2, 1243–1245 (1977).
- Taylor HA, Deumite NJ, Chaitman BR et al.: Asymptomatic LM coronary artery disease in the Coronary Artery Surgery Study (CASS) registry. Circulation 79, 1171–1179 (1989).
- Caracciolo EA, Davis KB, Sopko G et al.: Comparison of surgical and medical group survival in patients with LM coronary artery disease. Long-term CASS experience. Circulation 91, 2325–2334 (1995).
- Arnett EN, Isner JM, Redwood DR et al.: Coronary artery narrowing in coronary artery disease: comparison of cineangiographic and necropsy findings. Ann. Intern. Med. 91, 350–356 (1979).
- Waller BF: Anatomy, histology, and pathology of the major epicardial coronary arteries relevant to echocardiographic imaging techniques. J. Am. Soc. Echocardiogr. 2, 232–252 (1989).
- Isner JM, Donaldsen RF: Coronary angiographic and morphologic correlation. In: Cardiac Morphology. Waller BF (Ed.). Saunders, Philadelphia, PA, USA, 571–592 (1984).
- Marcus ML, Skorton DJ, Johnson MR, Collins SM, Harrison DG, Kerber RE: Visual estimates of percent diameter coronary stenosis: battered gold standard. J. Am. Coll. Cardiol. 11, 882–885 (1988).
- El Menyar A, Al Suwaidi J, Holmes DR: LM coronary artery stenosis: state of the art. Curr. Probl. Cardiol. 32, 103–193 (2007).
- Glagov S, Weisenberg E, Zarins CK et al.: Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316, 1371–1375 (1987).
- Alfonso F, Macaya C, Goicolea J et al.: Intravascular ultrasound imaging of angiographically normal coronary segments in patients coronary artery disease. Am. Heart J. 127, 536–544 (1994).
- Porter T, Sears T, Xie F et al.: Intravascular ultrasound study angiographically mildly diseased coronary arteries. J. Am. Coll. Cardiol. 22, 1858–1865 (1993).
- Fassa AA, Wagatsuma K, Higano ST et al.: Intravascular ultrasound-guided treatment for angiographically indeterminate LM coronary artery disease: a long-term follow-up study. J. Am. Coll. Cardiol. 45, 204–211 (2005).
- Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA: Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous LM coronary artery stenosis. Circulation 110, 2831–2836 (2004).
- Sano MD, Mintz G, Carlier S et al.: Assessing intermediate LM coronary lesions using intravascular ultrasound. Am. Heart J. 154, 983–988 (2007).
- Topol EJ, Nissen SE: Our preoccupation with coronary luminology: the dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 92, 2333–2342 (1995).
- Patil CV, Beyar R: Intermediate coronary artery stenosis: evidence-based decisions in interventions to avoid the oculostenotic reflex. Int. J. Cardiovasc. Intervent. 3, 195–206 (2000).
- Kuntz RE: Importance of considering atherosclerosis progression when choosing a coronary revascularization strategy: the diabetespercutaneous transluminal coronary angioplasty dilemma. Circulation. 99, 847–851 (1999).
- Mintz GS, Weissman NJ: Intravascular ultrasound in the drug-eluting stent era. J. Am. Coll. Cardiol. 48, 421 (2006).
- Bech GJW, Droste H, Pijls NHJ et al.: Value of fractional flow reserve in making decisions about bypass surgery for equivocal LM coronary artery disease. Heart 86, 547–552 (2001).
- Flohr T, Bruder H, Stierstorfer K et al.: New technical developments in multislice CT, part 2: sub-millimeter 16-slice scanning and increased gantry rotation speed for cardiac imaging. Rofo 174, 1022–1027 (2002).
- Ferencik M, Moselewski F, Ropers D et al.: Quantitative parameters of image quality in multidetector spiral computed tomographic coronary imaging with submillimeter collimation. Am. J. Cardiol. 92, 1257–1262 (2003).
- Leschka S, Alkadhi H, Plass A et al.: Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur. Heart J. 26, 1482–1487 (2005).
- Mollet NR, Cademartiri F, van Mieghem CA et al.: High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation 112, 2318–2323 (2005).
- Stein PD, Yaekoub AY, Matta F, Sostman HD: 64 slice CT for diagnosis of coronary artery disease: a systematic review. Am. J. Med. 121, 715–725 (2008).
- Schoenhagen P, Halliburton SS, Stillman AE et al.: Noninvasive imaging of coronary arteries: current and future role of multidetector row CT. Radiology 232, 7–17 (2004).
- Ligabue G, Rossi R, Ratti C, Favali M, Modena MG, Romagnoli R: Noninvasive evaluation of coronary artery stents patency after PTCA: role of Multislice Computed Tomography. Radiol. Med. (Torino) 108, 128–137 (2004).
- Gilard M, Cornily JC, Rioufol G et al.: Noninvasive assessment of LM coronary stent patency with 16-slice computed tomography. Am. J. Cardiol. 95(1), 110–112 (2005).
- Van Mieghem CAG, Cademartiri F, Mollet NR et al.: Multislice spiral computed tomography for the evaluation of stent patency after LM coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation 114, 645–653 (2006).
- Gerber TC, Manning W: Noninvasive coronary angiography with cardiac computed tomography and cardiovascular magnetic resonance. In: UpToDate Basow, DS (17.1), UpToDate, Waltham, MA, USA 2009.
- Bluemke DA, Achenbach S, Budoff M et al.: Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation 118, 586 (2008).
- Leber AW, Knez A, von Ziegler F et al.: Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J. Am. Coll. Cardiol. 46(1), 147–154 (2005).
- Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA: Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J. Am. Coll. Cardiol. 46(3), 552–557 (2005).
- Langer C, Wiemer M, Peterschroder A, Franzke K, Meyer H, Horstkotte D: Images in cardiovascular medicine. Multislice computed tomography and magnetic resonance imaging: complementary use in noninvasive coronary angiography. Circulation 112(23), E343–E344 (2005).
- Levine GN, Gomes AS, Arai AE et al.: Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation 116, 2878 (2007).
- Cutlip D: Management of LM coronary artery disease. In: UpToDate Basow, DS (17.1), UpToDate, Waltham, MA, USA 2009.
- Chaitman BR, Davis K, Fisher LD et al.: A life table and Cox regression analysis of patients with combined proximal left anterior descending and proximal left circumflex coronary artery disease: non-LM equivalent lesions (CASS). Circulation 68(6), 1163–1170 (1983).
- Caracciolo EA, Davis KB, Sopko G et al.: Comparison of surgical and medical group survival in patients with LM equivalent coronary artery disease. Long-term CASS experience. Circulation 91(9), 2335–2344 (1995).
- Takaro T, Peduzzi P, Detre KM et al.: Survival in subgroups of patients with LM coronary artery disease. Veterans Administration Cooperative Study of Surgery for Coronary Arterial Occlusive Disease. Circulation 66(1), 14–22 (1982).
- Eighteen-year follow-up in the Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for stable angina. The VA Coronary Artery Bypass Surgery Cooperative Study Group. Circulation 86, 121–130 (1992).
- Fischman DL, Leon MB, Baim DS et al.: A randomized comparison of coronary – stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 331, 496 (1994).
- Smith SC Jr, Feldman TE, Hirshfeld JW Jr et al.: ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention). Circulation 113, E166–E286 (2006).
- Silber S, Albertsson P, Aviles FF et al.: Task Force for PCI of the European Society of Cardiology. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur. Heart J. 26, 804–847 (2005).
- Teirstein PS: Percutaneous revascularization is the preferred strategy for patients with significant LM coronary stenosis. Circulation. 119, 1021–1033 (2009).
- Kornowski R, Klutstein M, Satler LF et al.: Impact of stents on clinical outcomes in percutaneous LM coronary artery revascularization. Am. J. Cardiol. 82, 32–37 (1998).
- Keeley EC, Aliabadi D, O’Neill WW, Safian RD: Immediate and long-term results of elective and emergent percutaneous interventions on protected and unprotected severely narrowed LM coronary arteries. Am. J. Cardiol. 83, 242–246 (1999).
- Kosuga K, Tamai H, Ueda K et al.: Initial and long-term results of angioplasty in unprotected LM coronary artery. Am. J. Cardiol. 83, 32–37 (1999).
- Takagi T, Stankovic G, Finci L et al.: Results and long-term predictors of adverse clinical events after elective percutaneous interventions on unprotected LM coronary artery. Circulation 106, 698–702 (2002).
- Black A, Cortina R, Bossi I, Choussat R, Fajadet J, Marco J: Unprotected LM coronary artery stenting: correlates of midterm survival and impact of patient selection, J. Am. Coll. Cardiol. 37, 832–838 (2001).
- Tan WA, Tamai H, Park SJ et al.: Long-term clinical outcomes after unprotected LM trunk percutaneous revascularization in 279 patients. Circulation 104, 1609–1614 (2001).
- Baim DS, Mauri L, Cutlip DC: Drug-eluting stenting for unprotected LM coronary artery disease: are we ready to replace bypass surgery? J. Am. Coll. Cardiol. 47, 878–881 (2006).
- Tamai H, Park S-J, Plokker T et al.: Directional atherectomy or stenting for unprotected LM coronary artery stenosis – the ULTIMA group experience (Abstract). J. Am. Coll. Cardiol. 31(Suppl. A), 101A (1998).
- Agostoni P, Valgimigli M, Van Mieghem CA et al.: Comparison of early outcome of percutaneous coronary intervention for unprotected LM coronary artery disease in the drug-eluting stent era with versus without intravascular ultrasonic guidance. Am. J. Cardiol. 95, 644–647 (2005).
- Biondi-Zoccai GG, Lotrionte M, Moretti C et al.: A collaborative systematic review and meta-analysis on 1278 patients undergoing percutaneous drug-eluting stenting for unprotected LM coronary artery disease. Am. Heart J. 155, 274–283 (2007).
- Valgimigli M, van Mieghem CA, Ong AT et al.: Short- and long-term clinical outcome after drug-eluting stent implantation for the percutaneous treatment of LM coronary artery disease: insights from the Rapamycin- Eluting and Taxus Stent Evaluated At Rotterdam Cardiology Hospital registries (RESEARCH and T-SEARCH). Circulation 111, 1383–1389 (2005).
- Chieffo A, Park SJ, Valgimigli M et al.: Favorable long-term outcome after drugeluting stent implantation in nonbifurcation lesions that involve unprotected LM coronary artery: a multicenter registry. Circulation. 116, 158–162 (2007).
- Ellis SG, Hill CM, Lytle BW: Spectrum of surgical risk for LM coronary stenoses: benchmark for potentially competing percutaneous therapies. Am. Heart J. 135, 335–338 (1998).
- Park SJ, Kim YH, Lee BK et al.: Sirolimuseluting stent implantation for unprotected LM coronary artery stenosis: comparison with bare metal stent implantation. J. Am. Coll. Cardiol. 45, 351–356 (2005).
- Chieffo A, Stankovic G, Bonizzoni E et al.: Early and mid-term results of drug-eluting stent implantation in unprotected LM. Circulation 111, 791–795 (2005).
- Arampatzis CA, Lemos PA, Tanabe K et al.: Effectiveness of sirolimus-eluting stent for treatment of LM coronary artery disease. Am. J. Cardiol. 92, 327–329 (2003).
- Price MJ, Cristea E, Sawhney N et al.: Serial angiographic follow-up of sirolimus-eluting stents for unprotected LM coronary artery revascularization. J. Am. Coll. Cardiol. 47(4), 871–877 (2006).
- Smith CR: Surgery, not percutaneous revascularization, is the preferred strategy for patients with significant LM coronary stenosis. Circulation 119, 1013–1020 (2009).
- Valgimigli M, Malagutti P, Aoki J et al.: Sirolimus-eluting versus paclitaxel-eluting stent implantation for the percutaneous treatment of LM coronary artery disease: a combined RESEARCH and T-SEARCH long-term analysis. J. Am. Coll. Cardiol. 47, 507–514 (2006).
- Mehilli J, Kastrati A, Byrne RA et al.: Paclitaxel – versus sirolimus eluting stents for unprotected LM coronary artery disease. J. Am. Coll. Cardiol. 53, 1760–1768 (2009).
- Williams DO, Abbott JD: Bifurcation intervention: is it crush time yet? J. Am. Coll. Cardiol. 46, 621–624 (2005).
- Iakovou I, Ge L, Colombo A: Contemporary stent treatment of coronary bifurcations. J. Am. Coll. Cardiol. 46, 1446–1455 (2005).
- Costa RA, Mintz GS, Carlier SG et al.: Bifurcation coronary lesions treated with the – crush – technique. J. Am. Coll. Cardiol. 46, 599–605 (2005).
- Steigen TK, Maeng M, Wiseth R et al.: Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation 114(18), 1955–1961 (2006).
- Khan MF, Athappan G, Popma JJ: LM coronary artery stenosis: a meta analysis of stents versus coronary artery bypass grafting. Catheter Cardiovasc. Interv. (74)1, 158 (2009).
- Buszman PE, Kiesz SR, Bochenek A et al.: Acute and late outcomes of unprotected LM stenting in comparison with surgical revascularization. J. Am. Coll. Cardiol. 51, 538–545 (2008).
- Serruys PW, Morice MC, Kappetein P et al.: Percutaneous coronary intervention versus coronary artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360, 961–972 (2009).
- Bravata DM, Gienger Al, McDonald KM et al.: Systematic review: the comparative effectiveness of PCI and CABG surgery. Ann. Intern. Med. 147, 703–716 (2007).
- Taggart DP, D’Amico R, Altman DG: Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 358(9285), 870–875 (2001).
- Ellis SG, Tamai H, Nobuyoshi M et al.: Contemporary percutaneous treatment of unprotected LM coronary stenosis: initial results from a multicenter registry analysis, 1994–1996. Circulation 96, 3867–3872 (1997).
- Wong SC, Sanborn T, Sleeper LA et al.: Angiographic findings and clinical correlates in patients with cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock? J. Am. Coll. Cardiol. 36, 1077 (2000)
- Marso SP, Steg G, Plokker T et al.: Catheter-based reperfusion of unprotected LM stenosis during an acute myocardial infarction (the ULTIMA experience). Unprotected LM Trunk Intervention Multi-center Assessment. Am. J. Cardiol. 83(11), 1513–1517 (1999).
- Jimenez-Navarro M, Hernandez-Garcia J, Alonso-Briales J et al.: Should we treat patients with moderately severe stenosis of the LM coronary artery and negative FFR results? J. Invas. Cardiol. 16, 398–400 (2004).
- Suemaru S, Iwasaki K, Yamamoto K et al.: Coronary pressure measurement to determine treatment strategy for equivocal LM coronary artery lesions. Heart Vessels 20, 271–277 (2005).
- Legutko J, Dudek D, Rzeszutko L, Wizimirski M, Dubiel JS: Fractional flow reserve assessment to determine the indications for myocardial revascularisation in patients with borderline stenosis of the LM coronary artery. Kardiol. Pol. 63(5), 499–506; discussion 507–508 (2005).
- Lindstaedt M, Yazar A, Germing A et al.: Clinical outcome in patients with intermediate or equivocal LM coronary artery disease after deferral of surgical revascularization on the basis of fractional flow reserve measurements. Am. Heart J. 152(1), 156 E1–156 E9 (2006).
- Barragan P, Silvestri M, Simeoni JB et al.: Stenting in unprotected LM coronary artery: immediate and follow up results (Abstract). Circulation 94(Suppl. I), I–672 (1996).
- Fajadet J, Brunel P, Jordan C et al.: Is stenting of LM coronary artery a reasonable procedure? (Abstract) Circulation 92(Suppl. I), I–74 (1995).
- Hausleiter J, Dirschinger J, Schuhlen H et al.: LM stenting (Abstract). Circulation 94(Suppl I), I–331 (1996).
- Hong MK, Mintz GS, Hong MK et al.: Intravascular ultrasound predictors of target lesion revascularization after stenting of protected LM coronary artery stenosis. Am. J. Cardiol. 83, 175–179 (1999).
- Itoh A, Colombo A, Hall P et al.: Stenting in protected and unprotected LM coronary artery: immediate and follow-up results (Abstract). J. Am. Coll. Cardiol. 27, 277A (1996).
- Karam C, Fajadet J, Cassagneau B et al.: Results of stenting of unprotected LM coronary artery stenosis in patients at high surgical risk. Am. J. Cardiol. 82, 975–978 (1998).
- Lopez JJ, Ho KK, Stoler RC et al.: Percutaneous treatment of protected and unprotected LM coronary artery stenoses with new devices: immediate angiographic results and intermediate-term follow up. J. Am. Coll. Cardiol. 29, 345–352 (1997).
- Park SJ, Park SW, Hong MK et al.: Stenting of unprotected LM coronary artery stenoses: immediate and late outcomes. J. Am. Coll. Cardiol. 31, 37–42 (1998).
- Tamura T, Masakiyo N, Nosaka H et al.: Palmaz-Schatz stenting in unprotected and protected LM artery: immediate and follow up results (Abstract). Circulation 94(Suppl. I), I-671 (1996).
- Tamura T, Kimura T, Nosaka M et al.: Palmaz-Schatz stenting in unprotected LM coronary artery stenosis: immediate and follow-up results (Abstract). J. Am. Coll. Cardiol. 31(Suppl. A), 273A (1998).
- De Lezo JS, Medina A, Pan M et al.: Rapamycin-eluting stents for the treatment of unprotected LM coronary disease. Am. Heart J. 148, 481–485 (2004).
- Dudek D, Heba G, Giszterowicz D et al.: Stenting of unprotected LM coronary artery in patients with low preoperative risk of coronary artery bypass grafting. Kardiol. Pol. 64, 929–936 (2006).
- Lee SH, Ko YG, Jang Y et al.: for the Korean Multicenter Angioplasty Team (KOMATE) Investigators: Sirolimus- versus paclitaxeleluting stent implantation for unprotected LM coronary artery stenosis. Cardiology 104, 181–185 (2005).
- Lozano I, Herrera C, Moris C et al.: Drug-eluting stents in patients with LM coronary lesions who are not candidates for surgical revascularization. Rev. Esp. Cardiol. 58, 145–152 (2005).
- Migliorini A, Moschi G, Giurlani L et al.: Drug-eluting stent supported percutaneous coronary intervention for unprotected LM disease. Catheter Cardiovasc. Interv. 68, 225–230 (2006).
- Wood F, Bazemore E, Schneider JE et al.: Technique of LM stenting is dependent on lesion location and distal branch protection. Catheter Cardiovasc. Interv. 65, 499–503 (2005).
- Carri D, Lhermusier T, Hmem M et al.: Clinical and angiographic outcome of paclitaxel-eluting stent implantation for unprotected LM coronary artery bifurcation narrowing. EuroIntervention 1, 396–402 (2006).
- Christiansen EH, Lassen JF, Andersen HR et al.: Outcome of unprotected LM percutaneous coronary intervention in surgical low-risk, surgical high-risk, and acute myocardial infarction patients. EuroIntervention 1, 403–408 (2006).
- Han YL, Wang SL, Jin QM et al.: Efficacy of stenting for unprotected LM coronary artery disease in 297 patients. Chin. Med. J. (Engl.) 119, 544–550 (2006).
- Sheiban I, Meliga E, Moretti C et al.: Sirolimus-eluting stents vs bare metal stents for the treatment of unprotected LM coronary artery stenosis. EuroIntervention 2, 356–362 (2006).
- Chieffo A, Morici N, Malsano F et al.: Percutaneous treatment with drug-eluting stent implantation versus bypass surgery for unprotected LM stenosis. A single-center experience. Circulation 113, 2542–2547 (2006).
- Cabau JR, Blois JD, Bertrand OF et al.: Nonrandomized comparison of coronary artery bypass surgery and percutaneous coronary intervention for the treatment of unprotected LM coronary artery disease in octogenarians. Circulation 118, 2374–2381 (2008).
- Hsu JT, Chu MC, Tai S et al.: Percutaneous coronary intervention versus coronary artery bypass graft surgery for the treatment of unprotected LM coronary artery stenosis. Int. Heart J. 355–370 (2008).
- Lee MS, Kapoor N, Jamal F et al.: Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected LM coronary artery disease. J. Am. Coll. Cardiol. 47, 864–870 (2006).
- Makikallio TH, Niemela M , Kervinen K et al.: Coronary angioplasty in drug eluting stent era for the treatment of unprotected LM stenosis compared with coronary artery bypass grafting. Ann. Med. 40, 437–443 (2008).
- Palmerini T, Barlocco F, Santarelli A et al.: A comparison between coronary artery bypass grafting surgery and drug eluting stent for the treatment of unprotected LM coronary artery disease in elderly patients (aged >75 years), Eur. Heart J. 28, 2714–2719 (2007).
- Sanmartin M, Baz JA, Claro R et al.: Comparison of drug-eluting stents versus surgery for unprotected LM coronary artery disease. Am. J. Cardiol. 100, 970–973 (2007).
- Seung KB, Park DW, Kim YH et al.: Stents versus coronary-artery bypass grafting for LM coronary artery disease. N. Engl. J. Med. 358, 1781–1792 (2008).
- Wu C, Hannan EL, Walford G, Faxon DP: Utilization and outcomes of unprotected LM coronary artery stenting and coronary artery bypass graft surgery. Ann. Thorac. Surg. 86, 1153–1159 (2008).
▪ Current American guidelines on the standard of care for the treatment of left main coronary artery (LMCA) disease.
▪ Current European guidelines on the standard of care for the treatment of LMCA disease.
▪▪ Comprehensive and well-laid out argument in favour of percutaneous coronary intervention (PCI) versus coronary artery bypass grafting (CABG).
▪ Helped distinguish between the safety of elective versus the higher risk of emergent left main (LM) PCI.
▪ Balanced editorial summarizing the literature at the time of publication.
▪▪ Meta-analysis that enabled interesting and important subgroup comparisons.
▪ Established standard in terms of outcomes with drug-eluting stent in nonbifurcational LM disease.
▪▪ Comprehensive review arguing in favour of CABG versus PCI.
▪▪ Systematic review and meta-analysis incorporating the most recent drug-eluting stent studies as compared with CABG.
▪▪ Randomized controlled trial whereby patients with unprotected LMCA disease treated with PCI had favourable early outcomes as compared to patients treated by CABG.
▪▪ Largest randomized controlled trial to date, in which the overall CABG remains the standard of care for patients with complex coronary artery disease. However, in the LMCA subgroup, except for the highest risk patients, CABG or PCI had comparable outcomes.
▪ Websites
TCTMD. IVUS guidance may improve survival in unprotected LMCA disease.
BOCA Radiology Group: Cardiac CT and coronary CTA.
TCTMD. BBC ONE: In bifurcation stenting, keep it simple.
Clinical trials. Bypass Surgery Versus Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease (PRECOMBAT). http://www.clinicaltrials.gov/ct2/show/NCT00422968?term=combat+and+left+main+disease&rank=1
Supplementary images
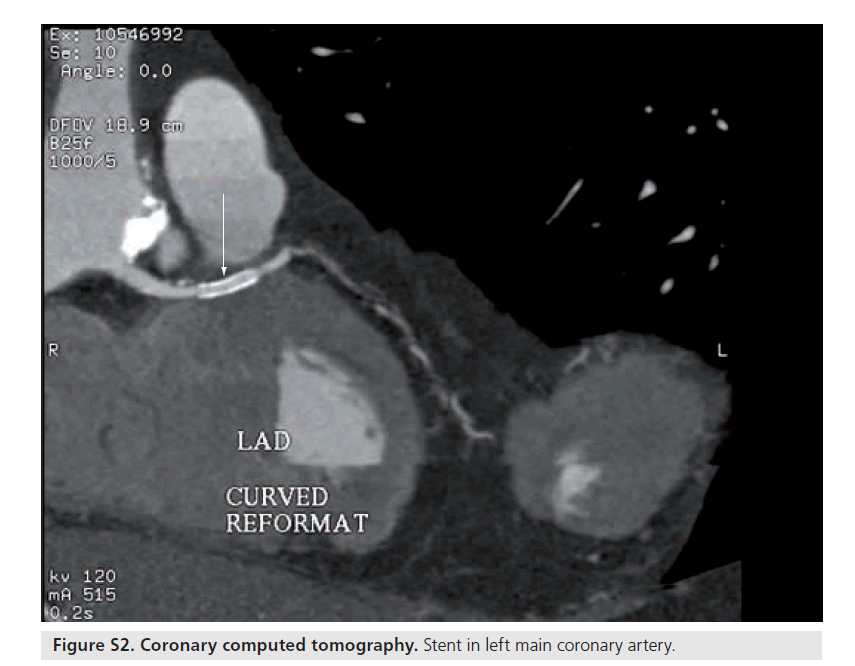
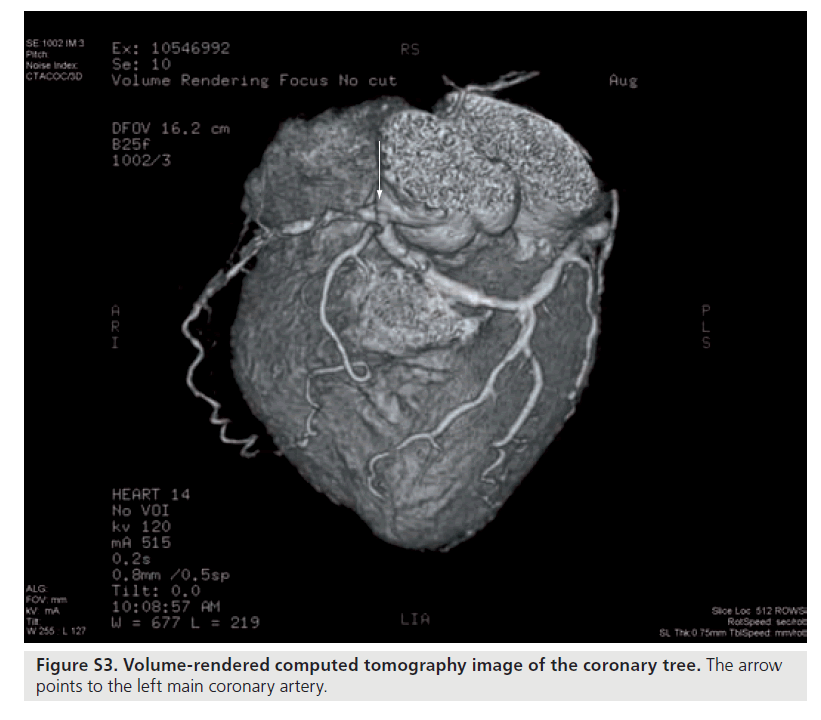
Figure S3. Volume-rendered computed tomography image of the coronary tree. The arrow points to the left main coronary artery.
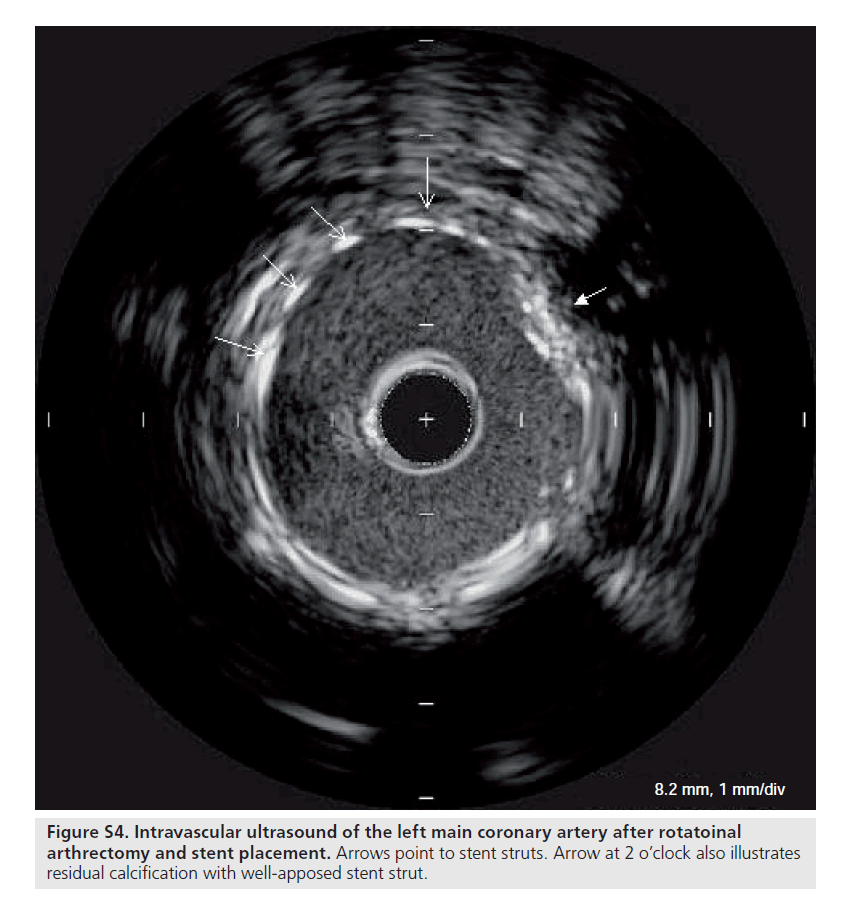
Figure S4. Intravascular ultrasound of the left main coronary artery after rotatoinal arthrectomy and stent placement. Arrows point to stent struts. Arrow at 2 o’clock also illustrates residual calcification with well-apposed stent strut.