Review Article - Interventional Cardiology (2021) Volume 13, Issue 3
Levoatriocardinal vein: A comprehensive interventional approach
- Corresponding Author:
- Mario Giordano
Department of Paediatric Cardiology,
“Ospedali dei Colli”, “Monaldi” Hospital,
University of Campania “Luigi Vanvitelli”,
Naples,
Italy,
E-mail: giordanomario1123@gmail.com
Received date: April 15, 2021; Accepted date: April 29, 2021; Published date: May 06, 2021
Abstract
Levoatriocardinal Vein (LACV) is a rare congenital vascular connection between the left atrium (or a pulmonary vein) and the cardinal venous system. This anomaly is usually associated with left-heart obstructive lesions allowing an adequate decompression of the left-sided heart. However, it may be found isolately or associated to congenital heart diseases without left-heart obstructive lesions. In these cases, the LACV develops a pre-tricuspid left-to-right shunt with consequent right-sided heart dilatation and pulmonary hypertension. The progresses of interventional cardiology allowed to develop various percutaneous strategies to approach these patients. The objective of this review is to highlight the role of interventional cardiology in this rare vascular anomaly and the various feasible strategy to adopt in order to the different settings: from the LACV stenting in the cases with associated left heart obstructive lesions up to the LACV embolization in the cases without associated congenital anomalies and with significant right-sided heart overload.
Keywords
Levoatriocardinal vein • Left-to-right shunt • Trans-catheter • Stenting • Coil • Vascular plug
Introduction
The levoatriocardinal vein (LACV) is a rare vascular connection between the left atrium (or pulmonary veins) and the superior vena cava (or innominate vein). In 1926, McIntosh et al. described the first report about this anomalous connection in a patient with cor triatriatum [1]. In 1950, Edwards and DuShane called “levoatrio-cardinal vein” a vascular structure connecting the left atrium and the innominate vein [2]. This nomenclature was adopted to highlight the embryology of this anomaly (i.e., a left atrial communication with the cardinal system).
In 1995, Bernstein et al. [3] described the largest series of patients with LACV, identifying two anatomic patterns: a direct communication between the left atrium and the cardinal venous system and a communication between a pulmonary vein and the cardinal venous system.
Usually, LACV remains patent in patients with left-sided heart obstructive lesion (cor triatriatum, hypoplastic left heart syndrome, mitral stenosis/atresia, Shone complex, aortic coarctation), ensuring a decompression of the left atrium [4,5]. In fact, both Bernsteint et al. [3] and Tosun et al. [6] identified a high prevalence of associated left- heart obstructive lesions in their large series (96% and 73%, respectively). However, a persistent LACV may be detected in patients without left-heart obstructive lesions and in this setting, it produces a pre-tricuspid left-to-right shunt with the risk of heart failure and pulmonary hypertension [7-10]. Rarely, the shunt inversion via levoatriocardinal vein may be the cause of recurrent cerebral thromboembolic events [11].
The aim of this review is to highlight the role of interventional cardiology in this rare congenital heart disease and the different features and tools useful for a comprehensive interventional approach.
Embriology and Anatomy
The LACV is a rare congenital vascular disease described as an anomalous connection between the left atrium (or a pulmonary vein) and the cardinal venous system.
Three main paired venous systems are detected in the human embryo:
• The umbilical, vitelline and omphalomesenteric veins, which carry the blood from the placenta, yolk sac, and intestine.
• The cardinal veins and their tributaries, which eventually give rise to the innominate and jugular veins, the superior and inferior vena cava and the coronary sinus.
• The pulmonary veins, which drain the blood from the lungs.
The pulmonary veins develop in the first month of embryonic life from the vascular plexus of the foregut, the splanchnic plexus. As pulmonary differentiation progresses, part of the splanchnic plexus forms the pulmonary vascular bed. At this stage, there is not a direct connection to the heart and the pulmonary vascular bed shares the routes of drainage of the splanchnic plexus via the umbilicovitelline and cardinal systems veins. Subsequently, the intraparenchymal pulmonary veins connect with the left atrium via the common pulmonary vein [12]. The primitive communications with the umbilicovitelline and cardinal systems are obliterating. The failure of this obliterating mechanism causes the persistence of a patent LACV.
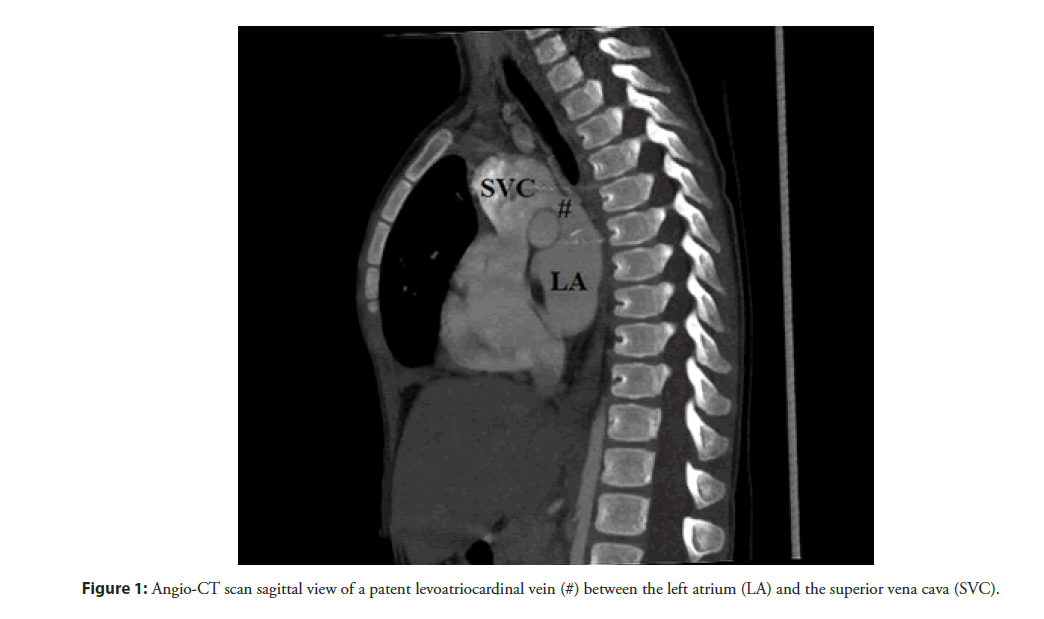
LACV has a broad anatomical spectrum with regard to origin, course and drainage. Bernstein et al. [3] identified two different anatomic patterns: a direct communication between the left atrium and the cardinal venous system (Figure 1) or between a pulmonary vein and the cardinal venous system. The more frequent anatomic pattern is the origin from the left atrium (68% of cases). When a LACV arises from the pulmonary veins, it was exclusively from the upper veins with an equal propensity for the right and left sides. In most cases, the LACV drains into the left innominate vein (48%), followed by the right superior vena cava (36%), and, less commonly, into the jugular veins and into a persistent left superior vena cava [6]. In no reported case, the anomalous vascular connection originated from either the trabeculated portion of the left atrium or the precardinal system, confirming a different identity from a persistent left superior vena cava. Moreover, the most of cases described showed a single LACV. Tuccillo et al. [10] reported the first case of “double” LACV in a child. The two anomalous vascular connections arose from the left upper pulmonary vein and the posterior wall of the left atrium, separately. The anatomy of these coexistent LACVs highlights a feasible persistency of two different anatomic patterns of LACV in the same patient. No cases of two or more LACV arising from the same structure (therefore with a same anatomical pattern) are described in literature.
In the cases of cor triatriatum, the persistent LACV arose from the proximal segment of the atrial chamber, providing an alternative route of flow.
The course of the anomalous vessel may be variable. The LACV draining into the left innominate vein usually runs laterally and posteriorly to the aortic arch and the left pulmonary artery, respectively. Despite the variability of its course a LACV can be differentiated from a left superior intercostal vein which is posterior to the aortic arch and descending aorta and connects with the accessory hemiazygos vein.
A persistent left superior vena cava can mimic a LACV connecting superiorly at the left internal jugular and left innominate vein junction and coursing laterally to the aortic arch. However, the left superior vena cava drains into the coronary sinus. In the heterotaxy syndrome (right isomerism), a left superior vena cava can drain directly into the left atrium because of the absence of coronary sinus. Moreover, the coexistence of left superior vena cava and extensive unroofing of the coronary sinus may mimic a LCAV (Raghib syndrome).
The Role of Interventional Cardiology
The LACV is a rare congenital vascular anomaly mostly associated with left-heart obstructive lesions (cor triatriatum, hyploplastic left heart syndrome, mitral stenosis/atresia, aortic coarctation). In this setting, the LACV is useful to achieve an adequate decompression of the left-chambers. The role of this vascular anomaly is crucial in the cases without a large atrial septal defect, particularly. In fact, in these patients the LACV is the only structure which allows complete decompression of the left-heart and its patency is necessary to keep the patient alive [13]. The presence of a LACV should be suspected in all patients with cor triatriatum or hypoplastic left heart syndrome and intact atrial septum [14,15]. In this setting, the LACV must be enough large to ensure an adequate decompression of the left-heart. However, the LACV may develop significant stenosis in the segments between the left main-stem bronchus and a patent ductus arteriosus which obstructs the blood- flow. In 2002, Vance [16] described the first case of LACV stenting in a neonate with hypoplastic left heart syndrome and intact atrial septum. In these frail newborns, the perforation and progressive dilatation with blade balloons of the atrial septum is hazardous and associated with high mortality rate [17]. The stenting of a stenotic LACV is a feasible and safer procedure to ensure the left atrium decompression. Vance used a Multi-Link Ultra coronary stent (Abbott Vascular Inc, Santa Clara, USA) dilated to 5.5 mm and adopted a femoral vein as vascular access. A homolateral jugular vein may be a useful and simple vascular access allowing a more linear course of catheters and delivery systems.
The diagnosis of persistent LACV is crucial in the patients with functionally univentricular heart before the bidirectional Glenn anastomosis. In fact, the LACV determines a direct vascular connection between superior vena cava (or left innominate vein) and the left atrium (or pulmonary veins) with a consequent Glenn circuit decompression. The right-to-left shunt cross the levoatriocardinal vein is responsible of a significant cyanosis. Kumar et al. [18] described a case of persistent LACV in a child (2 yearsold) with unbalanced atrioventricular septal defect undergone bidirectional Glenn anastomosis with consequent severe cyanosis (arterial oxygen saturation 65%). The trans-catheter closure of the LACV determined a significant improvement of the arterial oxygen saturation (80%). A similar setting was described by Alcibar et al. [19]: a child (6 years-old) with mitral atresia undergone total cavopulmonary anastomosis (Fontan operation). After the surgical operation, the patient showed a low oxygen arterial saturation (85%). The cardiac catheterization demonstrated the presence of a patent LACV with consequent decompression of the Fontan circuit and oxygen arterial desaturation. The percutaneous closure of the LACV with detachable coils allowed a significant improvement of arterial saturation (92-93%).
Moodley et al. [20] described a rare case of D-loop transposition of the great arteries with ventricular septal defect and intact atrial septum. The levoatriocardinal vein ensured an adequate interatrial shunt with improved arterial saturation, despite the absence of an interatrial defect. In this setting, a percutaneous stenting of a narrowed LACV (as Vance described) is an effective approach to improve the atrial mixing.
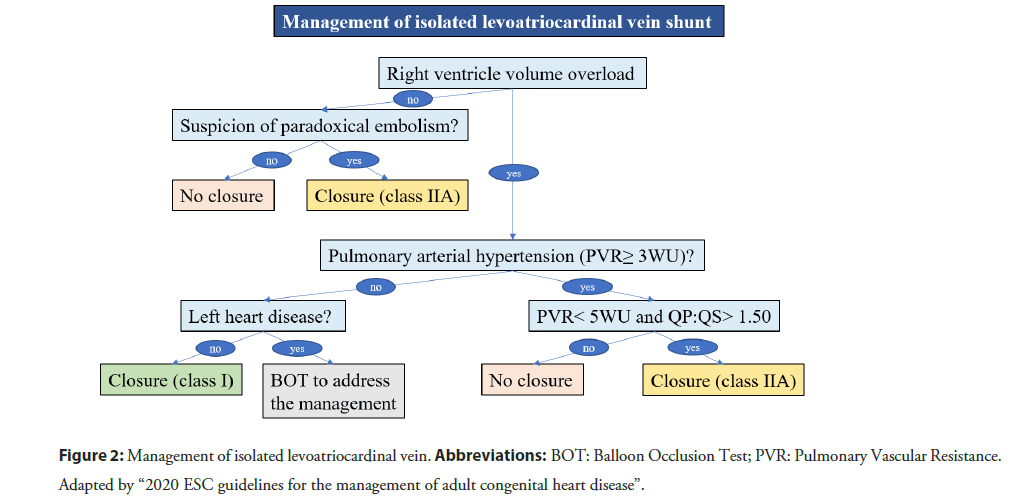
Though, LACV is usually associated to obstructive left-heart lesions, it may be both isolated [7,10] as well as associated to non-obstructive left-heart lesions [8,21]. In these settings, the LACV produces a left-to-right shunt with progressive dilation of right-heart and pulmonary hypertension [22]. In the current 2020 ESC guidelines for the management of adult congenital heart disease [23], there is not a specific chapter about the isolated LACV. However, this vascular anomaly is a pre-tricuspid shunt. The management algorithm about pre-tricuspid shunts (i.e., atrial septal defect and partial anomalous pulmonary venous drainage) may be reasonably extended to LACV (Figure 2).
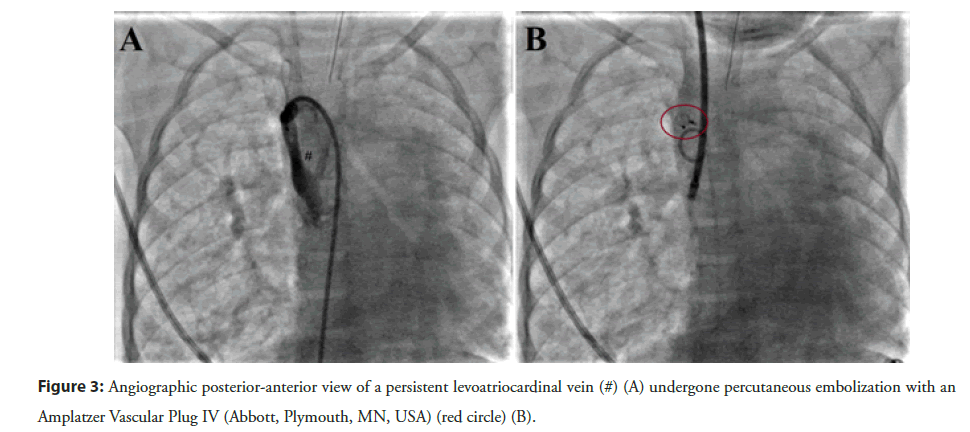
In the past, surgical ligation of the anomalous vascular connection was the most adopted strategy [24]. Nowadays, the transcatheter closure of the patent LACV is the first-line option to deal these patients. The detachable coils are useful to achieve the closure of the vascular connection, although the risk of residual shunt or coil embolization is not negligible [25]. Currently, the Amplatzer Vascular Plug II or IV (Abbott, Plymouth, MN, USA) are the most used devices to achieve an immediate complete closure [10,11,26] (Figure 3). The vascular plugs are easy to deploy and characterized by a low risk of residual shunt or embolization. The size of vascular plug should be 150%-180% of the LACV diameter because of the significant stretching ability of the venous vascular structure. The femoral vein is the preferred vascular access, though the internal jugular vein may be an excellent alternative in specific anatomy.
In rare circumstances, a persistent LACV may be the cause of cerebral thromboembolic events with a “patent foramen ovale like” mechanism. The occasional inversion of the LACV shunt (from left-to-right to right-to-left) is responsible of paradox embolisms with consequent recurrent cerebral thromboembolic events. The transcatheter LACV closure reduces the risk of cerebral ischemic attacks [11].
Conclusion
LACV is a rare congenital vascular anomaly, usually associated with left-heart obstructive lesions. In these cases, a patent LACV is useful to develop an adequate decompression of left-sided heart and a trans-catheter LACV stenting may be a feasible approach when the atrial septum is intact. The LACV assumes the same meaning in a setting of D-loop transposition of great arteries with intact atrial septum (or restrictive interatrial defect) because its patency allows a left-to-right shunt which increase oxygen arterial saturation. However, the LACV may be rarely isolated or associated to non-obstructive left-heart lesion. In these cases, the patent LACV is responsible of a pre-tricuspid left-to-right shunt with progressive right-heart dilatation and pulmonary hypertension. In this setting, a percutaneous closure of the patent vascular structure with detachable coils or vascular plugs is the preferred strategy to avoid an irreversible progression of right-heart dilatation and pulmonary hypertension. Moreover, the LACV shunt inversion may be a rare cause of cryptogenic cerebral ischemic attacks. A trans-catheter closure of the anomalous vascular connection reduces the risk of recurrences.
Nowadays, the percutaneous approach is the first strategy taken into account for patients with a persistent LACV. However, the vascular anomaly should be considered within the complex pathophysiology of the patient in order to adopt the most adequate therapeutic strategy.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgement
None
References
- McIntosh CA. Cor biatriatum triloculare. Am Heart J. 1: 735-44 (1926).
- Edwards JE, DuShane JW. Thoracic venous anomalies. Arch Pathol. 49: 517-37 (1950).
- Bernstein HS, Moore P, Stanger P, et al. The levoatriocardinal vein: Morphology and echocardiographic identification of the pulmonary-systemic connection. J Am Coll Cardiol. 26(4): 995-1001 (1995).
- Binsalamah ZM, De León LE, Heinle JS. Cor triatriatum sinister with an intact interatrial septum and a decompressing vein in a toddler. Cardiol Young. 27(6): 1221-4 (2017).
- Hellmund A, Berg C, Herberg U, et al. Levoatrial cardinal vein in a series of five prenatal cases with hypoplastic left heart syndrome and intact atrial septum. Ultraschall Med. 38(2): 206-11 (2017).
- Tosun Ö, Saygı M, Kasar T, et al. A rare pathology: Levoatriocardinal vein. Turk Kardiyol Dern Ars. 44(4): 315-9 (2016).
- Jaecklin T, Beghetti M, Didier D. Levoatriocardinal vein without cardiac malformation and normal pulmonary venous return. Heart. 89(12): 1444 (2003).
- Cullen EL, Breen JF, Rihal CS, et al. Levoatriocardinal vein with partial anomalous venous return and a bidirectional shunt. Circulation. 126(12): e174-7 (2012).
- Shet N, Maldjian P. Levoatriocardinal vein: An unusual cause of right-to-left shunting. J Clin Imaging Sci. 4: 68 (2014).
- Tuccillo A, Giordano M, Gaio G, et al. Trans-catheter closure of a rare cause of pre-tricuspid left-to-right shunt: A "double" levoatriocardinal vein without left heart obstructive lesions. J Cardiol Cases. 23(2): 65-68 (2020).
- Karangelis D, Avramidis D, Mousiama T, et al. Levoatriocardinal vein: A rarely recognized cause of recurrent cardiac and cerebral thromboembolic events. Can J Cardiol. 36(4): 589.e9-589.e11 (2020).
- Heart disease in infants, children, and adolescents including the fetus and young adult. Moss and Adams. 1, 8th edition. Lippincott Williams & Wilkins.
- Gomide M, Furci B, Mimic B, et al. Rapid 2-stage Norwood I for high-risk hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 146: 146-151 (2013).
- Paudel G, Ng BY, Law IH. Hypoplastic left heart with intact atrial septum and levoatriocardinal vein: A challenge in identifying aortic arch branches. Cardiol Young. 25(1): 171-3 (2015).
- Iida C, Muneuchi J, Watanabe M. Unique levoatriocardinal veins in neonates with hypoplastic left heart syndrome and intact atrial septum. Cardiol Young. 28(1): 150-152 (2018).
- Vance MS. Hypoplastic left heart syndrome with intact atrial septum: Levoatriocardinal vein stent placement as a bridge to surgery. Catheter Cardiovasc Interv. 57(1): 85-7 (2002).
- Atz AM, Feinstein JA, Perry SB, et al. Preoperative management of pulmonary venous hypertension in hypoplastic left heart syndrome with restrictive atrial septal defect. Am J Cardiol. 83(8): 1224-1228 (1999).
- Kumar V, Kumar G, Tiwari N, et al. Role of levoatrial cardinal vein plugging in single ventricle palliation. J Cardiol and Cardiovasc Ther. 11(4): 555819 (2018).
- Alcibar J, Gómez S, Vitoria Y, et al. Occlusion of the levoatrial cardinal vein with Gianturco coils after Fontan operation. Rev Esp Cardiol. 52(9): 733-6 (1999).
- Moodley S, Duncan W, Gandhi S. Levoatriocardinal vein in D-transposition of the great arteries. Cardiol Young. 26(6): 1235-7 (2016).
- Fujiwara K, Naito Y, Komai H, et al. Tetralogy of fallot with levoatrial cardinal vein. Pediatr Cardiol. 20(2): 136-8 (1999).
- Auti OB, Shetty V, Belaval V, et al. Levoatrial cardinal vein with normal left ventricle: A forgotten cause of pulmonary arterial hypertension. Indian J Radiol Imaging. 27(4): 413-416 (2017).
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 42(6): 563-645 (2021).
- Amoretti F, Cerillo AG, Chiappino D. The levoatriocardinal vein. Pediatr Cardiol. 26(4): 494-5 (2005).
- Lee ML, Chiu IS, Liao CY. Transvenous coaxial coil occlusion of the levoatriocardinal vein. Anatol J Cardiol. 20(3): 192-193 (2018).
- Ciliberti P, Taylor AM, Yates R, et al. Occlusion of persistent levoatrial cardinal vein without left heart hypoplasia utilizing an Amplatzer device. Eur Heart J Cardiovasc Imaging. 14(9): 857 (2013).