Research Article - Diabetes Management (2022) Volume 12, Issue 1
Long-term management of type 2 diabetes with faster aspart, degludec and flash glucose monitoring
- Corresponding Author:
- Bologna Carolina
Dipartimento di Medicina,
UOC Medicina Generale Ospedale del Mare di Napoli. ASL NA1 Centro,
Naples,
Italy
E-mail: carolina.bologna@libero.it
Received: 03-Jan-2022, Manuscript No. FMDM-21-0001; Editor assigned: 05-Jan-2022, PreQC No. FMDM-21-0001 (PQ); Reviewed: 19-Jan-2022, QC No. FMDM-21-0001; Revised: 18-Jan-2022, Manuscript No. FMDM-21-0001 (R); Published: 25-Jan-2022, DOI: 10.37532/1758-1907.2022.12(1).292-298
Abstract
Objective: To evaluate the clinical and prognostic impact of innovative therapies in hospitalized patients with poorly controlled type 2 diabetes (T2D).
Methods: Longitudinal, prospective study with a 6-month follow-up, involving patients with T2D admitted to an Internal Medicine ward for any reason. All patients, upon hospital admission, were treated with second generation rapid and basal insulins (faster apart and degludec). Flash glucose monitoring (FGM) through the Libre Free Style was used instead of self-monitoring of blood glucose through the glucometers. After hospital admission, patients continued the same approach and were evaluated after 6 months.
Results: Overall, 34 consecutive eligible patients were included in the study in the period between March 2019 and March 2021. Mean age was 67.2 ± 5.8 years and 73.3% were men. Of note, 12 out of 34 patients (35.3%) had been admitted for COVID-19. After six months from admission, a mean reduction of HbA1c levels of 1.3 ± 0.7% was documented. Fasting blood glucose levels, obesity indices, renal and liver function also significantly improved. Episodes of mild hypoglycemia occurred in 10 (29.4%) patients, while no severe episodes were reported. A highly statistically significant improvement was also found in the overall EQ-5D Index value and VAS score, suggesting a possible cost-effective profile of this approach.
Conclusion: The study represents an important proof-of-concept that also inpatients, a category usually not included among those eligible for innovative therapeutic options, can benefit of second generation insulin’s and FGM from a clinical and economic viewpoint.
Keywords
■ hyperglycaemia ■ faster aspart ■ degludec ■ flash glucose monitoring
Introduction
The aim of diabetes therapy is to optimize glycemic control, usually measured by glycated hemoglobin (HbA1c) levels, to reduce the risk of macro-and microvascular complications, the incidence and progression of which strongly correlate with HbA1c levels. However, in practice, many patients do not achieve the recommended HbA1c targets. A recent analysis of clinical data from over 500,000 patients with type 2 diabetes in Italy documented that 52.8% of patients had HbA1c levels ≤ 7.0%, 29.2% had HbA1c levels between 7.1% and 8.0%, and 18.0% had HbA1c levels>8.0% [1].
Among the reasons patients do not reach or maintain HbA1c targets, factors related to bolus insulin therapy and post-prandial glucose (PPG) control play an important role. Both fasting and post-prandial hyperglycemia contribute to HbA1c levels, and post-prandial hyperglycemia represents an independent risk factor for cardiovascular disease [2]. Furthermore, post- prandial hyperglycemic excursions contribute to glycemic variability, which may be related to diabetes complications independently of HbA1c levels [3]. In addition, the risk for hypoglycemia is directly related to increased glycemic variability in type 1 and type 2 diabetes, an important limiting factor for therapy optimization [4].
Despite the relevance of monitoring and targeting PPG, Italian data on self-monitoring of blood glucose (SMBG) relative to over 13,000 patients with T2D showed that, while the frequency of fasting blood glucose control was adequate, self-monitoring of postprandial blood glucose was rarely performed. Furthermore, two thirds of people treated with insulin had average postprandial blood glucose values above the recommended target of 140 mg/dl [5].
These problems can be at least partially overcome with modern insulin therapies, able to replicate normal physiology more closely, allowing to lower HbA1c levels and to stabilize glucose fluctuations.
Among the new insulins, faster aspart (FIASP) is a formulation of Insulin Aspart (IAsp) containing niacinamide and L-arginine. FIASP exerts a greater early glucose-lowering effect than IAsp, and this has been associated with increased early suppression of hepatic glucose production and early glucose disappearance [6]. The efficacy and safety of FIASP in adults with type 2 diabetes was evaluated in 2 treat-to-target, active-controlled randomized controlled trials (onset 2 and onset 3) [7,8].
In addition, degludec represents second generation basal insulin with improved pharmacodynamic/pharmacokinetic profile; this latter determinates a lower intra-patient variability, an extended duration of action providing full and stable 24 hour basal insulin coverage with once daily dosing, and comparable efficacy to first generation basal insulin’s with lower rates of hypoglycemia [9]. Furthermore, the growing use of flash glucose monitoring (FGM) instead of SMBG can increase the awareness of the need for optimizing PPG control [10].
The study aims to evaluate the clinical and prognostic impact of the use of FIASP+degludec U100 FGM (Libre Free Style system TM) in hospitalized patients with poor glycemic control assessed during hospitalization in an Internal Medicine ward and for 6 months after discharge. Our goals were to improve metabolic control, reducing glycemic variability with FGM, reduce hypoglycemic events, improve impact on risk factors, improve quality of life and reduce costs.
Methodology
This is a longitudinal, prospective study with a follow-up duration of 6 months, involving patients with T2D admitted to an Internal Medicine ward. All patients, upon hospital admission, were treated with FIASP and with degludec U100 as basal insulin. FGM through the Libre Free Style was used instead of self-monitoring of blood glucose through the glucometers. After hospital admission, patients continued the same approach and were evaluated after 6 months.
Consecutive patients meeting the following inclusion criteria were enrolled: age>18 years, diagnosis of T2D, need of basal-bolus insulin therapy (both naïve and switchers patients were eligible), hospitalized in the internal medicine ward for any cause. Exclusion criteria were current neoplasms, chronic infectious diseases (HIV-HCV-HBV), pregnancy, psychiatric diseases, and patients needing dialysis.
The primary endpoint of the study was represented by the change in HbA1c levels 6 months after the initiation of therapy with second generation insulins, as compared to baseline levels.
Secondary endpoints included the evaluation after 6 months of follow-up as compared to baseline values of body mass index (BMI), waist circumference, albuminuria, intima media thickness (IMT), hepatic steatosis, quality of life. The number of hypoglycemic episodes and the number of strips and glucose sensors used during 6 months were also assessed.
After the signature of the informed consent, the following data were collected at hospital admission (T0): anamnestic data, anthropometric data [waist circumference, BMI, heart rate (HR)], fasting blood glucose, HbA1c, albuminuria, ultrasound assessment of IMT, ultrasound assessment of fatty liver disease [according to “Italian Society of Ultrasonology in Medicine and Biology” (SIUMB) criterion].
Patients were asked to fill-in the EuroQoL/EQ- 5D-5L quality of life questionnaire [11]. The EQ-5D-5L descriptive system comprises five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), and each dimension has five response levels: no problems, slight problems, moderate problems, severe problems, unable to/extreme problems. The respondent is asked to indicate his/her health state by checking the box next to the most appropriate response level for each of the five dimensions. EQ-5D health states can also reported as a single summary number (index value), which reflects how good or bad a health state is according to the preferences of the general population of a country/region. Since the value set for the Italian population is not available, UK and France value sets were used. The EQ-5D instrument also contains a Visual Analogue Scale (VAS). The EQ-5D VAS records the respondent’s self-rated health on a vertical VAS where the endpoints are labeled ‘The best health you can imagine’ (score=100) and ‘The worst health you can imagine’ (score=0).
After hospital admission, patients were recommended to continue the prescribed therapy and blood glucose monitoring after discharge (FIASP, degludec and LibreTM positioning). The use of food diary was also recommended. After 6 months (T6), during an outpatient follow-up visit, Hb1Ac, BMI, waist circumference, albuminuria, EQ-5D, IMT, fatty liver disease, hypoglycemic episodes (mild-blood glucose<70 mg/dl-and severe-requiring third part intervention) were evaluated.
Furthermore, the number of strips and sensors utilized and the number of subjects experiencing hypoglycemic episodes during 6 months was assessed.
■Statistical methods
1) A minimum sample size of 31 achieved 90% power to detect a mean change of 0.5 in HbA1c levels, assuming a standard deviation of differences of 0.8 and with a significance level (alpha) of 0.05 using a two-sided Wilcoxon test.
2) Descriptive data were summarized as mean and standard deviation (SD) in case of continuous variables or percentages in case of categorical variables. The comparison between 6 months vs. baseline values was performed using the Wilcoxon signed rank test for continuous variables and the McNemar test or the Fisher exact test for categorical variables.
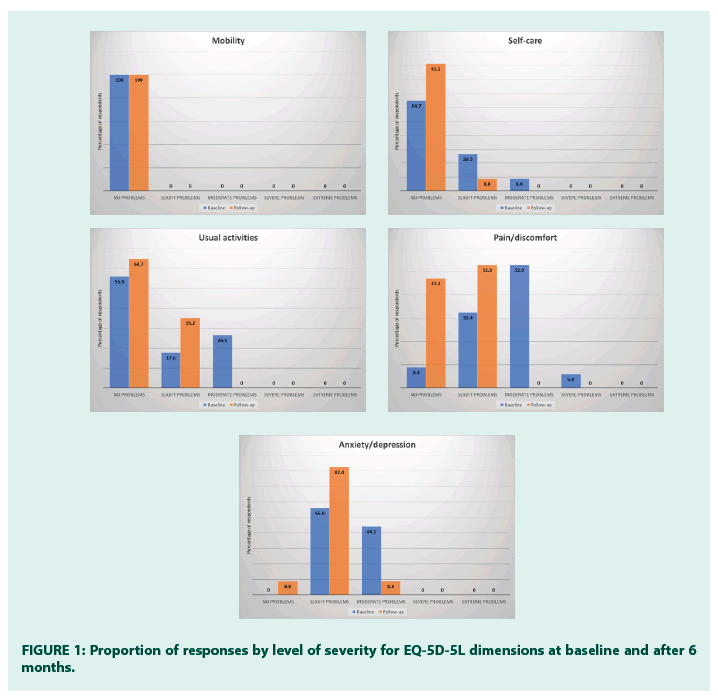
3) Results relative to the EQ-5D were shown as percentage of patients reporting each level of problem on each dimension. EQ-5D health states were also reported as a single summary number (mean and standard deviation).
Results
Overall, 34 consecutive eligible patients were included in the study in the period between march/2019 and march/2021. Mean age was 67.2 ± 5.8 years and 73.3% were men. Of note, 12 out of 34 patients (35.3%) had been admitted for COVID-19. At admission all patients started a basal-bolus regimen with 3 injection day of FIASP and 1 injection day of insulin degludec. Insulin was injected by the nurse during the admission (FIASP could be administered even after the meals). After the discharge, all patients continued the same therapy (2nd generation insulins+SMBG with Free Style) at home. Patients were reassessed after 6 months during an outpatient visit.Clinical outcomes at T0 and T6 are reported in Table 1.
| At admission (T0) | At 6-month follow-up (T6) | *p-value | |
|---|---|---|---|
| HbA1c (%) | 8.3 ± 0.8 | 7.0 ± 0.5 | <0.0001 |
| Fasting blood glucose (mg/dl) | 143 ± 18 | 115 ± 11 | <0.0001 |
| BMI (kg/m2) | 29.2 ± 4.3 | 27.8 ± 3.8 | <0.0001 |
| Waist circumference (cm) | 95.6 ± 9.6 | 90.3 ± 9.1 | <0.0001 |
| Albuminuria (%) | |||
| Normo | 5.9 | 29.4 | <0.0001 |
| Micro | 50.0 | 58.8 | |
| Macro | 44.1 | 11.8 | |
| Fatty liver disease (%) | |||
| Absent/Moderate | 44.1 | 82.4 | 0.001 |
| Severe | 55.9 | 17.6 | |
| IMT (mm) | 1.2 ± 0.2 | 1.1 ± 0.2 | <0.0001 |
| EQ-5D Mobility (%) | |||
| No problems | 100 | 100 | 1.00 |
| Slight problems | - | - | |
| Moderate problems | - | - | |
| Severe problems | - | - | |
| Extreme problems | - | - | |
| EQ-5D Self-care (%) | |||
| No problems | 64.7 | 91.2 | 0.03 |
| Slight problems | 26.5 | 8.8 | |
| Moderate problems | 8.8 | - | |
| Severe problems | - | - | |
| Extreme problems | - | - | |
| EQ-5D Usual activities (%) | |||
| No problems | 55.9 | 64.7 | 0.16 |
| Slight problems | 17.6 | 35.3 | |
| Moderate problems | 26.5 | - | |
| Severe problems | - | - | |
| Extreme problems | - | - | |
| EQ-5D Pain/discomfort (%) | |||
| No problems | 8.8 | 47.1 | 0.49 |
| Slight problems | 32.4 | 52.9 | |
| Moderate problems | 52.9 | - | |
| Severe problems | 5.9 | - | |
| Extreme problems | - | - | |
| EQ-5D Anxiety/depression (%) | |||
| No problems | - | 8.8 | 0.04 |
| Slight problems | 55.9 | 82.4 | |
| Moderate problems | 44.1 | 8.8 | |
| Severe problems | - | - | |
| Extreme problems | - | - | |
| EQ-5D Index value** | 0.70 ± 0.10 | 0.81 ± 0.10 | <0.0001 |
| EQ-5D Index value*** | 0.69 ± 0.18 | 0.83 ± 0.14 | <0.0001 |
| EQ-5D VAS | 37.4 ± 10.2 | 49.4 ± 9.6 | <0.0001 |
Note: Data are mean and standard deviation or proportion. No missing data. Statistical significant p-values are in bold. *Wilcoxon signed rank test (continuous variables); McNemar test or fisher exact test (categorical variables);** UK value set; *** France value set.
Table 1. Patient characteristics at hospital admission and after 6 months of follow-up.
Metabolic control markedly improved after six months of treatment with FIASP supported by FGM, with an average reduction of HbA1c levels of 1.3 ± 0.7%. Fasting blood glucose levels also significantly decreased by an average of 27.8 ± 13.2 mg/dl. BMI (-1.39 ± 0.93) and waist circumference (-5.3 ± 4.4 cm) were also significantly reduced. The proportion of patients with normoalbuminuria increased from 5.9% at baseline to 29.4% after 6 months, while the proportion of patients with no or moderate fatty liver disease increased from 42.1% to 82.4%. IMT was slightly but significantly reduced (-0.08 ± 0.10 mm). Episodes of mild hypoglycemia occurred in 10 (29.4%) patients, while no severe episodes were reported [12-16].
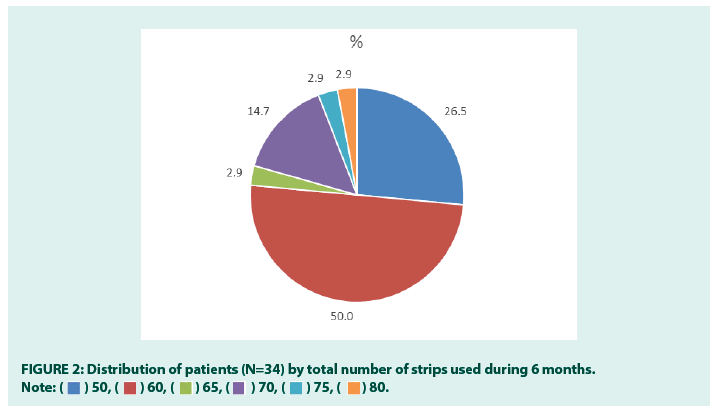
As for quality of life, a significant improvement was documented in the self-care and anxiety/ depression domains of EQ-5D (Table 1 and Figure 1). A highly statistically significant improvement was also found in the overall EQ- 5D Index value and VAS score. On average, patients used 59.4 ± 7.5 SMBG strips during 6 months, and half of patients (50.0%) used 60 strips (Figure 2). All patients used between 12 and 14 sensors during 6 months (average 12.9 ± 0.9) [17-21].
Discussion and Conclusion
The study shows that, in T2D hospitalized patients with poor glycemic control, the use of recent innovative options for the management of T2D, i.e. second generation basal and short-acting insulin and flash glucose monitoring, during the hospital stay and after the discharge, is associated with statistically significant improvements in metabolic control, obesity indices, and renal, liver, and cardiovascular parameters. Moreover, the use of these options is associated with statistically significant improvements in EQ-5D dimensions and score, indicating the impact on general health status of high-quality care. It is also known that EQ-5D can be translated in cost-utilities, suggesting a possible cost-effective profile of this approach to be investigated with ad hoc studies.
Hyperglycemia in inpatients is a common, serious, and costly health care problem. Hyperglycemia is associated with adverse events including prolonged length of stay, infection risk, disability after discharge, and death. On the other hand, hypoglycemia and elevated glycemic variability related to suboptimal management of insulin therapy in inpatients increase morbidity, mortality, and overall costs of care. In the past, sliding scale insulin (SSI) was the common approach to manage in hospital hyperglycemia. Existing literature suggests that this approach should be abandoned in favor of inpatient diabetes management programs involving effective and well-tolerated basal-bolus insulin regimens. The use of second generation basal and short-acting insulin’s can represent a key strategy to reduce hypoglycemia in these frail patients. They also have the potential to simplify the management of insulin therapy for nurses during hospitalization.
The study has strengths and limitations. The major strength is represented by the innovative therapeutic approach, which showed to be feasible and safe. Major limitations are represented by the lack of a control group, and specific data on length of stay, glycemic variability, and direct and indirect costs associated with the study approach.
In conclusion, the study represents an important proof-of-concept that also inpatients, i.e. a category usually not included among those eligible for these options, can benefit of second generation insulins and FGM from a clinical and economic viewpoint.
Acknowledgements
■Funding
No funding for this study.
■Editorial assistance
The editorial assistance was provided by CORESEARCH SRL (Maria Chiara Rossi, Giusi Graziano) through a Novo Nordisk S.p.A. Unconditional grant. The authors of the publication are fully responsible for the contents and conclusions. Novo Nordisk S.p.A. did not influence and has not been involved in the data interpretation presented in the manuscript.
■ Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
■Author contributions
➢ Marina Lugarà contributed to study concept and design of data.
➢ Carolina Bologna contributed to acquisition of data.
➢ Mariella Naddeo contributed to drafting of the manuscript, to interpretation of data, to statistical analysis and to critical revision of the manuscript for important intellectual content.
➢ All authors read and approved the final manuscript. Carolina Bologna is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
■Disclosures
Has nothing to disclosey.
■Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by the ethics committee of the participating center. Informed consent was obtained from all patients for being included in the study.
■ Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Associazione Medici Diabetologi. Valutazione Degli Indicatori Amd Di Qualità Dell’assistenza Al Diabete Di Tipo 1 E 2 in Italia. Annali AMD, Roma RM, Italy (2020).
- DECODE Study Group. Glucose Tolerance and Cardiovascular Mortality: Comparison of Fasting and 2-hour Diagnostic Criteria. Arch Intern Med. 161(6):397e405 (2001).
- Ceriello A, Monnier L, Owens D. Glycaemic Variability in Diabetes: Clinical and Therapeutic Implications. Lancet Diabetes Endocrinol. 7(3):221-230 (2019).
- Monnier L, Wojtusciszyn A, Colette C. The Contribution of Glucose Variability to Asymptomatic Hypoglycemia in Persons with Type 2 Diabetes. Diabetes Technol Ther. 13(8):813e8 (2011).
- Rossi MC, Lucisano G, Ceriello A, et al. Real-world Use of Self-monitoring of Blood Glucose in People with Type 2 Diabetes: An Urgent Need for Improvement. Acta Diabetol. 55(10):1059-1066 (2018).
- Basu A, Pieber TR, Hansen AK, et al. Greater Early Postprandial Suppression of Endogenous Glucose Production and Higher Initial Glucose Disappearance Is Achieved with Fast-acting Insulin Aspart Compared with Insulin Aspart. Diabetes Obes Metab. 20(7):1615e22 (2018).
- Bowering K, Case C, Harvey J, et al. Faster Aspart Versus Insulin Aspart as Part of a Basal-bolus Regimen in Inadequately Controlled Type 2 Diabetes: The Onset 2 Trial. Diabetes Care. 40(7):951e7 (2017).
- Rodbard HW, Tripathy D, Vidrio Velazquez M, et al. Adding Fast-acting Insulin Aspart to Basal Insulin Significantly Improved Glycaemic Control in Patients with Type 2 Diabetes: A Randomized, 18-week, Open-label, Phase 3 Trial (Onset 3). Diabetes Obes Metab. 19(10):1389e96 (2017).
- Lajara R, Cengiz E, Tanenberg RJ. The Role of the New Basal Insulin Analogs in Addressing Unmet Clinical Needs in People with Type 1 and Type 2 Diabetes. Curr Med Res Opin. 33(6):1045-1055 (2017).
- Leelarathna L, Wilmot EG. Flash Forward: A Review of Flash Glucose Monitoring. Diabet Med. 35(4):472-482 (2018).
- Herdman M, Gudex C, Lloyd A, et al. Development and Preliminary Testing of the New Five-level Version of Eq-5d (Eq-5d-5l). Qual Life Res. 20(9):1727-36 (2011).
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of Hyperglycemia in Hospitalized Patients in Non-critical Care Setting: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 97(1):16-38 (2012).
- Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: An Independent Marker of In-hospital Mortality in Patients with Undiagnosed Diabetes. J Clin Endocrinol Metab. 87(3):978-82(2002).
- Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early Postoperative Glucose Control Predicts Nosocomial Infection Rate in Diabetic Patients. J Parenter Enteral Nutr. 22(2):77-81 (1998).
- Clement S, Braithwaite SS, Magee MF, et al. Management of Diabetes and Hyperglycemia in Hospitals. Diabetes Care. 27(2):553–97 (2004).
- Eiland L, Goldner W, Drincic A, et al. Inpatient Hypoglycemia: A Challenge That Must Be Addressed. Curr Diabetes Rep. 14(4):445 (2014).
- Mendez CE, Mok K-T, Ata A, et al. Increased Glycemic Variability Is Independently Associated with Length of Stay and Mortality in Noncritically Ill Hospitalized Patients. Diabetes Care. 36(12):4091-4097 (2013).
- Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of Basal-bolus Insulin Regimens in the Inpatient Management of Non-critically Ill Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Metab Res Rev. 33(5): e2885 (2017).
- Simioni N, Filippi A, Scardapane M, et al. Efficacy and Safety of Insulin Degludec for Hyperglycemia Management in Noncritical Hospitalized Patients with Diabetes: An Observational Study. Diabetes Ther. 8(4):941-946 (2017).
- D'Souza SC, Kruger DF. Considerations for Insulin-treated Type 2 Diabetes Patients During Hospitalization: A Narrative Review of What We Need to Know in the Age of Second-generation Basal Insulin Analogs. Diabetes Ther. 11(12):2775-2790 (2020).
- Vliebergh J, Lefever E, Mathieu C. Advances in Newer Basal and Bolus Insulins: Impact on Type 1 Diabetes. Curr Opin Endocrinol Diabetes Obes. 28(1):1-7 (2021).