Research Article - Diabetes Management (2016) Volume 6, Issue 6
Low rate of intensification in type 2 diabetic patients inadequately controlled with basal insulin: the INTERDIA study
- Corresponding Author:
- Detournay B
Cemka Eval, 43 Bd du Maréchal Joffre, 92 340 Bourg-la-Reine, France
E-mail: bruno.detournay@cemka.fr
Abstract
To estimate the delay before treatment intensification in poorly controlled basal insulintreated patients with type 2 diabetes mellitus (T2DM), with or without oral antidiabetic drugs (OAD) but no Glucagon-Like Peptide-1 receptor agonist (GLP-1-RA). Methods: A retrospective analysis of prescribing records of a sample of general practitioners was conducted. Inadequate glycaemic control in T2DM was defined by two successive HbA1c values over 7% (scenario 1) or 8% (scenario 2) at least 3 months apart. Treatment intensification was defined either by a change in drug regimen (either the addition of a non-basal insulin, GLP-1-RA or any OAD), or by increasing the dose of basal insulin by at least 10% when represcribing. Cumulated insulin dose increase over the observation period by at least 20% was also tested. The delay to intensification was calculated from the first HbA1c value higher than 7% or 8%, respectively. Results: Two populations with documented poorly controlled diabetes consisting of 500 patients in Scenario 1 and 684 in Scenario 2 were selected. After one year, treatment was intensified (modification of drug regimen) in 16.3% [95% CI: 12.2%, 21.8%] of patients in scenario 1 and 24.0% [17.3%, 32.7%] in scenario 2. Including insulin dose increase of at least 10%, this proportion was increased to 55.5% [49.5%; 61.7%] and 66.1% [57.6%, 74.5%], respectively. Conclusion: Lack of intensification remains high in patients with poorly controlled T2DM treated with basal insulin. Further investigations are needed to understand whether it is related to therapeutic inertia, or to very loose objectives, or both.
Keywords
France, primary care, therapeutic inertia, treatment, type 2 diabetes
Introduction
In France, patients with Type 2 diabetes mellitus (T2DM) are principally treated by general practitioners (GPs), with less than 10% consulting a diabetologist or endocrinologist over one year [1]. Practice guidelines have been developed to assist physicians with the process of managing patients with T2DM [2-5], and these are regularly updated. Up until 2013, French national guidelines included a treatment algorithm based on HbA1c values (Table 1). The HbA1c target was set at <6.5% or <8.0% depending on the initial HbA1c level and the treatment delivered. In the most recent French guidelines, published in 2013 [4], an HbA1c target of ≤7% is recommended for most people with T2DM.
| Current HbA1c | Treatment | HbA1c target |
|---|---|---|
| 6-6.5% Despite lifestyle recommendations |
Monotherapy by metformin (or α-glucosidase inhibitor in case of intolerance or contra-indication for metformin), insulin secretagogue if BMI <27kg/m2 |
<6.5% |
| >6.5% Despite lifestyle recommendations |
Monotherapy by insulin secretagogue or metformin or α-glucosidase inhibitor | Maintain <6.5% |
| >6.5% Despite monotherapy and lifestyle recommendations |
Bitherapy | Reduce to <6.5% |
| >7% Despite bitherapy and lifestyle recommendations |
Oral tritherapy or insulin + metformin ± other oral hypoglycaemic treatments except glitazones | Reduce to <7% |
| >8% Despite tritherapy and lifestyle recommendations |
Insulin + metformin ± other oral diabetic treatments except glitazones | Reduce to <7% |
Table 1. Therapeutic recommendations for patients with T2DM in France before 2013.
Over the last decade, mean glycaemic control has improved in France, as shown by the results of the two ENTRED Surveys [6,7], performed in 2001 and in 2007, of a national representative sample of patients with diabetes. Nonetheless, despite numerous public health initiatives, levels of HbA1c still remain high and above the recommended thresholds in a large proportion of patients [7]. In 2007, the ENTRED Survey demonstrated that 16% of patients with T2DM had an HbA1c above 8% (35% among insulin treated patients), 56% an HbA1c above 7% while only one third (36%) optimally controlled (last reported HbA1c ≤6.5%) [6,7].
In order to increase the proportion of patients who are optimally controlled, practice guidelines recommend treatment intensification, either by adding another treatment or up-titration of the dose (or both) [2,4]. However, in everyday practice, this is not implemented as promptly as would be desired, with the consequence that patients may remain for long periods with their glycaemia inadequately controlled. The long delay before treatment intensification in a patient who requires it is referred to as therapeutic or clinical inertia [8]. The importance of early control of glucose through a treat-to-target strategy with timely treatment switches has been illustrated in several large studies [9-12] and is reflected in current practice guidelines [2,13]. For these reasons, the therapeutic inertia remains an important issue in terms of public health.
In France, the principal data on treatment intensification in patients with uncontrolled T2DM with oral anti-diabetic drugs (OAD) come from the DIAttitude survey [14,15]. This study reported that only 39% of patients who needed intensification because of two consecutive inadequate HbA1c measurements, had changed their treatment six months later, and only 59% had changed treatment after one year. However, no information is yet available on therapeutic inertia in patients treated with insulin. The objective of the present analysis was to quantify therapeutic inertia in patients with T2DM treated with basal insulin.
Patients and methods
▪ Data collection and inclusion criteria
Data were extracted from a representative panel, the LPD (Longitudinal Patient Data) Database (CEGEDIM Group) of 1,200 French GPs who document clinical data on their activity in an electronic database. The panel was representative of all GPs in France with respect to age, sex and region of practice (eight different regions). The database was updated continuously with new data and allowed all consultations by an individual patient to be followed. Treatments were identified through their EPHMRA codes (basal insulin: class A10C5; OADs: classes A10H to A10N2; GLP1 analogues: class A10X).
Adult patients (≥18 years) with T2DM documented by the GP in the LPD database were identified in the LPD database and enrolled in the study if they had consulted their GP at least once during the observation period of the study (January 2009 to December 2011), had been treated with a basal insulin for at least six months and had at least two measurements of HbA1c were documented during the observation period. Patients treated with Glucagon-Like Peptide-1 Receptor Agonists (GLP-1-RA), rapid insulins, or premixed insulins were excluded.
Patients with poorly controlled diabetes requiring treatment intensification were identified as all included patients with two consecutive measurements of HbA1c above a threshold of 7% (scenario 1) or 8% (scenario 2) at least 3 months apart. Patients with poorly controlled diabetes at baseline were only included if the starting date of the loss of diabetes control (as estimated through HbA1c measurements) was documented.
▪ Treatment intensification
Treatment intensification was defined by addition of another insulin to the long-acting insulin, increase of the insulin dose, switch to another non-basal insulin (including NPH insulin), addition of a GLP-1-RA or addition of an OAD. Stopping insulin was not considered as a treatment intensification whatever the other treatment prescribed simultaneously. An increase in insulin dose was defined as an increase in dose of at least 10% in a single step.
In patients with uncontrolled diabetes, the first treatment modification following the index date for uncontrolled diabetes was identified. If the treatment modification corresponded to a treatment intensification, the period of time between the index date of uncontrolled diabetes (first abnormally-elevated HbA1c measurement) and the date of the initiation of treatment intensification was estimated.
In a complementary analysis, treatment intensification was alternatively defined as a cumulated increase of ≥ 20% between the first and the last treatment documented over one year.
▪ Statistical methods
The data were collected before the publication of the 2013 guidelines. At this time, for a given patient the recommended target HbA1c level depended on the initial HbA1c (either 7% or 8% for a patient on insulin) (Table 1). Therefore the statistical analysis took into account both these thresholds in two different scenarios.
In patients requiring treatment intensification, the period of time between the index date of uncontrolled diabetes (first measurement of above-target HbA1c) and the date of the initiation of treatment intensification was determined from Cox models that expressed the risk of the occurrence of treatment intensification according to time.
Two separate analyses were performed. In the first, increasing the insulin dose was not considered as a treatment intensification whereas, in the second, dose increases were also taken into account.
All data were analysed using SAS version 9.2.
▪ Ethical considerations
Procedures for data collection and management were approved by the Commission Nationale de l’Informatique et des Libertés (CNIL), which ensures that all medical information is kept confidential and anonymous. Since this was a retrospective database analysis, ethical committee approval and patient consent were not required.
Results
▪ Study populations
The flow chart for the study population is presented in Figure 1. The population covered by the LPD Database included 55,368 with diabetes, of whom 49,592 (89.6%) had T2DM. In all, 5,840 of these patients received at least one prescription of insulin during the observation period and 3,665 (7.4%) fulfilled the selection criteria of treatment with a basal insulin for at least six months. After exclusion of patients without two documented HbA1c measurements during the observation period, data from 849 patients with T2DM were available for analysis.
This group consisted of 442 patients already treated at the beginning of the observation period and 407 who started treatment during the observation period itself. Some of the already treated patients had uncontrolled diabetes at baseline. Since it was not possible to establish the time from loss of diabetes control to treatment intensification in these patients, they were excluded from the analysis. This concerned 349 (79%) patients with HbA1c >7% (Scenario 1) and 165 (37%) with HbA1c >8% (Scenario 2). The remaining already treated patients were pooled with the 407 patients starting basal insulin treatment during the course of the observation period; these groups consisted in 500 patients in Scenario 1 and 684 patients in Scenario 2. Of these patients, 336 patients (67.2%) in Scenario 1 and 185 patients (27.0%) in Scenario 2 were subsequently documented as having uncontrolled diabetes and these patients constituted the analysis population. Moreover, 70.8% of uncontrolled patients in scenario 2 (HbA1c values over 8%), were not at FPG target as defined by their physicians. The characteristics of these patients are summarised in Table 1.
The duration of follow-up was respectively 32.5 months and 32.7 months on average in Scenario 1 and Scenario 2.
▪ Treatment intensification
Treatment intensification without taking into account changes in insulin dose: scenario 1
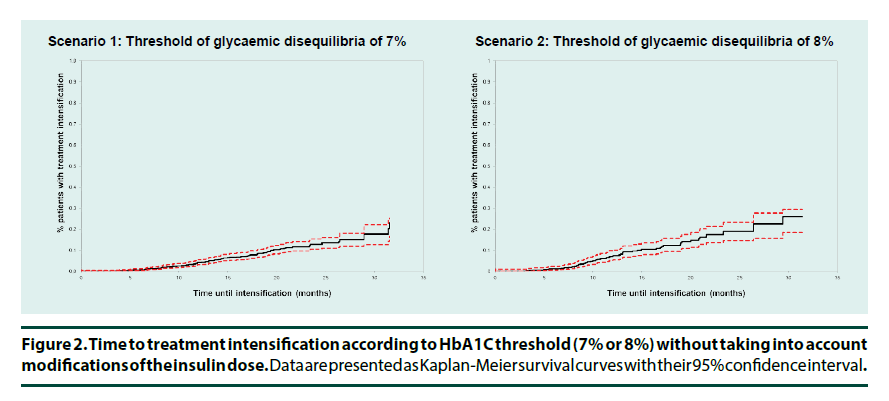
In Scenario 1 (HbA1c>7%), 86 (25.6%) of the 336 patients who lost or failed to achieve glycaemic control after starting a basal insulin underwent treatment intensification over the study period, on average 14.1 ± 6.7 months after the documentation of inadequate glycaemic control. After one year, only 4.2% (3.1%-5.6%) of the patients underwent treatment intensification (Figure 2). The principal changes documented were adding an OAD (52.3%) or adding another insulin (40.7%) (Table 2). In addition, 71 patients (21.1%) changed their treatment regimen without intensification. These changes involved principally discontinuation of basal insulin and less frequently OAD switch.
| Scenario 1 (HbA1C>7%) N=336 |
Scenario 1 (HbA1C>8%) N=185 |
|
|---|---|---|
| Age (years) | ||
| Mean (SD) | 67.1 (11.2) | 66.1 (11.3) |
| (18-60) | 82 (24.4%) | 54 (29.2%) |
| (60-65) | 57 (17.0%) | 30 (16.2%) |
| (65-70) | 54 (16.1%) | 31 (16.8%) |
| (70-75) | 45 (13.4%) | 20 (10.8%) |
| ≥75 | 98 (29.2%) | 50 (27.0%) |
| Gender | ||
| Female | 134 (39.9%) | 77 (41.6%) |
| Male | 202 (60.1%) | 108 (58.4%) |
| Full health coverage (100%) | 334 (99.4%) | 185 (100.0%) |
| BMI | ||
| Mean (SD) | 30.1 (5.6) | 30.4 (5.6) |
| ≤25 kg/m2 | 46 (17.8%) | 24 (16.2%) |
| ]25-30] kg/m2 | 87 (33.7%) | 51 (34.5%) |
| >30 kg/m2 | 125 (48.4%) | 73 (49.3%) |
| Duration of the observation period (in months) | ||
| Mean (SD) | 32.5 (4.3) | 32.7 (4.2) |
| Number of HbA1C measurements during the observation period | ||
| Mean (SD) | 7.4 (3.1) | 7.1 (3.0) |
| Duration of the basal insulin ± OAD regimen (in months) | ||
| Mean (SD) | 18.2 (8.3) | 20.2 (8.9) |
| Number of HbA1C measurements during the basal insulin ± OAD regimen | ||
| Mean (SD) | 4.3 (2.4) | 4.7 (2.5) |
Table 2. Patient characteristics according to the threshold of uncontrolled diabetes.
Treatment intensification without taking into account changes in insulin dose: scenario 2
In Scenario 2 (HbA1c>8%), 55 (29.7%) of the 185 patients losing glycaemic control underwent treatment intensification with a 13.0 ± 6.8 months mean duration of the period of therapeutic inertia among this patients (Table 3). Twelve months following the loss of diabetes control only 7.1% (5.1-9.7%) of the overall population with uncontrolled diabetes underwent treatment intensification (Figure 2). This principally involved addition of an OAD (52.7%) or of another insulin (40.0%) (Table 3). In addition, 85 patients (45.9%) changed their treatment regimen without intensification, again principally discontinuing their basal insulin.
| Scenario 1 (HbA1C> 7%) N=336 |
Scenario 1 (HbA1C> 8%) N=185 |
|
|---|---|---|
| Duration of follow-up (months: mean, SD) | 32.5 (4.3) | 32.7 (4.2) |
| Patient with treatment intensification | 86 (25.6%) | 55 (29.7%) |
| Add-on insulin | 35 (40.7%) | 22 (40.0%) |
| Add-on GLP1 | 6 (7.0%) | 4 (7.3%) |
| Add-on OAD | 45 (52.3%) | 29 (52.7%) |
| Time until intensification in months among patients with intensification (observed) | ||
| Mean (SD) | 14.1 (6.7) | 13.0 (6.8) |
| <9 months | 23 (26.7%) | 17 (30.9%) |
| Between 9 and 12 months | 15 (17.4%) | 13 (23.6%) |
| Between 12 and 18 months | 24 (27.9%) | 13 (23.6%) |
| Between 18 and 24 months | 17 (19.8%) | 8 (14.5%) |
| ≥24 months | 7 (8.1%) | 4 (7.3%) |
| Time until intensification in months (Kaplan-Meier estimate) | ||
| Median (CI-95%) | Not reached | Not reached |
Table 3. Treatment changes according to HbA1c threshold without taking into account modifications of the insulin dose.
Treatment intensification taking into account changes in insulin dose: scenario 1
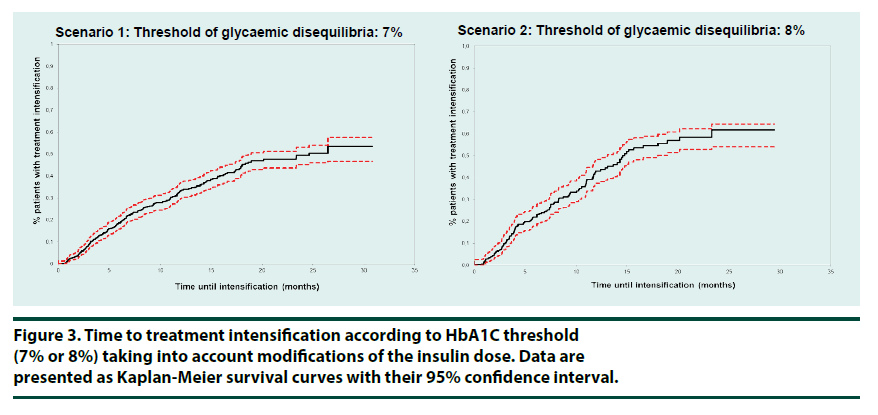
When significant increases in insulin dose (+10% in one step) were also considered as treatment intensification, the proportion of patients in Scenario 1 who underwent treatment intensification was 59.5% over the study duration (32.5 months on average) (Table 4). One year after losing glycaemic control 33.0% (29.5- 36.7%) of patients had their treatment intensified (Figure 3). Median time to intensification was 24.6 (19.0-NA) months. Taking simultaneously into account any cumulated insulin dose increase over the observation period by at least 20% as a treatment intensification the percentage of patients who had their treatment intensified one year after losing glycaemic control increased to 39.9%.
Treatment intensification taking into account changes in insulin dose: scenario 2
In Scenario 2, the proportion of patients who underwent therapeutic intensification including insulin dose changes one year after the first measurement of above-target HbA1c and over the study duration, 32.7 months on average, was 42.9% (37.4-48.3%) (Figure 3) (47.0% taking into account a 20% cumulated dose increase over the observation period). Median time until intensification was 14.6 months (13.1-18.0) (Table 4).
| Scenario 1 (HbA1C>7%) N=336 |
Scenario 1 (HbA1C>8%) N=185 |
|
|---|---|---|
| Duration of follow-up (months: mean, SD) | 32.5 (4.3) | 32.7 (4.2) |
| Patient with treatment intensification | 2001 (59.5%) | 120 (64.9%) |
| Add-on insulin | 25 (12.5%) | 15 (12.5%) |
| Add-on GLP1 | 4 (2.0%) | 2 (1.7%) |
| Add-on OAD | 22 (11.0%) | 11 (9.2%) |
| Increase of the insulin dose by >10% | 151 (75.5%) | 93 (77.5%) |
| Time until intensification in months among patients with intensification (observed) | ||
| Mean (SD) | 7.6 (6.0) | 7.4 (5.7) |
| <9 months | 138 (69.0%) | 82 (68.3%) |
| Between 9 and 12 months | 22 (11.0%) | 18 (15.0%) |
| Between 12 and 18 months | 27 (13.5%) | 14 (11.7%) |
| Between 18 and 24 months | 9 (4.5%) | 4 (3.3%) |
| ≥24 months | 4 (2.0%) | 2 (1.7%) |
| Time until intensification in months (Kaplan-Meier estimate) | ||
| Median (CI-95%) | 24.6 (19.0-NA) | 14.6 (13.1-18.0) |
Table 4. Treatment changes according to HbA1c threshold taking into account modifications of the insulin dose.
Discussion
In this database study, only 4.2% of the patients treated with basal insulin and with two documented HbA1c measurements demonstrating uncontrolled diabetes underwent treatment intensification, not including insulin dose change, one year after the first measurement of an HbA1c over 7% (7.1% with a 8% threshold). Considering at least 10% insulin dose increase in a single step as a therapeutic intensification these percentages were clearly improved (33.0% and 42.9% respectively), but left a majority of patients unintensified Moreover, median time until intensification exceeds one year (14.6 months (13.1-18.0)) with the 8% HbA1c threshold.
Taken together, these observations provide clear evidence of significant therapeutic inertia in patients with T2DM treated with basal insulin.
Therapeutic inertia has now been documented at every stage of the therapeutic trajectory in T2DM [16]. In a recent survey assessing treatment intensification in 17,493 patients treated with oral anti-diabetic drugs in France [17], it was shown that 18% had unacceptable control that should have prompted treatment intensification. However, intensification occured within 6 months in 39% of cases and after one year in 59% of cases.
Khunti et al. [18] used a large family practice database to show a clear pattern of delay in treatment intensification up to the initiation of insulin. As in the current analysis, they acknowledged that HbA1c targets had to be personalised and presented the proportion of patients intensified for a range of HbA1c thresholds between 7 and 8%. For example, median time to intensification to insulin was 6 years for 8%. These studies provide consistent evidence for a significant delay in intensifying oral anti-diabetic treatments.
Intensification of treatment in basal insulintreated patients has received much less attention in real-life settings. In Canada, using a large database, Harris et al. [19] found that once insulin was initiated, patients experienced an average reduction in HbA1c levels from 9.5% to 7.9%. Nonetheless, after one year of insulin therapy, approximately 20% of patients still had very poor glycaemic control (HbA1c >9.0%) and more than 70% of patients were above the target HbA1c threshold of 7.0%. No analysis of treatment changes was conducted. Another analysis of a large reimbursement database in the US showed no evidence of treatment intensification following insulin initiation in the majority of patients, although the absence of information on HbA1c levels limits the interpretation of this study [20].
The reasons for a persistently poor glycaemic control, in patients with a recent basal insulin initiation, were recently explored in an interview-based qualitative study [21]. Issues such as compliance to regular meal and medication times, fear of hypoglycaemia, needles and pain, lack of knowledge and insufficient self-efficacy in diabetes care were found to be barriers to glycaemic control in people with T2DM using insulin. Solutions targeting some of these barriers have been proposed, and some of them have been evaluated in clinical trials. For example, multidisciplinary care involving diabetologists, General Practicioners (GPs) and specialist diabetes nurses may be helpful in ensuring a successful and effective transition from oral therapies to injections of insulin [22]. In addition, it is possible that some physicians still have a conservative approach to prescribing insulin, for example due to concerns about hypoglycaemia or weight gain, and this may lead to prescription of low initial doses. This would be consistent with the finding that the most frequent intensification strategy was an increase in insulin dose. Indeed, if insulin dose increases were excluded from the definition of therapeutic intensification, the mean duration of the period of therapeutic inertia doubled to over one year. It should be noted that in our study, the choice of a threshold of a 10% increase in insulin dose to identify therapeutic intensification is arbitrary, and in in this type of database analysis it is not possible to distinguish unequivocally between active therapeutic intensification and dose titration. The development of practice guidelines to help physicians decide when and how to increase the dose of insulin may be useful.
Our study revealed the extent of suboptimal care of patients with T2MD treated with basal insulins with respect to current practice guidelines. The majority of patients were not monitored regularly for HbA1c levels, the majority of those that were monitored failed to achieve their HbA1c target of ≤7% on treatment, and these treatment failures were not moved promptly to a more intensive treatment regimen. It is expected that the resulting inadequate glycaemic control would have significant patient and public health consequences, with higher rates of associated diabetic complications. Health care professionals need to be aware of the importance of overcoming therapeutic inertia in order to ensure treatment initiation or treatment intensification in an appropriate and timely manner [5,16].
There are a number of limitations which should be taken into account in interpreting our results. The characteristics of the GPs in the CSD LPD Database are similar to those in France in general practice setting, but we cannot exclude some differences in their current daily practice. The population was restricted to patients with documented initial loss of control of diabetes during the observation period. This design was chosen to enable calculation of the duration of uncontrolled diabetes before intensification. However, a consequence of this choice is that patients who were already treated by basal insulins at the beginning of the observational period and whose first documented HbA1c measurement was already above target were excluded from the analysis. With regard to the 7% HbA1c threshold, this was the case for the majority of patients already treated with a basal insulin at inclusion. It is not possible to determine the duration of the period of therapeutic inertia for these patients, and our assessment of this parameter may thus by under-estimated. The study makes the assumption that treatment changes are motivated only by inadequate HbA1c and conversely do not take into consideration some other clinical aspects which sometimes may object to such a decision (i.e. comorbidities, age). However, treatment intensification can improve glycaemic control with no worsening of health status, especially in elderly, lower-income, and minority patients with type 2 diabetes, as it was shown in the TRIAD study [23]. Finally, by design we did not capture the rate of intensification in people without HbA1c values available on the recruitment period; for these poorly monitored patients, inertia is expected to be even higher than in our study population.
Conclusion
In everyday practice, lack of appropriate titration and treatment intensification is frequent in patients treated with basal insulin, leading to loss of confidence in the efficacy of care, favouring the development of diabetic complications and diminishing quality of life. The barriers to timely monitoring and treatment adjustment need to be identified and addressed in both patients and health care providers.
Acknowledgements
Sanofi France funded this study. This study has been presented as a poster at the 2013 SFD (Société Francophone du Diabète.
References
- Robert J, Roudier C, Poutignat N et al. Prise en charge des personnes diabétiques de type 2 en France en 2007 et tendances par rapport à 2001. Bull. Epidémiol. Hebd. 42(43), 455-460 (2009).
- Inzucchi SI, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: 2015: A patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes. Care. 38(1), 140-149 (2015).
- Haute Autorité de la Santé, A physician's guide to type II diabetes – A chronic disorder. HAS Paris (2007).
- http://www.has-sante.fr/portail/jcms/c_1022476/fr/strategie-medicamenteuse-du-controle-glycemique-du-diabete-de-type-2
- American Diabetes Association. Standards of Medical Care in Diabetes 2015. Diabetes. Care. 38, S1-S89.
- Druet C, Bourdel-Marchasson I, Weill A et al. Le diabète de type 2 en France: épidémiologie, évolution de la qualité de la prise en charge, poids social et économique. Entred. Presse. Med. 42: 830-838 (2013).
- Fagot-Campagna A, Fosse S, Roudier C et al. Pour le Comité scientifique d'Entred. Caractéristiques, risque vasculaire et complications chez les personnes diabétiques en France métropolitaine: d’importantes évolutions entre Entred 2001 et Entred 2007. Bull. Epidemiol. Hebd. 42-43, 450-455 (2009).
- Phillips LS, Branch WT, Cook CB et al. Clinical Inertia. Ann. Intern. Med. 135, 825-834 (2001).
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New. Engl. J. Med. 329, 683-689 (1993).
- UK Prospective Diabetes Study Group, Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 352(9131), 837-853 (1998).
- Holman RR, Paul SK, Angelyn Bethel M et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359(15), 1577-1589 (2008).
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358(24), 2560-2572 (2008).
- Skyler JS, Bergenstal R, Bonow RO et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes. Care. 32(1), 187-192 (2009).
- Balkau B, Bouée S, Avignon A et al. Type 2 diabetes treatment intensification in general practice in France in 2008-2009: the DIAttitude Study. Diabetes. Metab. 38, S29-S35 (2012).
- Halimi S, Balkau B, Attali C et al. Therapeutic management of orally treated type 2 diabetic patients, by French general practitioners in 2010: the DIAttitude Study. Diabetes. Metab. 38(Suppl 3): S36-S46 (2012).
- Zafar A, Davies M, Azhar A et al. Clinical inertia in management of T2DM. Prim. Care. Diabetes. 4(4), 203-207 (2010).
- Bouée S, Detournay B, Balkau B et al. Diabète de type 2: pratiques d'intensification thérapeutique chez les médecins généralistes en France en 2008-2009. Bull. Epidemiol. Hebd. 42(43), 436-440 (2010).
- Khunti K, Wolden ML, Thorsted BL et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes. Care. 36(11), 3411-3417 (2013).
- Harris SB, Kapor J, Lank CN et al. Clinical inertia in patients with T2DM requiring insulin in family practice. Can. Fam. Physician. 56(12), e418-e424 (2010).
- Patrick AR, Fischer MA, Choudhry NK et al. Trends in insulin initiation and treatment intensification among patients with type 2 diabetes. J. Gen. Intern. Med. 29(2), 320-327 (2014).
- Tong WT, Vethakkan SR, Ng CJ. Why do some people with type 2 diabetes who are using insulin have poor glycaemic control? A qualitative study. BMJ. Open. 5(1), e006407 (2015).
- Manski-Nankervis JA, Blackberry I, Young D et al. Relational coordination amongst health professionals involved in insulin initiation for people with type 2 diabetes in general practice: an exploratory survey. BMC. Health. Serv. Res. 14, 515 (2014).
- McEwen LN, Bilik D, Johnson SL et al. Predictors and impact of intensification of antihyperglycemic therapy in type 2 diabetes: translating research into action for diabetes (TRIAD). Diabetes. Care. 32(6), 971-976 (2009).