Review Article - Imaging in Medicine (2010) Volume 2, Issue 6
Low-dose pulmonary CT angiography: reduced radiation exposure and iodine load at low tube kilovoltage
Zsolt Szucs-Farkas†1, Peter Vock1 & Sebastian T Schindera11Department of Diagnostic, Interventional & Pediatric Radiology, University Hospital & University of Berne, Freiburgstrasse 4. Berne, CH-3010, Switzerland
- Corresponding Author:
- Zsolt Szucs-Farkas
Department of Diagnostic
Interventional & Pediatric Radiology
University Hospital & University of Berne
Freiburgstrasse 4. Berne, CH-3010, Switzerland
Fax: +41 31 632 0570
E-mail: zsolt.szuecs@insel.ch
Abstract
CT pulmonary angiography is the currently accepted standard in ruling out acute pulmonary embolism. Issues of radiation dose received by patients via CT have been extensively disputed by radiologists and reported by the media. In recent years there has been considerable research performed to find ways for reducing radiation exposure from CT. Herein, we will discuss specific measures that have been shown to be valuable for CT pulmonary angiography. The limitations and the potential benefits of reduced CT peak tube kilovoltage will be detailed as this method is capable of reducing both radiation exposure and iodine load to the patient simultaneously. We discuss some of the emerging tools, which will hopefully play a significant role in wider acceptance of low-dose CT pulmonary angiography protocols.
Keywords
CT angiography ▪ image quality ▪ low kilovoltage ▪ pregnancy ▪ pulmonary embolism ▪ radiation dose ▪ radiation protection
Pulmonary embolism (PE) is a very common cause of death in the USA, with at least 600,000 cases occurring annually [1–3]. In association with laboratory findings and clinical symptoms, imaging is a mainstay of diagnostic evaluation of patients with suspected PE. The confident diagnosis or ruling out of filling defects in the pulmonary arteries may be crucial in the process of making therapeutic decisions. In patients with suspected high-risk PE due to right ventricular failure, a significant delay in the introduction of thrombolytic therapy may aggravate the patient’s health status. This delay can have lethal consequences. On the other hand, unnecessary treatment with thrombolytic agents or long-term thrombosis prophylaxis may result in bleeding complications [4–9]. As in several other fields of diagnostic radiology, the imaging of PE has been revolutionized by the wide availability of multidetector-row CT units enabling a rapid scanning of the pulmonary arterial system with a high spatial resolution.
To date, CT pulmonary angiography (CTPA) using multidetector-row CT scanners has replaced invasive pulmonary angiography as the primary imaging modality to exclude acute PE as it provides similar sensitivity and specificity with lower radiation exposure [10–14]. The large scale Prospective Investigation of PE Diagnosis (PIOPED) II trial, mostly using CT scanners with four detector rows, demonstrated high sensitivity (83%) and specificity (96%) with CTPA in the detection of PE [15]. Although the radiation exposure from CTPA is generally not as high as with a CT examination of the abdomen or pelvis, the mean estimated effective dose can still reach 15 mSv on average (range, 13–40 mSv) as reported by Mettler et al. [16]. Other authors have published lower observed effective radiation doses ranging between 3 and 5 mSv [14,17]. As the benefit:risk ratio for using CT is high and the health benefit of the CT-derived information is immediate, the number of CTPAs being used as a diagnostic tool will not decrease [18]. Furthermore, there are a growing number of young adults with suspected PEs who are examined using CTPA. The reliability of formulas used to estimate the relative risk of cancer development, resulting from low-dose ionizing radiation (i.e., <100 mSv), is subject to debate [19–21]. Nonetheless, the radiological community is making great efforts, especially in young patients, to reduce exposure dose in order to minimize the risk of developing radiationinduced cancers during later life. There are several reviews that have recently been published on various tools that can be used to reduce patient exposure from CT [22–26]. The applicability of these low-dose tools is highly dependent on the patients’ characteristics, the body region imaged and the clinical indication. Low-dose CT images are a disadvantage as they are often of lower quality than their normal-dose counterpart images, owing to the increased imaging noise. Thus, specific countermeasures are often necessary to prevent decreased image quality and loss of diagnostic confidence. It should be noted that the most effective way to reduce radiation exposure to the population from diagnostic CT is to perform CT studies only when there is a clear medical necessity. Therefore, by justifying the indication of the CT need, the requesting physician also takes responsibility for dose reduction to the patient.
The iodinated contrast medium (CM) used with CTPA may cause contrast-induced nephropathy in elderly patients, who often have impaired renal function or diabetes [27]. The probability of developing contrast-induced nephropathy, the third most common cause of hospital-acquired renal failure, is directly proportional to the injected iodine mass [27]. Thus, reducing the iodine load in this patient group can efficiently prevent contrast-induced nephropathy and help patients avoid dialysis and reduce medical costs. Furthermore, reduced CM volume is also advantageous in patients with impaired right heart function, as even lowosmolar CM will pull fluid from the interstitium in its venous return and may lead to volume overload of the right ventricle.
In summary, the ideal CTPA:
▪ Is ordered based on clinical suspicion of PE (justification)
▪ Is tailored to the patient’s characteristics (e.g., body weight [BW] and body diameter, age, gender, renal and circulatory function)
▪ Uses as low a radiation dose as is reasonably achievable
▪ Uses the least amount of CM needed for the diagnosis
▪ Provides a good image quality to enable high diagnostic confidence
▪ Rules out or demonstrates other diseases as differential diagnosis for PE
Instead of a comprehensive systematic review of available low-dose tools for CT, this article will emphasize specific measures that have been shown to be valuable for CTPA. Discussion will center on the potential benefits and limitations of reducing CT peak tube voltage and how this method is capable of reducing both radiation exposure and iodine load to the patient simultaneously. There will also be an explanation of the role of additional tools available to improve image quality with low-dose CTPA. We will not delve into the discussion of factors that impact radiation exposure dose and image quality, which are not or cannot be changed routinely by the operator (e.g., focal spot size, x-ray filtration, sensitivity and geometry of CT detector elements). Despite the increasing use of dual-source CT scanners, we will focus on single-energy CTPA, thus facilitating the ability to rapidly implement the discussed tools on the most commonly used CT units with 16–64 detector rows. In the final section of the paper, ‘Future perspective’, some new and emerging tools that may play an important role in radiation dose reduction from CTPA will be discussed. As these tools are not significantly different from the measures used for CT in other body regions and have been extensively described in two recent publications by Yu and McCollough et al. [22,23] we will omit an in-depth description of technical details and limit the discussion in order to highlight important points.
Low CT tube voltage
On most modern CT scanners, the peak tube voltage can typically be selected between 80 and 140 peak kilovoltage (kVp). Until recently, CTPA protocols have typically used a tube voltage of 120–140 kVp and tube current time of up to 320 mAs [28,29]. In the last 5 years, increasing attention has been put on the use of reduced tube voltage for CT angiography in various body regions, including the chest [30–37].
Physical basis
The relationship between dose and tube energy is exponential (i.e., reduction of the tube voltage from 120 to 100 and 80 kVp reduces dose by 41 and 74%, respectively) if all other scanning parameters are kept constant. The x-ray photons emitted by the CT tube at lower energy have lower velocity and, thus, decreased kinetic energy. Compared with ‘normal’ energy photons emitted at 120 kVp tube voltage, fewer low energy photons will reach the detector due to increased absorption by the patient’s body. The lower number of photons contributing to the image will result in increased image noise (mottle). If the dose becomes too low, photon starving artifacts will be generated and electronic noise will no longer be negligible. Increased image noise at low kVp can be compensated for by setting the tube current time product to a higher level. However, the ability to compensate for image noise with higher tube current is limited by the capacity of the CT tube and the increased dose absorbed in the skin and breasts.
In contrast to tube current, the tube voltage has an impact on the x-ray absorption of substances with high atomic number, such as bone or iodine, while the attenuation by the soft tissues is not significantly dependent on kVp. When peak tube voltage decreases from 120 to 80 kVp, the representative x-ray photon energy decreases from 66 to 52 keV, respectively. As the x-ray photon energy approaches the absorption maximum (k-edge) of iodine, which is 33.2 keV, the photoelectric effect will be more pronounced in the image generated and iodine attenuation will increase (Table 1) [38].
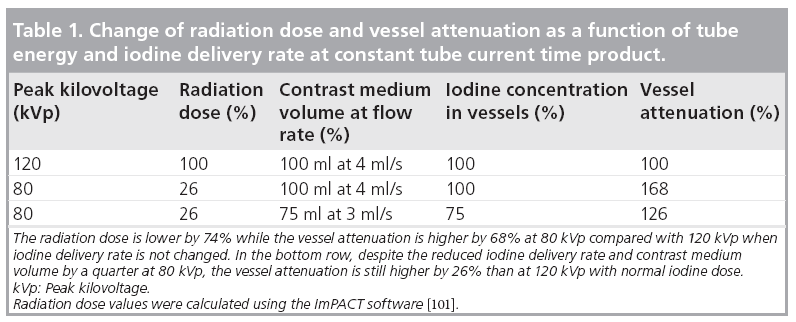
Image quality at reduced kVp with constant iodine dose
The net effect of reduced CT tube voltage on the contrast:noise ratio (CNR) in the contrastenhanced vessels depends on the cross-sectional area of the patient’s body region being imaged, which strongly correlates with the BW. In lightand mid-weight patients, the gain in vessel attenuation at 80 kVp compensates, or even outweighs, the increase in image noise, resulting in a constant or improved CNR compared with the normal tube voltage images [23]. In a phantom study of the chest it was shown that patients with BWs in excess of 100 kg had a significant increase in image noise at 80 kVp, which would result in a nondiagnostic image quality with CTPA. This result necessitates the use of 100 kVp for CTPA in these larger-weight patients [37].
As mentioned previously, setting the tube current at a higher level impedes the increase in image noise and thus, improves CNR at low kVp. On the other hand, the x-ray absorption of air-filled lungs is quite low compared with other body regions. Therefore, the increase in image noise with low-dose CT is significantly less expressed for the chest than for the abdomen or pelvis, which favors the use of low-dose techniques for CTPA.
Two recent publications demonstrated that image quality did not decrease when peak tube voltage with CTPA was lowered from 140 and 120 to 100 kVp, resulting in a dose reduction of 44% and more than 50%, respectively [30,31]. Schueller-Weidekamm et al. found more pulmonary segmental arteries, which were able to be analyzed at 100 kVp [31], compared with 140 kVp. A retrospective analysis of 400 CTPAs showed a significantly higher signal in the contrast-enhanced vessels at 100 and 110 kVp compared with 120 and 130 kVp, while leading to no deterioration of image quality [39].
Image quality at low kVp with reduced iodine dose
The increase of the CT number in the contrastenhanced vessel at reduced tube voltage can be traded to reduce the iodine load given to the patient with no loss of CNR. For example, if we reduce the iodine concentration in the vessels by 25%, by reducing both the volume and the flow rate of injected CM by a quarter, the vessel signal at 80 kVp will still be higher by 26% compared with that measured at 120 kVp using a normal iodine dose (Table 1). This very simple theory also appears to work well in practice.
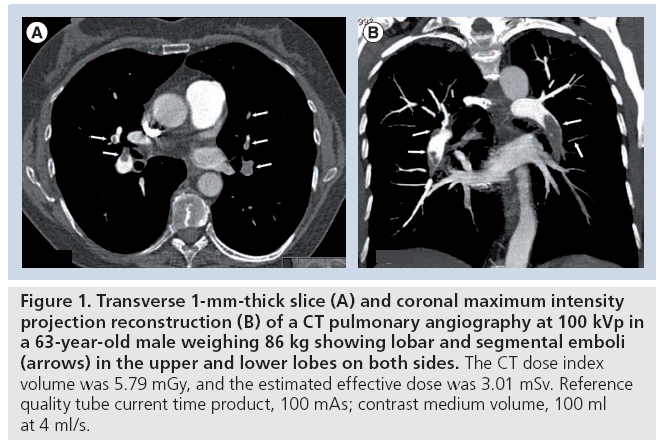
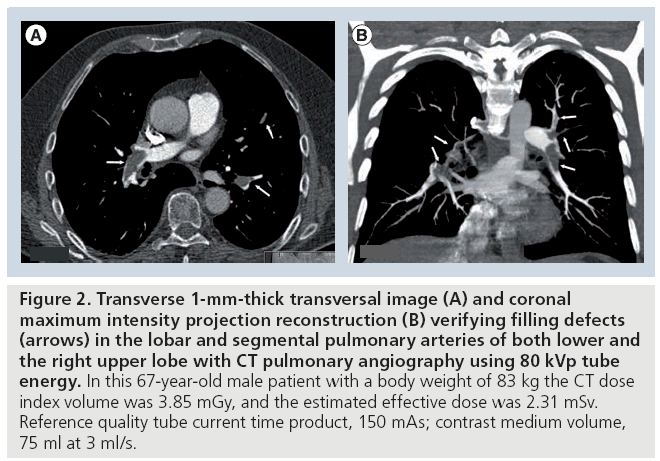
In a publication from 2004, Sigal-Cinqualbre et al. drew attention to the possibility of reducing the injected CM volume for routine chest CT at 80 kVp [34]. In doing so they found that 50–60 ml of CM with an iodine concentration of 300 mg/ml, corresponding to approximately 15–18 g of iodine, resulted in a noninferior image quality compared with a standard chest CT protocol using 90 ml CM at 120 kVp. Holmquist et al. used an 80 kVp CTPA protocol with the primary goal of reducing iodine load in patients with impaired renal function [40,41]. This group showed that the injected CM dose with CTPA could be reduced to 150 mg/kg of BW in patients with moderate to severe renal impairment. This decreased amount of iodine, corresponding to a volume of 37.5 ml of 300 mgI/ml CM in a patient weighing 75 kg, resulted in an acceptable image quality and no elevation of creatinine. Despite significantly elevating tube current time product up to 380 mAs, the CT dose index volume was 5.8 mGy (mean estimated effective dose, 2.5 mSv at a scan length of 22 cm); however, the mean BW of patients was rather low at 65 kg [42]. An 80 kVp CTPA protocol was recently introduced and uses a quality reference tube current of 150 mAs in patients with BW less than 100 kg. Compared with the 100 kVp CTPA protocol with reference quality tube current time product of 100 mAs, the low-dose protocol using 80 kVp enabled a reduction of both the applied iodine dose by 25% and the radiation dose by 40%, with no loss in the CNR or subjective image quality (Figures 1 & 2) [36]. A retrospective analysis showed diagnostic image quality of the 80 kVp protocol in 100 consecutive patients weighing up to 99 kg, while keeping the average radiation dose at 3.6 mGy, corresponding to a mean effective dose of 1.9 mSv [43]. This dose is lower than that from a ventilation/perfusion (V/Q) scan or the annual background radiation dose in most countries [16].
Figure 1: Transverse 1-mm-thick slice (A) and coronal maximum intensity projection reconstruction (B) of a CT pulmonary angiography at 100 kVp in a 63-year-old male weighing 86 kg showing lobar and segmental emboli (arrows) in the upper and lower lobes on both sides. The CT dose index volume was 5.79 mGy, and the estimated effective dose was 3.01 mSv. Reference quality tube current time product, 100 mAs; contrast medium volume, 100 ml at 4 ml/s.
Figure 2: Transverse 1-mm-thick transversal image (A) and coronal maximum intensity projection reconstruction (B) verifying filling defects (arrows) in the lobar and segmental pulmonary arteries of both lower and the right upper lobe with CT pulmonary angiography using 80 kVp tube energy. In this 67-year-old male patient with a body weight of 83 kg the CT dose index volume was 3.85 mGy, and the estimated effective dose was 2.31 mSv. Reference quality tube current time product, 150 mAs; contrast medium volume, 75 ml at 3 ml/s.
Diagnostic accuracy with low-kilovoltage CTPA
Sensitivity data for CTPA in the literature are based on standard protocols using 120 kVp tube voltage. MacKenzie et al. investigated the diagnostic accuracy in simulated low-dose images, where the simulation involved decreasing the effective tube current and thus, resulted in lower CNR values compared with normal dose images. The authors found no significant deterioration of accuracy in the images from the half-dose simulation, but did observe deterioration in the images from the one-fourth, or less, dose simulations [44].
Although there are more data on the image quality with low kVp CTPA, there are very few results on the sensitivity of this method. Before the widespread introduction of low kVp CTPA protocols, the noninferiority of these low-dose protocols, with respect to diagnostic accuracy, should be determined in a large patient population. In theory, the higher intravascular attenuation at low kVp should result in an increased contrast between the vessel and the embolus material, suggesting that no significant filling defects would be missed. In a retrospective blinded study we compared the diagnostic accuracy with a normal-dose protocol using 100 ml CM at 300 mg/ml iodine concentration at 120 kVp and a low-dose CTPA protocol using 75 ml CM of same iodine concentration at 80 kVp. The study consisted of four groups of 30 patients matched by age, gender, BW and thorax dimension. The blinded analysis of the 120 CT studies by two independent radiologists yielded no significant difference, both in the sensitivity and specificity at all levels of the pulmonary arteries [45]. There are also some preliminary intra-individual results from the comparison of the two datasets acquired simultaneously at different tube energies with dual energy CT scanners, suggesting that the accuracy at the lower tube energy is very similar to that in the normal dose images [46]. However, we are still waiting for results from prospective randomized studies.
Image quality for lung parenchyma & mediastinum
Although CTPA focuses on the vessels, accidental findings in the lungs such as atelectasis, consolidation, lung nodules and pulmonary edema are quite common. Therefore, diagnostic confidence to exclude those findings should not suffer from radiation dose reduction. The image quality and diagnostic accuracy for the lung parenchyma with routine chest CT using low mAs have been investigated [47–51]; however, data on low kVp applications are much more limited. In one study, image quality for the parenchyma with 120 and 80 kVp was found to be similar in a small number of patients [34], but the diagnostic accuracy with low kVp protocols has yet to be appropriately analyzed. In our clinical experience, lung nodules of reasonable size (>2 mm) or consolidations can be confidently ruled out at 80 kVp, but there are no prospective data to support this observation.
The mediastinum is more severely affected by the increase in image noise at 80 kVp compared with the lungs, especially in patients with a BW of 75–100 kg, which results in reduced image quality. However, the increased noise in the mediastinum does not restrict the evaluation of the pulmonary arteries and so does not prevent answering the primary clinical question for CTPA. For routine chest CT applications that are specifically targeting the mediastinum, we suggest using tube voltages of 100–120 kVp.
How to deal with the increased noise from low kVp CTPA?
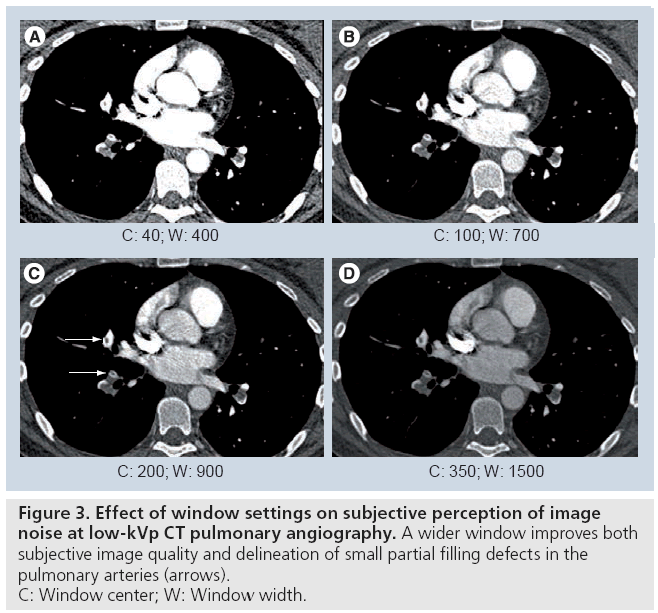
The maximum acceptable noise level is largely dependent on the reader of the image. The use of wider CT window settings (e.g., center: 80–200 HU; width: 750–900 HU) compared with a common mediastinal window (center: 40 HU; width: 450 HU) not only improves the subjective perception of noise, but also helps to avoid missing small partial filling defects in the pulmonary arteries by reducing ‘overshine’ from the contrast-enhanced vessels (Figure 3). This wider window also effectively reduces streak artifacts that are especially common in the right upper lobe pulmonary artery, dorsal to the superior vena cava. Although the window center and level above work well with the 80 kVp CTPA using a reduced amount of CM in our experience, the best window settings should be selected individually at the readers’ discretion.
Figure 3: Effect of window settings on subjective perception of image
noise at low-kVp CT pulmonary angiography. A wider window improves both
subjective image quality and delineation of small partial filling defects in the
pulmonary arteries (arrows).
C: Window center; W: Window width.
An increased reconstruction thickness of the CT images can significantly reduce image noise. For example, doubling the reconstruction thickness from 0.625 to 1.25 mm reduces image noise by 30% as the noise is inversely proportional to the square root of the reconstruction thickness. The trade-off for the lower image noise is reduced spatial resolution in the z-axis, which may compromise the detection rate of subsegmental PE to a certain extent.
Schoepf et al. found 14% more emboli in the subsegmental pulmonary arteries when using 1-mm-thick images compared with 2 mm slice thickness with a four-row CT scanner [52]. We think that 1-mm-thick transverse reconstructions are usually sufficient to rule out subsegmental PE and no submillimeter slices are required for this task. However, we are not aware of any comparative studies on this topic.
Overlapping maximum intensity projection reconstructions of 5–10 mm thickness in the transverse/coronal/sagittal plane(s) are still used as these offer good delineation of pulmonary arteries at low noise. However, small emboli, or those surrounded by contrast agent, can easily be overlooked in maximum intensity projection images. Therefore, maximum intensity projection reconstructions should always be used in conjunction with the original transverse images to avoid missing PE.
The use of soft kernels for image reconstruction can reduce the noise content of the images at the cost of decreased sharpness and spatial resolution [53]. Therefore, we do not use these reconstructions on a regular basis.
Various noise filters and alternate image reconstruction algorithms were recently introduced as very potent tools for noise reduction and are yet to be widely implemented. These filters will be discussed in more detail in the ‘Future perspective’ section.
Further possibilities to reduce patient dose with CTPA
The use of reduced tube current time product is a widely used tool to help with dose reduction during routine chest CT or high-resolution CT of the lung parenchyma, but not for CTPA [49,54–58]. As mentioned earlier, the obvious cause for this is the decreased CNR compared with a normal radiation dose protocol. This is due to the fact that the iodine signal remains constant at an increased image noise level when using low mAs. Alternatively, the automatic real time adjustment of the tube current, preferably both in the x–y plane and the z-axis, can effectively reduce patient dose and is already part of the routine CTPA protocol in most facilities.
Reducing the scan length is a very simple and effective method of reducing radiation dose with CTPA. Limiting the scan range from the aortic arch to the top of the diaphragm, thus omitting the lung apex and base, can reduce dose by up to a third and may not miss significant peripheral emboli [59]. This is an issue of local and personal preference, if one is willing to omit the image information in those anatomic regions in favor of lower radiation dose. Reduced scan length can be used together with low kVp and other tools to reach optimal results (i.e., low radiation exposure and decreased iodine dose), while maintaining high diagnostic image quality (Table 2).
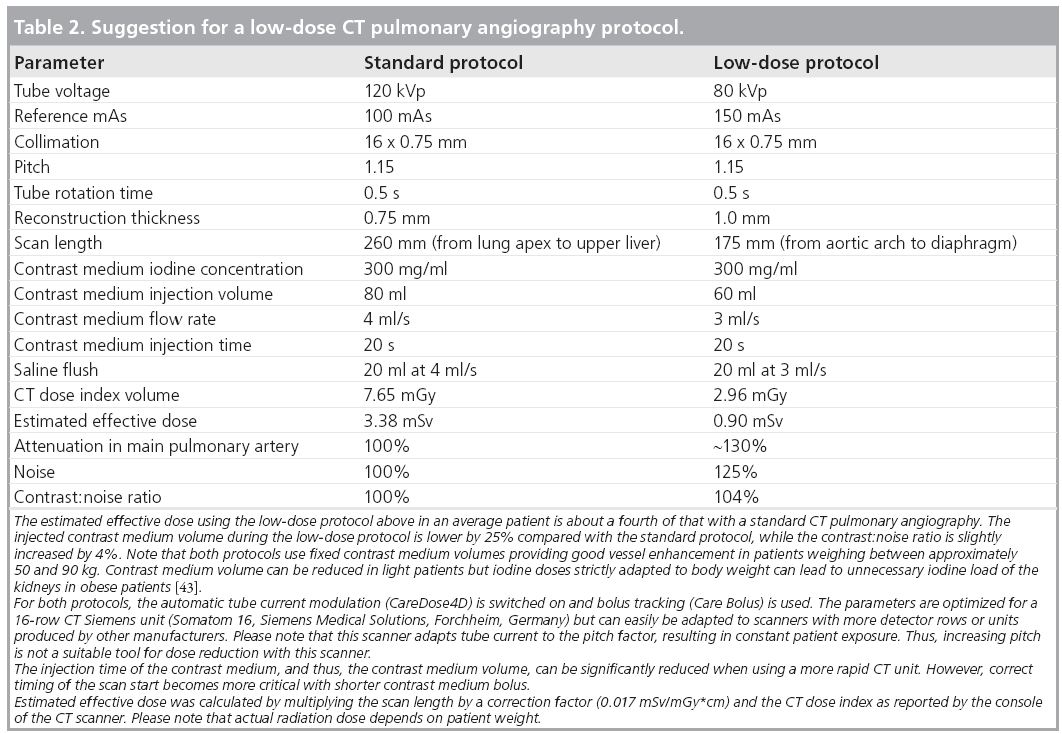
Correct positioning of the patient in the isocenter of the CT gantry is often overlooked as a significant contributor to reducing patient radiation dose. It has been shown that small inaccuracies of 3–6 cm can lead to increased surface dose by 18–49% and lead to reduction of image quality when using automatic tube current modulation [60,61].
Low-dose CTPA in patients with special conditions: high BW & pregnancy
High BW causes both increased noise and reduced attenuation from iodinated CM due to both the high proportion of absorbed x-ray photons and beam hardening [23,37,62]. Therefore, tube voltage and current cannot be reduced arbitrarily in order to avoid reduced image quality. In our experience, patients with BWs between 100 and 130 kg can be examined with 100 kVp tube voltage and 150 mAs quality reference using a 16-row scanner (Somatom 16, Siemens Medical Solutions, Forchheim, Germany) with automatic exposure control (CareDose4D).
In pregnant women with suspected PE, it is especially important to keep patient dose as low as possible. Lung scintigraphy is currently accepted to exclude PE in cases of an elevated d-dimer, if sonography of the lower extremities is negative for deep vein thrombosis. Low-dose CTPA at 80 kVp is a good alternative for lung scintigraphy at similar patient dose, especially in cases of known chronic lung disease or pathologic changes on a conventional chest radiograph, as a V/Q scan would probably yield a nondiagnostic result. Stopping the lower end of the scan range at the diaphragm and using oral barium as internal shielding are effective methods to keep radiation dose to both the mother and fetus reasonably low. Wrapping a lead apron over the lower abdomen is unlikely to reduce the radiation exposure because most of the scatter radiation comes from inside of the body [63–66]. An estimated dose to the fetus of less than 0.1 mGy is very low, with only minimal, if any, increased probability of developing cancer or malformations [67–69]. More importantly, pregnant women have demonstrated increased amounts of radiosensitive glandular breast tissue as compared with nonpregnant women. The impact of using breast shields in preventing higher absorbed breast dose is controversial in the literature [70,71]. In addition, the CM injection protocol should be adapted to account for the hypercirculatory state that arises during pregnancy, which is due to high blood volume, elevated cardiac output and physiologic AV shunts in the placenta. These factors, along with increased intra-abdominal pressure, result in rapid dilution of injected CM in the vessels, with lower and earlier peak enhancement [72–74]. Thus, the iodine delivery rate must be increased in pregnant women by increasing the flow rate and/or the iodine concentration of the contrast agent. Moreover, during the second and third trimester it may be nearly impossible for the patient to hold their breath throughout the duration of the examination, especially when using a CT scanner with 64 or less detector rows. Therefore, a shallow but continuous breathing regimen is a good alternative, which additionally helps in preventing further increases in the intra-abdominal pressure and may also improve opacification of the pulmonary vessels. The latest generation CT scanners are able to scan the whole chest in less than 1 s, making breath holds unnecessary.
Since iodinated contrast agents have been shown to cross the human placenta and enter the fetus, the Guidelines of the European Society of Urogenital Radiology suggest that thyroid function should be checked in the neonate during the first week [75]. However, Atwell et al. found normal serum thyroid-stimulating hormone levels in 21 neonates exposed in utero [76]. We are not aware of adequate and well-controlled teratogenic studies on the effects of iodinated CM in pregnant women.
Future perspective
In the last decade, tremendous efforts have been made by CT manufacturers to improve image quality and reduce patient dose. More advanced collimator designs allowing for significant dose reduction by adaptive shielding (i.e., elimination of overscanning at the beginning and end of the scan range) have recently become available [77,78]. A novel scanning algorithm minimizing the tube current when the x-ray tube travels over the ventral surface of the patients’ body is a very promising method to reduce radiation dose to the most radiosensitive organs, such as the breast, lens and thyroid gland [70]. The development of detectors consisting of more sensitive and rapidly responding detector elements with advanced geometry will lead to improved detection efficiency of x-ray photons, all allowing for a significant dose and/ or noise reduction in the near future. The use of advanced x-ray beam-shaping filters adapted to the scan region can contribute to significant radiation dose reduction [79]. The commercial introduction of the photon-counting detector systems is expected to revolutionize CT imaging, both in terms of dose reduction and tissue characterization [80,81]. All these promising techniques can be used with CTPA alone or in conjunction with other low-dose tools. However, comprehensive scientific investigations should precede their routine implementation.
Besides the improved detection of the roentgen quanta, optimizing reconstruction from the available image data is an important topic of current clinical and industrial research. Although it has been widely used in nuclear medicine imaging for a long time, only recently have iterative image reconstruction techniques become available for CT. There are numerous advantages for using iterative image reconstruction instead of the currently used filtered back projection method, which include reduced noise and beam hardening effects, improved handling of incomplete image datasets and improved spatial resolution [82–84]. Owing to the extensive computing capacity required, it was available for research only, but not for commercial use. To date, most of the major CT vendors have implemented iterative image reconstruction in their newest generation models of scanners, by using either original raw data or image data. The improved image quality due to iterative reconstruction could enhance the acceptance of low-dose CT protocols, both among radiologists and referring clinicians, who often disfavor using low-dose protocols owing to increased image noise. Furthermore, reduced image noise might facilitate the extension of the indication of low-kVp CTPA to obese patients and may improve the diagnostic confidence for pathologic changes in the mediastinum and lung parenchyma. A similar impact is to be expected from the use of more advanced noise filters such as the nonlinear 3D optimized reconstruction algorithm, multiband filtering or highly constrained back projection local reconstruction [85– 87]. Contrary to most of the widely available noise filters, the previously referenced techniques are capable of improving the image quality with no loss of spatial resolution. Although the effect of these filters on image quality is explored by scientific investigations for abdominal CT, data on the impact with CTPA, and especially on the diagnostic accuracy of these techniques, are limited. Therefore, further research and careful analyses are necessary before these techniques find their way into routine diagnostic approaches to exclude acute PE.
Although it does not reduce radiation dose alone, calculation of the iodine content from the datasets acquired by two x-ray tubes working at two different energies in dual-source CT scanners might reduce diagnostic errors with CTPA. This is especially true in small lung arteries that can appear as hypodense structures due to volume averaging with the surrounding lung parenchyma, and can mimic filling defects. In this last case the calculated iodine map can help to decide if the vessel is perfused or not. The extension of this method to the lung parenchyma gives a scintigraphy-like appearance of perfusion maps and is promising to fully replace V/Q scans as a one-stop test [88–95].
Conclusion
Low kVp, in combination with other techniques, enables a significant reduction of both the radiation dose and iodine load at good diagnostic image quality with CTPA. The limitations of the method and the individual adjustment of the CT protocol to the specific patient conditions will help avoid diminished image quality. Noise reduction techniques will play an important role in the wider distribution and acceptance of low- dose CTPA protocols.
Financial & competing interests disclosure
S Schindera received grant from Siemens Healthcare. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• • of considerable interest
- Dalen JE, Alpert JS: Natural history of pulmonary embolism. Prog. Cardiovasc. Dis. 17(4), 259–270 (1975).
- Tapson VF, Humbert M: Incidence and prevalence of chronic thromboembolic pulmonary hypertension: from acute to chronic pulmonary embolism. Proc. Am. Thorac. Soc. 3(7), 564–567 (2006).
- Torbicki A, Perrier A, Konstantinides S et al.: Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 29(18), 2276–2315 (2008).
- Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W: Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am. J. Cardiol. 82(8), 966–970 (1998).
- Kanter DS, Mikkola KM, Patel SR, Parker JA, Goldhaber SZ: Thrombolytic therapy for pulmonary embolism. Frequency of intracranial hemorrhage and associated risk factors. Chest 111(5), 1241–1245 (1997).
- Meyer G, Sors H, Charbonnier B et al.: Effects of intravenous urokinase versus alteplase on total pulmonary resistance in acute massive pulmonary embolism: a European multicenter double-blind trial. The European Cooperative Study Group for Pulmonary Embolism. J. Am. Coll. Cardiol. 19(2), 239–245 (1992).
- Dalla-Volta S, Palla A, Santolicandro A et al.: Paims 2: alteplase combined with heparin versus heparin in the treatment of acute pulmonary embolism. Plasminogen Activator Italian Multicenter Study 2. J. Am. Coll. Cardiol. 20(3), 520–526 (1992).
- Levine M, Hirsh J, Weitz J et al.: A randomized trial of a single bolus dosage regimen of recombinant tissue plasminogen activator in patients with acute pulmonary embolism. Chest 98(6), 1473–1479 (1990).
- The urokinase pulmonary embolism trial. A national cooperative study. Circulation 47(Suppl. 2), II1–II108 (1973).
- Coche E, Vynckier S, Octave-Prignot M: Pulmonary embolism: radiation dose with multi-detector row CT and digital angiography for diagnosis. Radiology 240(3), 690–697 (2006).
- Winer-Muram HT, Rydberg J, Johnson MS et al.: Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology 233(3), 806–815 (2004).
- Cronin P, Weg JG, Kazerooni EA: The role of multidetector computed tomography angiography for the diagnosis of pulmonary embolism. Semin. Nucl. Med. 38(6), 418–431 (2008).
- Qanadli SD, Hajjam ME, Mesurolle B et al.: Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 217(2), 447–455 (2000).
- Remy-Jardin M, Pistolesi M, Goodman LR et al.: Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the fleischner society. Radiology 245(2), 315–329 (2007).
- Stein PD, Fowler SE, Goodman LR et al.: Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 354(22), 2317–2327 (2006).
- Mettler F Jr, Huda W, Yoshizumi TT, Mahesh M: Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248(1), 254–263 (2008).
- Kuiper JW, Geleijns J, Matheijssen NA, Teeuwisse W, Pattynama PM: Radiation exposure of multi-row detector spiral computed tomography of the pulmonary arteries: comparison with digital subtraction pulmonary angiography. Eur. Radiol. 13(7), 1496–1500 (2003).
- Boone JM: Multidetector CT: opportunities, challenges, and concerns associated with scanners with 64 or more detector rows. Radiology 241(2), 334–337 (2006).
- Brenner DJ, Hall EJ: Computed tomography – an increasing source of radiation exposure. N. Engl. J. Med. 357(22), 2277–2284 (2007).
- Shrimpton PC, Hillier MC, Lewis MA, Dunn M: National survey of doses from CT in the UK: 2003. Br. J. Radiol. 79(948), 968–980 (2006).
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation NRC: Health risks from exposure to low levels of ionizing radiation: Beir VII Phase 2. The National Academies Press, DC, USA (2006).
- Yu L, Liu X, Leng S et al.: Radiation dose reduction in computed tomography: techniques and future perspective. Imaging Med. 1(1), 65–84 (2009).
- McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J: Strategies for reducing radiation dose in CT. Radiol. Clin. North Am. 47(1), 27–40 (2009).
- Kubo T, Lin PJ, Stiller W et al.: Radiation dose reduction in chest CT: a review. AJR Am. J. Roentgenol. 190(2), 335–343 (2008).
- Kalender WA, Buchenau S, Deak P et al.: Technical approaches to the optimisation of CT. Phys. Med. 24(2), 71–79 (2008).
- Mccollough CH, Bruesewitz MR, Kofler JM Jr: CT dose reduction and dose management tools: overview of available options. Radiographics 26(2), 503–512 (2006).
- Bartorelli AL, Marenzi G: Contrast-induced nephropathy. J. Interv. Cardiol. 21(1), 74–85 (2008).
- Wittram C, Waltman AC, Shepard JA, Halpern E, Goodman LR: Discordance between CT and angiography in the PIOPED II study. Radiology 244(3), 883–889 (2007).
- Gottschalk A, Stein PD, Goodman LR, Sostman HD: Overview of prospective investigation of pulmonary embolism diagnosis II. Semin. Nucl. Med. 32(3), 173–182 (2002).
- Heyer CM, Mohr PS, Lemburg SP, Peters SA, Nicolas V: Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 245(2), 577–583 (2007).
- Schueller-Weidekamm C, Schaefer- Prokop CM, Weber M, Herold CJ, Prokop M: CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. Radiology 241(3), 899–907 (2006).
- Wintersperger B, Jakobs T, Herzog P et al.: Aorto-iliac multidetector-row CT angiography with low kv settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur. Radiol. 15(2), 334–341 (2005).
- Bahner ML, Bengel A, Brix G, Zuna I, Kauczor HU, Delorme S: Improved vascular opacification in cerebral computed tomography angiography with 80 kvp. Invest. Radiol. 40(4), 229–234 (2005).
- Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF: Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 231(1), 169–174 (2004).
- Bjorkdahl P, Nyman U: Using 100- instead of 120-kvp computed tomography to diagnose pulmonary embolism almost halves the radiation dose with preserved diagnostic quality. Acta Radiol. 51(3), 260–270 (2010).
- Szucs-Farkas Z, Kurmann L, Strautz T, Patak MA, Vock P, Schindera ST: Patient exposure and image quality of low-dose pulmonary computed tomography angiography: comparison of 100- and 80-kvp protocols. Invest. Radiol. 43(12), 871–876 (2008).
- Szucs-Farkas Z, Verdun FR, Von Allmen G, Mini RL, Vock P: Effect of x-ray tube parameters, iodine concentration, and patient size on image quality in pulmonary computed tomography angiography: a chest-phantom-study. Invest. Radiol. 43(6), 374–381 (2008).
- Huda W, Scalzetti EM, Levin G: Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 217(2), 430–435 (2000).
- Matsuoka S, Hunsaker AR, Gill RR et al.: Vascular enhancement and image quality of MDCT pulmonary angiography in 400 cases: comparison of standard and low kilovoltage settings. AJR Am. J. Cardiol. 192(6), 1651–1656 (2009).
- Holmquist F, Hansson K, Pasquariello F, Bjork J, Nyman U: Minimizing contrast medium doses to diagnose pulmonary embolism with 80-kvp multidetector computed tomography in azotemic patients. Acta Radiol. 50(2), 181–193 (2009). nn Provides evidence of good image quality at reduced radiation dose and contrast medium amount.
- Holmquist F, Nyman U: Eighty-peak kilovoltage 16-channel multidetector computed tomography and reduced contrast-medium doses tailored to body weight to diagnose pulmonary embolism in azotaemic patients. Eur. Radiol. 16(5), 1165–1176 (2006).
- Kristiansson M, Holmquist F, Nyman U: Ultralow contrast medium doses at CT to diagnose pulmonary embolism in patients with moderate to severe renal impairment: a feasibility study. Eur. Radiol. 20(6), 1321–1330 (2009).
- Szucs-Farkas Z, Strautz T, Patak MA, Kurmann L, Vock P, Schindera ST: Is body weight the most appropriate criterion to select patients eligible for low-dose pulmonary CT angiography? Analysis of objective and subjective image quality at 80 kvp in 100 patients. Eur. Radiol. 19(8), 1914–1922 (2009).
- 44 MacKenzie JD, Nazario-Larrieu J, Cai T et al.: Reduced-dose CT: effect on reader evaluation in detection of pulmonary embolism. AJR Am. J. Roentgenol. 189(6), 1371–1379 (2007).
- Szucs-Farkas Z, Schaller C, Bensler S, Patak MA, Vock P, Schindera ST: Detection of pulmonary emboli with CT angiography at reduced radiation exposure and contrast material volume: comparison of 80 kvp and 120 kvp protocols in a matched cohort. Invest. Radiol. 44(12), 793–799 (2009).
- Pontana F, Faivre JB, Remy-Jardin M et al.: Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad. Radiol. 15(12), 1494–1504 (2008).
- Mayo JR, Kim KI, Macdonald SL et al.: Reduced radiation dose helical chest CT: effect on reader evaluation of structures and lung findings. Radiology 232(3), 749–756 (2004).
- Mayo JR, Hartman TE, Lee KS, Primack SL, Vedal S, Muller NL: CT of the chest: minimal tube current required for good image quality with the least radiation dose. AJR Am. J. Roentgenol. 164(3), 603–607 (1995).
- Mori K, Tominaga K, Hirose T, Sasagawa M, Yokoyama K, Moriyama N: Utility of low-dose helical CT as a second step after plain chest radiography for mass screening for lung cancer. J. Thorac. Imaging 12(3), 173–180 (1997).
- Takahashi M, Maguire WM, Ashtari M et al.: Low-dose spiral computed tomography of the thorax: comparison with the standard-dose technique. Invest. Radiol. 33(2), 68–73 (1998).
- Wildberger JE, Mahnken AH, Schmitz- Rode T et al.: Individually adapted examination protocols for reduction of radiation exposure in chest CT. Invest. Radiol. 36(10), 604–611 (2001).
- Schoepf UJ, Holzknecht N, Helmberger TK et al.: Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology 222(2), 483–490 (2002).
- Kalra MK, Wittram C, Maher MM et al.: Can noise reduction filters improve low-radiation-dose chest CT images? Pilot study. Radiology 228(1), 257–264 (2003).
- Funama Y, Awai K, Liu D et al.: Detection of nodules showing ground-glass opacity in the lungs at low-dose multidetector computed tomography: phantom and clinical study. J. Comput. Assist. Tomogr. 33(1), 49–53 (2009).
- Gierada DS, Pilgram TK, Whiting BR et al.: Comparison of standard- and low-radiationdose CT for quantification of emphysema. AJR Am. J. Roentgenol. 188(1), 42–47 (2007).
- Wormanns D, Ludwig K, Beyer F, Heindel W, Diederich S: Detection of pulmonary nodules at multirow-detector CT: effectiveness of double reading to improve sensitivity at standard-dose and low-dose chest CT. Eur. Radiol. 15(1), 14–22 (2005).
- Dinkel HP, Sonnenschein M, Hoppe H, Vock P: Low-dose multislice CT of the thorax in follow-up of malignant lymphoma and extrapulmonary primary tumors. Eur. Radiol. 13(6), 1241–1249 (2003).
- Jung KJ, Lee KS, Kim SY, Kim TS, Pyeun YS, Lee JY: Low-dose, volumetric helical CT: image quality, radiation dose, and usefulness for evaluation of bronchiectasis. Invest. Radiol. 35(9), 557–563 (2000).
- Kallen JA, Coughlin BF, O’Loughlin MT, Stein B: Reduced z-axis coverage multidetector CT angiography for suspected acute pulmonary embolism could decrease dose and maintain diagnostic accuracy. Emerg. Radiol. 17(1), 31–35 (2010).
- Toth T, Ge Z, Daly MP: The influence of patient centering on CT dose and image noise. Med. Phys. 34(7), 3093–3101 (2007).
- Li J, Udayasankar UK, Toth TL, Seamans J, Small WC, Kalra MK: Automatic patient centering for MDCT: effect on radiation dose. AJR Am. J. Roentgenol. 188(2), 547–552 (2007).
- Wolbarst A: The formation of a radiographic image. In: Physics of Radiology. Appleton and Lange, CO, USA, 147–205 (1993).
- Litmanovich D, Boiselle PM, Bankier AA, Kataoka ML, Pianykh O, Raptopoulos V: Dose reduction in computed tomographic angiography of pregnant patients with suspected acute pulmonary embolism. J. Comput. Assist. Tomogr. 33(6), 961–966 (2009).
- Doshi SK, Negus IS, Oduko JM: Fetal radiation dose from CT pulmonary angiography in late pregnancy: a phantom study. Br. J. Radiol. 81(968), 653–658 (2008).
- Kennedy EV, Iball GR, Brettle DS: Investigation into the effects of lead shielding for fetal dose reduction in CT pulmonary angiography. Br. J. Radiol. 80(956), 631–638 (2007).
- Yousefzadeh DK, Ward MB, Reft C: Internal barium shielding to minimize fetal irradiation in spiral chest CT: a phantom simulation experiment. Radiology 239(3), 751–758 (2006).
- Wagner LK, Hayman LA: Pregnancy and women radiologists. Radiology 145(2), 559–562 (1982).
- Wagner LK, Huda W: When a pregnant woman with suspected appendicitis is referred for a CT scan, what should a radiologist do to minimize potential radiation risks? Pediatric Radiol. 34(7), 589–590 (2004).
- Ames Castro M, Shipp TD, Castro EE, Ouzounian J, Rao P: The use of helical computed tomography in pregnancy for the diagnosis of acute appendicitis. Am. J. Obstet. Gynecol. 184(5), 954–957 (2001).
- Vollmar SV, Kalender WA: Reduction of dose to the female breast in thoracic CT: a comparison of standard-protocol, bismuthshielded, partial and tube-current-modulated CT examinations. Eur. Radiol. 18(8), 1674–1682 (2008).
- Hurwitz LM, Yoshizumi TT, Goodman PC et al.: Radiation dose savings for adult pulmonary embolus 64-MDCT using bismuth breast shields, lower peak kilovoltage, and automatic tube current modulation. AJR Am. J. Roentgenol. 192(1), 244–253 (2009).
- Andreou AK, Curtin JJ, Wilde S, Clark A: Does pregnancy affect vascular enhancement in patients undergoing CT pulmonary angiography? Eur. Radiol. 18(12), 2716–2722 (2008).
- U-King-Im JM, Freeman SJ, Boylan T, Cheow HK: Quality of CT pulmonary angiography for suspected pulmonary embolus in pregnancy. Eur. Radiol. 18(12), 2709–2715 (2008).
- Schaefer-Prokop C, Prokop M: CTPA for the diagnosis of acute pulmonary embolism during pregnancy. Eur. Radiol. 18(12), 2705–2708 (2008).
- Thomsen HS: European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur. J. Radiol. 60(3), 307–313 (2006).
- Atwell TD, Lteif AN, Brown DL, Mccann M, Townsend JE, Leroy AJ: Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am. J. Roentgenol. 191(1), 268–271 (2008).
- Deak PD, Langner O, Lell M, Kalender WA: Effects of adaptive section collimation on patient radiation dose in multisection spiral CT. Radiology 252(1), 140–147 (2009).
- Christner JA, Zavaletta VA, Eusemann CD, Walz-Flannigan AI, Mccollough CH: Dose reduction in helical CT: dynamically adjustable z-axis x-ray beam collimation. AJR Am. J. Roentgenol. 194(1), W49–W55 (2009).
- Mccollough CH, Primak AN, Saba O et al.: Dose performance of a 64-channel dualsource CT scanner. Radiology 243(3), 775–784 (2007).
- Shikhaliev PM: Computed tomography with energy-resolved detection: a feasibility study. Phys. Med. Biol. 53(5), 1475–1495 (2008).
- Shikhaliev PM, Xu T, Molloi S: Photon counting computed tomography: concept and initial results. Med. Phys. 32(2), 427–436 (2005).
- Thibault JB, Sauer KD, Bouman CA, Hsieh J: A three-dimensional statistical approach to improved image quality for multislice helical CT. Med. Phys. 34(11), 4526–4544 (2007).
- Nuyts J, De Man B, Dupont P, Defrise M, Suetens P, Mortelmans L: Iterative reconstruction for helical CT: a simulation study. Phys. Med. Biol. 43(4), 729–737 (1998).
- Wang G, Snyder DL, O’Sullivan JA, Vannier MW: Iterative deblurring for CT metal artifact reduction. IEEE Trans. Med. Imaging 15(5), 657–664 (1996).
- Bai M, Chen J, Raupach R, Suess C, Tao Y, Peng M: Effect of nonlinear threedimensional optimized reconstruction algorithm filter on image quality and radiation dose: validation on phantoms. Med. Phys. 36(1), 95–97 (2009).
- Supanich M, Tao Y, Nett B et al.: Radiation dose reduction in time-resolved CT angiography using highly constrained back projection reconstruction. Phys. Med. Biol. 54(14), 4575–4593 (2009).
- Liu X, Primak AN, Krier JD, Yu L, Lerman LO, Mccollough CH: Renal perfusion and hemodynamics: accurate in vivo determination at CT with a 10-fold decrease in radiation dose and HYPR noise reduction. Radiology 253(1), 98–105 (2009).
- Krissak R, Henzler T, Reichert M, Krauss B, Schoenberg SO, Fink C: Enhanced visualization of lung vessels for diagnosis of pulmonary embolism using dual energy CT angiography. Invest. Radiol. 45(6), 341–346 (2010).
- Lu GM, Wu SY, Yeh B, Zhang LJ: Dualenergy computed tomography in pulmonary embolism. Br. J. Radiol. 83(992), 707–718 (2010).
- Nikolaou K, Thieme S, Sommer W, Johnson T, Reiser MF: Diagnosing pulmonary embolism: new computed tomography applications. J. Thorac. Imaging 25(2), 151–160 (2010).
- Remy-Jardin M, Faivre JB, Pontana F et al.: Thoracic applications of dual energy. Radiol. Clin. North Am. 48(1), 193–205 (2010).
- Thieme SF, Johnson TR, Lee C et al.: Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am. J. Roentgenol. 193(1), 144–149 (2009).
- Zhang LJ, Chai X, Wu SY et al.: Detection of pulmonary embolism by dual energy CT: correlation with perfusion scintigraphy and histopathological findings in rabbits. Eur. Radiol. (2009) (Epub ahead of print).
- Zhang LJ, Zhao YE, Wu SY et al.: Pulmonary embolism detection with dual-energy CT: experimental study of dual-source CT in rabbits. Radiology 252(1), 61–70 (2009).
- Fink C, Johnson TR, Michaely HJ et al.: Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. Rofo 180(10), 879–883 (2008).
- ImPACT: Imaging Performance Assessment of CT Scanners www.impactscan.org
• • Estimates lifetime risk of cancer caused by radiation exposure from CT.
• • Overview of various techniques for dose reduction with CT.
• • Summary of dose-reduction strategies and available automatic exposure control CT tools.
• • Provides evidence of good image quality at reduced radiation dose and contrast medium amount.
• • Provides evidence of good image quality at reduced radiation dose and contrast medium amount.
• • Provides evidence of good image quality at reduced radiation dose and contrast medium amount.
• • Retrospective interindividual study on the diagnostic accuracy of low-dose and normal-dose CT pulmonary angiography protocols.
• • Discusses the need to adapt contrast injection protocols in pregnancy.
• • Discusses the need to adapt contrast injection protocols in pregnancy.
• • Discusses the need to adapt contrast injection protocols in pregnancy.
Website