Review Article - Imaging in Medicine (2022) Volume 14, Issue 6
Magnetic Nanoparticles in Medical Imaging
1Department of Chemical Engineering, Arak Branch, Islamic Azad University, Arak, Iran
2Young Researchers and Elite Club, Gachsaran Branch, Islamic Azad University, Gachsaran, Iran
- *Corresponding Author:
- Ehsan Kianfar
Department of Chemical Engineering, Arak Branch, Islamic Azad University, Arak, Iran
E-mail: e-kianfar94@iau-arak.ac.ir/ ehsan_ kianfar2010@yahoo.com
Abstract
Unique Features and Benefits of Magnetic Nanoparticles The superiority of these particles as contrast agents in magnetic resonance imaging (MRI). MRI is based on the interaction between a magnetic field and tissue protons. Studies have shown that the use of magnetic nanoparticles in MRI provides better contrast of images and allows imaging at the cellular and molecular levels. This article gives a brief description of how MRI works, also compares magnetic nanoparticles and other contrast agents, and discusses the reason for the superiority and increased contrast of magnetic nanoparticles. First, the required properties for these particles are named Factors affecting these properties are discussed, including particle size and its effect on image contrast, particle preparation method and its effect on their physical and chemical properties, and the types of coatings used and the benefits. In the following, the types of nanoparticles used are briefly introduced and finally the way of transfer of particles to the target tissue is passively investigated. The study of inflamed tissue, angiogenesis, apoptosis, gene expression, cancer, and cellular tracing is described, and a brief explanation of how and applications of surface comfort modification is given, as well as a major discussion on the properties and characteristics of magnetic nanoparticles in medical imaging.
Keywords
Magnetic Nanoparticles • Angiogenesis • Apoptosis • Gene Expression • Cancer • Medical Imaging
Introduction
■ Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is a powerful medical tool in the field of diagnosis [1]. The basis of the phenomenon of nuclear magnetic resonance with the appropriate contrast factor, allows early detection of cancer and various diseases [2].
■ Principles of MRI contrast
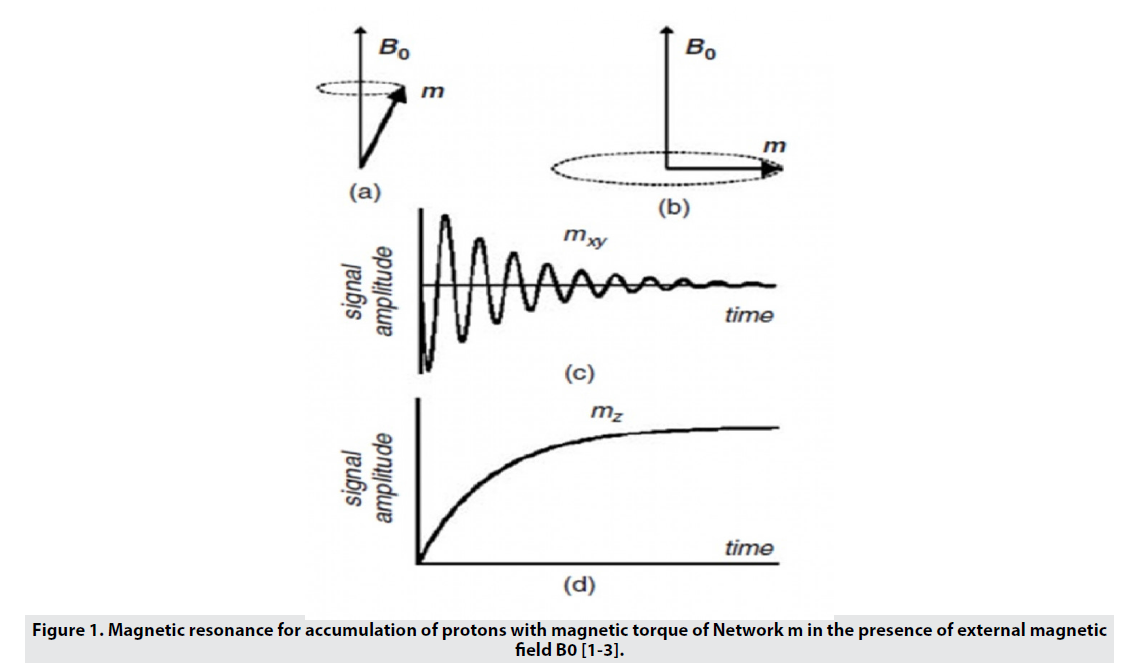
MRI uses a strong magnetic field that aligns the proton magnetic moments in the sample and produces a magnetism of magnitude M0 along the z-axis (Mz) (Figure 1). A radio frequency (RF) pulse is amplified at a frequency that transmits energy to the protons by rotating the protons' magnetic moments, causing them to move away from the Z axis and into an angle called the flip angle. The choice of flip angle depends on the sequence of images applied, but is generally located on the transverse plane (xy plane) and causes pure Mxy magnetism. By removing RF, the proton magnetic moments are relieved to their original state (equilibrium). The time required for the comfort of the magnetic moments in equilibrium, which is called the comfort time, depends on the type of tissue [3,4]. MRI contrast in soft tissues is due to differences in proton density, spin-lattice relaxation time (T1), and proton spin-spin relaxation time (T2). is. Fast-proton protons (short T1) regenerate full magnetism in the Z-axis direction and produce high-intensity signals. For protons that relax more slowly (have a longer T2), full magnetization does not recur before the RF pulse is picked up, and so these protons inherently produce a weaker signal, leading to a phenomenon called an effect. They are saturated. Images of T1 weight show anatomy well and are preferred when clear images of the structure are required [4]. T2 is the time constant of exponential loss of transverse magnetism (Mxy) after RF pulse application. T2 is related to the amount of time it takes for the protons' magnetic moments to change direction and randomly align with the xy plane after RF, eventually leading to a pure zero magnetic moment on the xy plane. This phase less process can be caused by the combination of local heterogeneities of the magnetic field as a result of the interaction of adjacent molecules, as well as by the macroscopic effects associated with small changes in the external magnetic field. When the phase-out time is calculated for both molecular interactions and external magnetic field heterogeneities, it is called * T2 and the images produced are called * T2 weight images. Images with a weight of T2 are generated by removing the phase effects related to external field heterogeneities and are calculated only for molecular interactions. T2 weight images give good pathological information when abnormal fluids appear against a bright normal tissue background. Since there is a difference between the water content of organs and tissues, and also in many diseases the process of damage leads to changes in water content, this imaging method is widely used in medicine [1]. The MRI machine is a tube surrounded by a circular magnet (Figure 2). This magnet creates a magnetic field. Here, radio waves with different wavelengths scan the surface of the sample. When a uniform magnetic field is used, it has a rotating core at the Larmor frequency and consists of two energy levels, high and low [3]. By absorbing energy from the same frequency radio wave with a difference of two levels, the sample goes to a higher energy state and is in the direction of the external magnetic field. When the field is cut off, these nuclei return to their original state [1] and return to They emit the previous level of radio waves (RF) at Larmor frequency. The RF signal is received by the radiofrequency coil and it shows the difference between the two levels [3]. The wire then converts the received waves into electric current. These currents are amplified and transmitted to the computer as MRI signals. Computers convert this data into an image using a conversion system called Fourier transform. This image is very accurate and can show very small changes [1].
■ Contrast factors in MRI
In most tissues, the intrinsic changes of T1 and T2 are small, and often in clinical applications, external materials are used to enhance the contrast between the target tissue and surrounding tissues. While almost all MRI contrast agents affect both T1 and T2 times, the effect of contrast agents is usually more pronounced at one of the T1 or T2 times, leading to the division of these probes into T1 or T2 contrast agents. Contrast factors T1 are used to increase the signal intensity, which amplifies the positive contrast in T1 weight images, while T2 contrast factors reduce the signal intensity and amplify the negative contrast in T2 weight images. Currently, most of the contrast agents used in the clinic is based on paramagnetic chelates of lanthanide metals such as gadolinium. The presence of paramagnetic ions near water protons reduces their T1 comfort time by coordinating with water molecules and increases contrast. Although gadolinium chelates are widely used, their short circulation time, poor tracking sensitivity, and toxicity concerns have led to the continued development of magnetic nanoparticles as contrast enhancers [5].
■ Advantages of magnetic nanoparticles as contrast agents
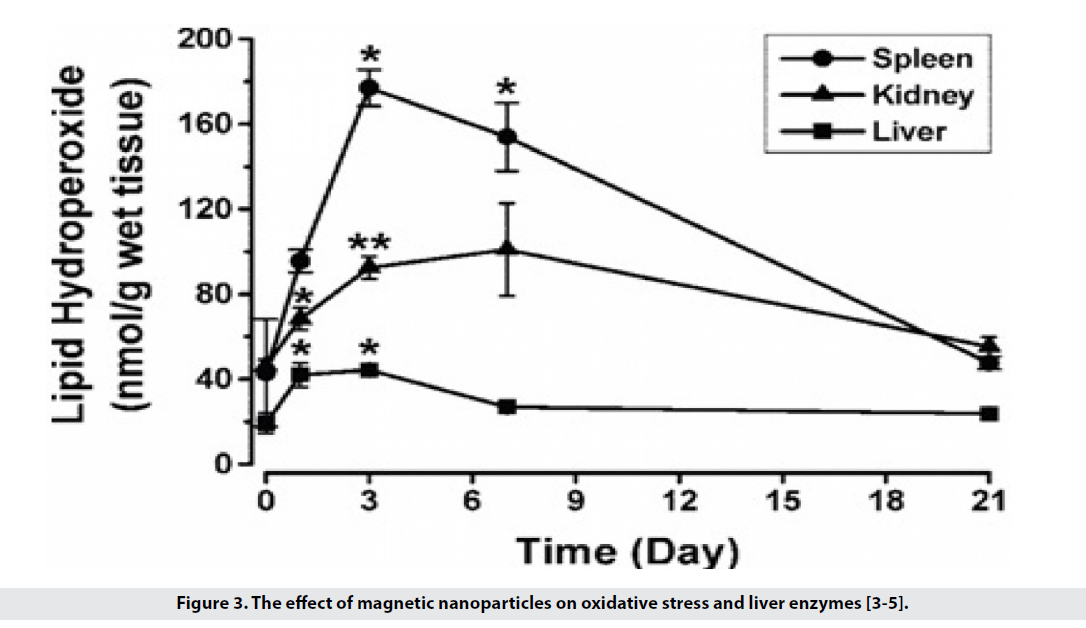
• They have low toxicity and are biocompatible, for example in the use of iron oxide because its amount is much less than iron in the body. According to the hemostatic mechanisms, physiological iron is metabolized and does not cause much change in liver enzymes and oxidative stress (Figure 3). Applying the coating on the surface reduces its toxicity.
Figure 3: The effect of magnetic nanoparticles on oxidative stress and liver enzymes [3-5].
• They have a long presence in the blood and by using the coating on the surface, the clearance of particles can be delayed and the duration of the presence of blood can be increased.
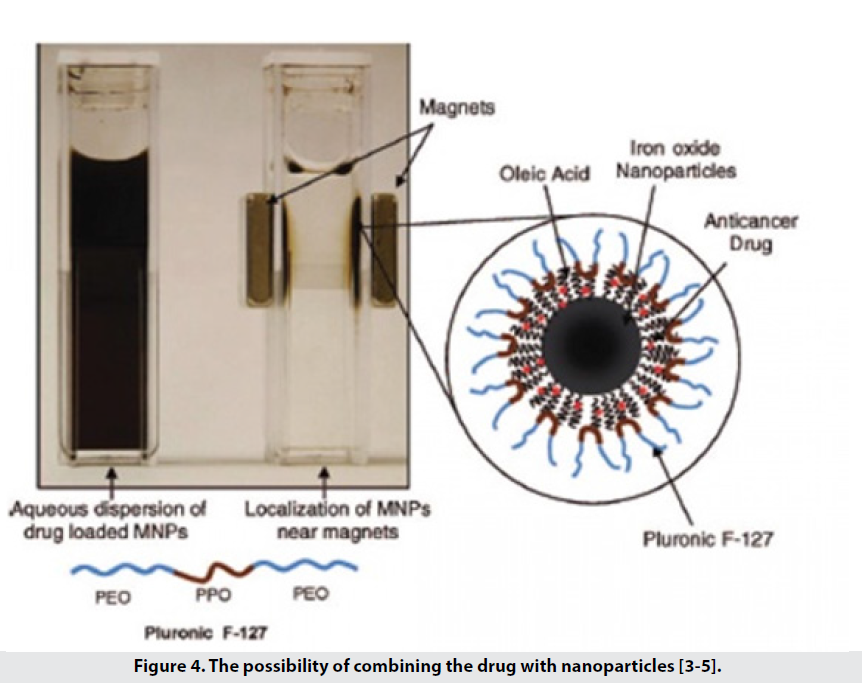
• They are also able to carry hydrophobic drugs such as paclitaxel, doxorubicin to the tissue (Figure 4).
Figure 4: The possibility of combining the drug with nanoparticles [3-5].
• They have good contrast [5,6].
■ How Do Magnetic Nanoparticles Improve Image Contrast
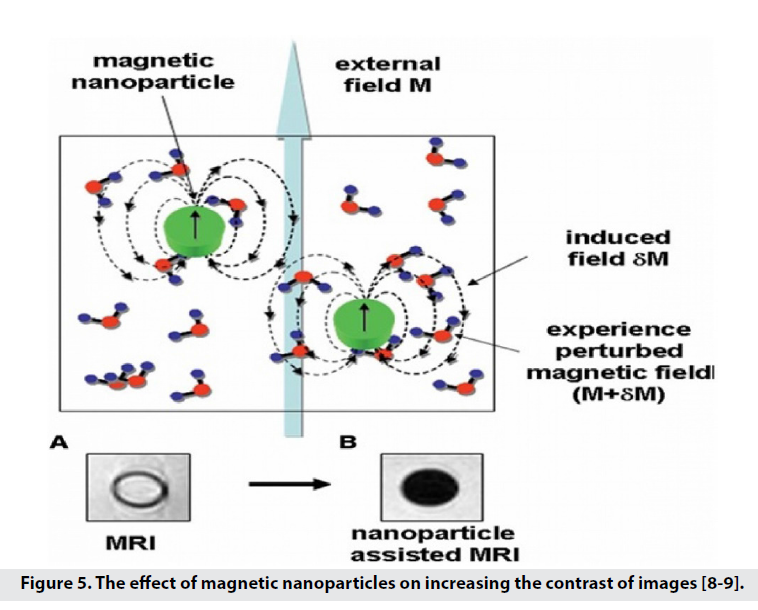
With their small structure, these particles reduce the time of T1 and T2, which leads to darkening of the image background [7-9] and increase the contrast and sensitivity. Super paramagnetic nanoparticles (SPM) in the magnetic field used in MRI, are magnetically saturated and are able to produce a local disruptive dipole field, thus reducing the amount of T1, T2 [3]. The term super paramagnet is derived from the strong paramagnetic nature of particles of this size. When these particles are exposed to an external magnetic field, the momentum of the particles is in the direction of the field and increases the magnetic flux. Therefore, it is not the imaging of SPIO particles but its effect on the longitudinal and transverse comfort of the surrounding nuclei [8-11]. SPIOs are iron oxide particles with superparamagnetic properties that originate in the nanoscale. When particles enter the superparamagnetic range, they lose the hysteresis loop, so they are strongly affected by the external magnetic field. After removing the magnetic field, in order to remove the field residues, Brownian motions cause the SPIOs to randomize. Brownian movements prevent the accumulation of SPIOs due to magnetic attraction [8]. They also increase the specificity of diagnosis and hydrophilicity in MRI applications by coating the surface of nanoparticles with specific functional groups such as monoclonal antibodies and proteins [2]. For these reasons, magnetic nanoparticles and nanocrystals are used to amplify the signal (with properties such as small size, strong magnetism, biocompatibility, and active performance for the receiver) [3]. In 2005, the idea of using superparamagnetic iron oxide nanoparticles (SPIO)3 in MRI was proposed [9]. Magnetic nanoparticle probes for biomedical imaging applications include Nano-sized SPIO cores of magnetite (Fe3O4)4 or magnesite (γ Fe2O3)5 (Figure 5) [8,9].
Figure 5: The effect of magnetic nanoparticles on increasing the contrast of images [8-9].
Molecular Imaging
Expanding molecular imaging technology plays a key role in the research and applied sciences. Molecular imaging is the boundary between life sciences and physics and creates an image at the molecular level by facilitating the interaction of the technique used with the biological structure. Molecular imaging has different definitions and as a non-invasive, quantitative and reproducible method, it provides imaging of the desired macromolecules or biological processes in living organisms. Given that many pathogenic processes are characterized by changes in molecular profiles or changes in cellular behavior before the effects of anatomy, this method [5-9]:
• Ability to diagnose the disease quickly.
• More accurate prediction of the level of the disease and the possibility of treatment by the patient to reduce treatment processes.
• Ability to show the effect of the therapeutic agent and it improves our understanding of the cell's interaction with the environment.
• Therefore, it is predictable that molecular imaging will have a profound effect in both laboratory and therapeutic areas [8].
Various techniques of optical imaging, nuclear imaging and magnetic resonance imaging are the most widely used molecular imaging techniques, which is our discussion of magnetic resonance imaging. MRI provides anatomical and morphological information with high spatial resolution and unlimited penetration depth, which is improved to the cellular and molecular level using contrast enhancers such as MNPs [1,12-14]. Although iron oxide particles have been used as a contrast agent for about 45 years, the development of the synthesis and coating of magnetic nanoparticles in the last decade has increased their applications in biological studies, including blood fusion, as specific cell and tissue contrast agents in imaging. Magnetism has made cell tracking and the detection of biomolecules possible.
■ Properties Required for Nanoparticles in Magnetic Imaging
• High torque and superparamagnetic uniformity.
• High colloidal stability under physiological conditions (high salt concentration, pH changes).
• Ability to escape the reticuloendothelial system.
• Low toxicity and high biocompatibility.
• Functionality for binding to bioactive species such as protein and nucleic acid [1,15-17].
Factors Affecting the Properties of Particles
Quality and properties required for nanoparticles as contrast agents to particulate matter, particle size distribution, particle surface charge, stability in aqueous solution and physiological liquids, spatial chemistry of nanoparticles, optimum magnetic properties and chemical properties of molecules The operator depends on the particle level [1,2].
■ Particle size
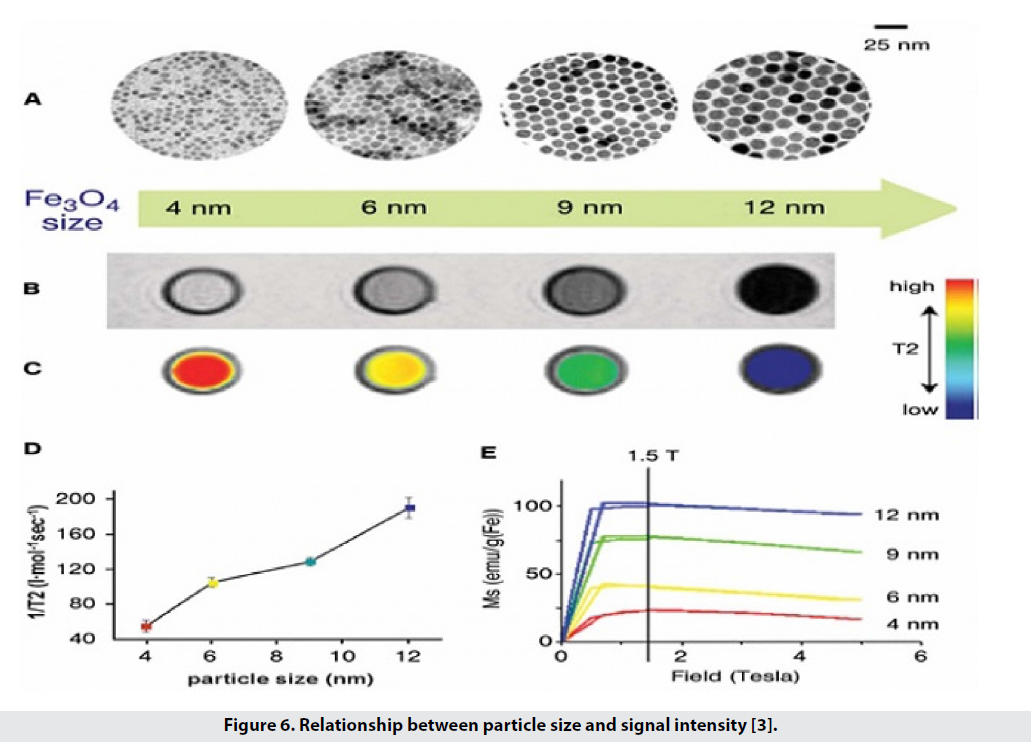
Experiments show that the diameter of magnetic nanoparticles affects the signal intensification and the half-life of the particles in the body. Studies have shown that particles with a diameter of 10 nm have a longer half-life than particles with a diameter of 30nm and larger [3].
γ: Proton gyromagnetic ratio in water, M: Molecularity of nanoparticle solution, r: Particle radius, N: Avogadro number, μ: Magnetic moment of nanoparticles, Ws: Larmore frequency of electron solution and wI: Larmore frequency of water protons [6].
According to Formula 1, there is a relationship between particle size and T2 reduction and therefore image contrast. We have particles with sizes of 12, 9, 6 and 4 nanometers and each has its own magnetic moment, so that under the field T 1.5, the magnetic moment is 102, 80, 43 and 25 emug-1 Fe, respectively. The comfort gradient, or T2 / 1, increases from 56 to 106, 130, and 190, which is the contrast of the image. As the particle size increases from 4 to 12, the color difference from white to black is seen, and as the particle size increases, the intensity of the MRI signals decreases. Figure 6 shows the relationship between signal size and intensity [3]. On the other hand, studies have shown that particles with a diameter of 10 nm have a longer half-life than particles with a diameter of 30 nm and larger [1].
■ Particle classification
Magnetic nanoparticles are classified based on their overall diameter, which includes the core and the coating [18-21]:
• Oral SPIOs with a size between 300 nm and 3.5 μ that are used through the oral route and are often used to image the gastrointestinal tract.
• Standard SPIOs (SSPIO) with a size of 150- 60 nm.
• Very small SPIOs (USPIO) with a size of about 100-40 nm.
• MION iron oxide monocrystalline nanoparticles, which is a subset of USPIO with a size of about 10-30 nm.
• Particles larger than 50 nm that contain multiple crystals.
• Particles smaller than 50 nm are used in molecular imaging in vivo conditions, so SPIOs include all types of MIONT, USPIO, and CLIO (MIONs that cross-link with dextran coatings) [4].
■ Method of preparation of magnetic nanoparticles for use in MRI
Conventional nanoparticle fabrication methods, such as micro emulsions and sol-gels, do not have precise size control and monodispersing. They have relatively weak crystallinity nanoparticles and extensive spatial chemistry compositions. In contrast, methods without water and high temperature conditions show better size control, produce large single crystals, and have good spatial chemistry. The only problem with these methods is the low solubility in water; For this purpose, different coatings are used [1,22-24].
■ Particle coating
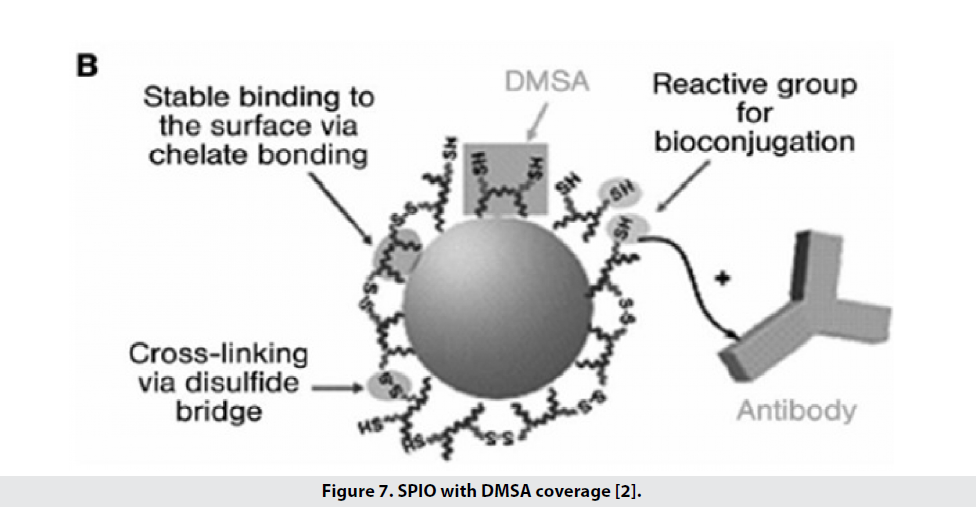
Types of coatings are used to increase biocompatibility, change the surface charge of particles, increase their half-life in the blood and improve the performance and specificity of particles [2]. Types of coatings (Table 1) including bi-functional ligands, polymer, siloxane, phospholipid micelles (which have the ability to bind to Tat peptides for cellular marking) and sometimes 2,3-dimercaptosuccinic acid (DMSA). Is used (Figure 7). DMSA binds carboxylate chelates to iron, creates disulfide crosslinking between ligands, and also places thiol groups on the surface, allowing it to bind to specific and functional markers. These particles have high stability in water and saline phosphate buffer and saline conditions and are therefore a good option for binding to cancer markers in both intrinsic and extrinsic conditions [1]. To improve the biocompatibility of iron oxidebased superparamagnetic (SPM) nanoparticles, the particles are coated with a polymer of dextran that is easily excreted by the liver after treatment [2]. One of the coatings used to increase the hydrophilicity of iron oxide nanoparticles is silica, which has a problem with agglomeration in the blood, which is why they have used dextran instead, which is more stable under physiological conditions. Table 2 shows the types of nanoparticles based on iron oxide that can be used in MRI, mentioning their functional properties.
Coating material |
Advantages |
|---|---|
| Citric,gluconic,oleic | Large SPIO nucleus with thin coating of organophilic composition |
| Dextran | High plasma half-life |
| Polycarboxymethyl dextran | Decreased diameter, increased plasma half-life |
| Polyvinyl alcohol | Long half-life in blood plasma |
| Starches | Biocompatibility, bioavailability and pH stability, with the ability to change the surface |
| PMMA | Carrier of magnetic drug delivery |
| PLGA | Biocompatible coverage approved by the Food and Drug Administration |
| PAM | Matrix with the ability to trap multiple particles |
| PEG | Increased half-life in blood plasma, ability to chemically change the surface |
| PEG-lipid | Thin coatings and bio-extensions available |
| Silane | Reacts to alcohol and silane coupling agents |
| Silica | Neutral and biocompatible coating |
Table 1. Types of coatings used in SPIO 6 [1-2].
Types of Magnetic Nanoparticles Based on Spio
■ Single crystal iron oxide nanoparticles (MION) and cross-linked iron oxide (CLIO)
Smaller iron oxide particles, including monocrystalline iron oxide (MION) or crosslinked iron oxide (CLIO) with dextran coating, have been found to have a longer half-life and greater ability to bind to biologically active molecules. Inner conditions work better. In these particles, the size of the iron oxide center is 2.8nm and the size with dextran coating is about 10-30nm [1].
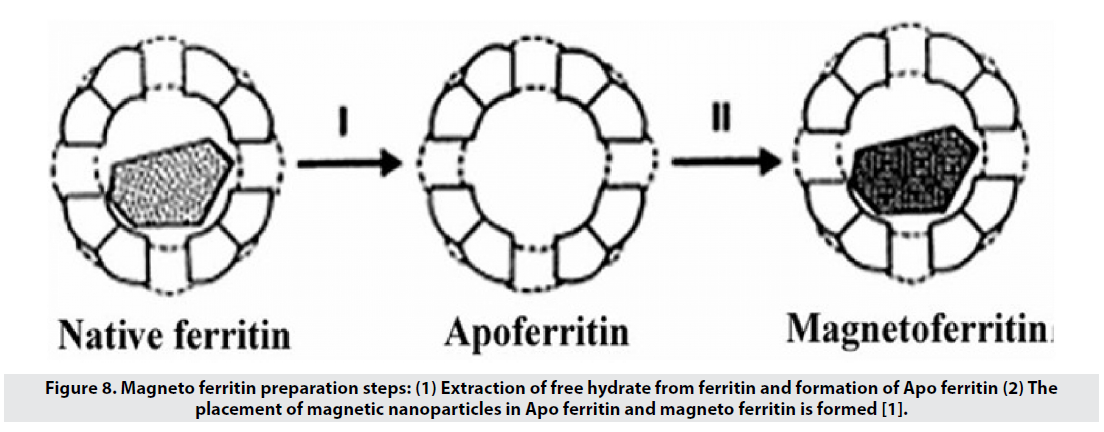
■ Magneto ferritin
Ferritin is an iron-storage protein in the body that has a 6-nm hydrated free nucleus (5Fe2O3- 9H2O) and an Apo ferritin polypeptide shell (Figure 8). Magneto ferritin is also used in imaging and has a T2 tolerance of about 157 L mmol-1 s-1 and is expected to have high biocompatibility and colloidal stability in the blood. However, the results show a clearance of less than 10 minutes for these particles, because they go to the liver, spleen and lymph nodes through the reticuloendothelial system, and therefore are a good option for imaging these organs, but not for molecular imaging [1].
Figure 8: Magneto ferritin preparation steps: (1) Extraction of free hydrate from ferritin and formation of Apo ferritin (2) The placement of magnetic nanoparticles in Apo ferritin and magneto ferritin is formed [1].
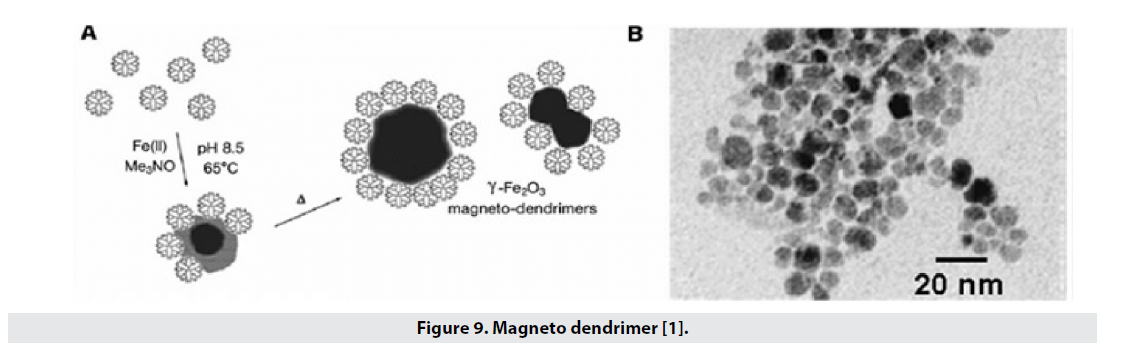
■ Magnetodendrimer
The multifunctional structure due to the single end and pore groups makes dendrimers desirable for drug delivery in the drug delivery, which can similarly carry magnetic nanoparticles; These particles show more magnetic properties (magnetic saturation around emu g_1 Fe 94) and have a high T2 tolerance of 200 to 406 L mmol_1 s_1 and easily penetrate into the cell without the need for a transfer agent (Figure 9). Thus, these factors can be used in cell marking and cell tracking. The size of iron oxide nucleus reaches 7nm-8nm and nucleus with dendrimer coating reaches 20nm-30nm [1,25,26].
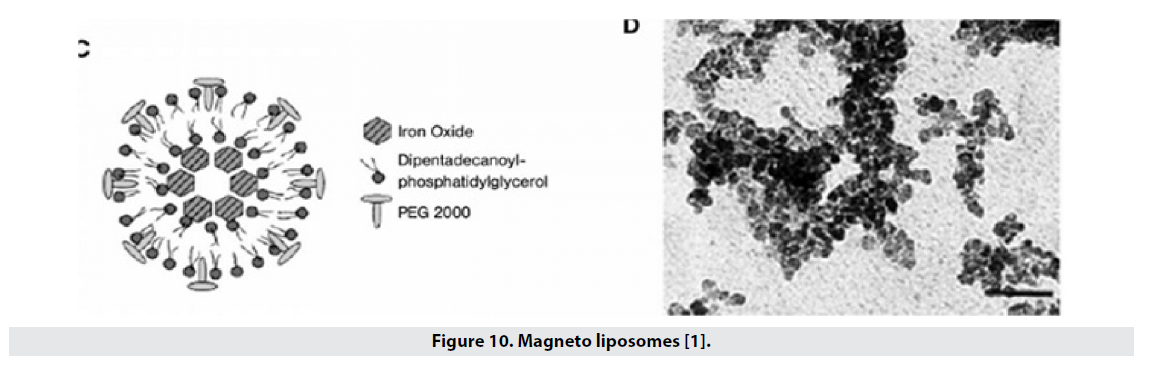
■ Magneto liposomes
Liposomes are also widely used to transport drugs and are a good coating for the hydrophilicity of magnetic nanoparticles (Figure 10). The particles are located in the center of the liposome, the size of the oxide iron nucleus is 16 nm and the particle size in the liposomal state is about 60 nm, and in this structure the comfort of T2 is 240 mmol-1 s-1L [1].
Transfer Magnetic Nanoparticles to Tissues
■ Inactive transfer
In passive transfer, the hydrodynamic diameter and surface charge of SPIOs (which are dependent factors on the surface coating material) overshadow the nanoparticle circulation time, tissue access, opsonization, and cell uptake. The following are cases of passive transmission of magnetic MRI probes [3]. Different pathways of magnetic nanoparticles in different tissues determine the contrast of MRI. Small particles are collected by the reticuloendothelial system. In tumor cells, due to the absence of the reticuloendothelial system, the comfort time does not change despite the MNP contrast agents. Therefore, the detection of lymph node malignancy, liver and brain tumors is possible by comparing the contrast images obtained from MRI with the help of magnetic nanoparticles [3]. SPIO probes lack molecular specificity for imaging biological systems by physiological processes involving nonspecific cell harvest, entrapment within phagocytic macrophages of tumor and inflamed tissues, or accumulation in the spleen, liver, and lymph nodes naturally.
■ Liver and spleen imaging
Research has shown that particles larger than 150 nm with dextran coating are excreted nonspecifically by Kupffer cells in a healthy liver. Due to the absence of Kupffer cells in malignant livers, it is possible to detect healthy and defective tissue [4].
■ Lymph node imaging
The smallest particles, 30 nm in size, are collected by the lymph vessels and clustered in the lymph nodes. Accumulation of SPIO in normal nodes is associated with a decrease in T2, and the absence of SPIOs in MRI images accurately indicates flow disturbance or metastasis of the nodes, thus allowing, without a specific marker, to distinguish between diseased and healthy structures [4]; Thus, a macrophage containing particles produces a black image and the cancerous tissue is clear [1].
Cancer Imaging
Numerous studies have shown that in addition to detecting lymph node metastases, SPIOs can also detect solid tumors directly. These particles can be inactive by leaking from arteries and removing macrophages. The amount of leakage depends on the degree of porosity of the tumor vessels. AMI- 25 is used to examine the bone marrow and highsensitivity bone marrow damage is diagnosed (AIM is a type of magnetic nanoparticle that can be used in MRI, the specifications of which are listed in Table 2) [27-30].
Factor |
Iron oxide core size (nm) | Overall particle size (nanometers) | Covering material | Comfort T2 (L mol-1 S-1) |
Half-life in the blood |
|---|---|---|---|---|---|
| AMI-121(Lumirem; Gastromark) | 10 | 300 | Silicon | 72 | Less than 5 minutes |
| AMI-25 Feridex; Endorm | 5-6 | 80-150 | Dextran | 98 | 6 minutes |
| SHU 555A (Resorvist) | 2-4 | 62 | Carbodextran | 151 | 3.5 minutes |
| AMI-227(Combidex; Sinerem) | 4-6 | 20-40 | Dextran | 53 | Less than 3600 minutes |
Table 2. Types of SPIO shapes with different surface coatings [1-2].
■ Macrophage imaging
MRI in the diagnosis of inflammation and destructive diseases with high macrophage activity is based on the effective removal of particles by macrophages and other phagocytic cells and can identify the site of transplant rejection, the location of atherosclerotic plaques and the possibility of pre-stenosis diagnosis. It certainly brings with it a more desirable treatment. The role of macrophages in diseased tissues of the central nervous system allows imaging to detect stroke, multiple sclerosis, brain tumor, and carotid atherosclerosis. It should be noted that the contrast of images largely depends on the time of blood circulation and the surface charge of the particles. Experience has shown that small particles with a size of 15nm-30nm with a longer circulation time show better contrast at the site of inflammation compared to larger particles [4].
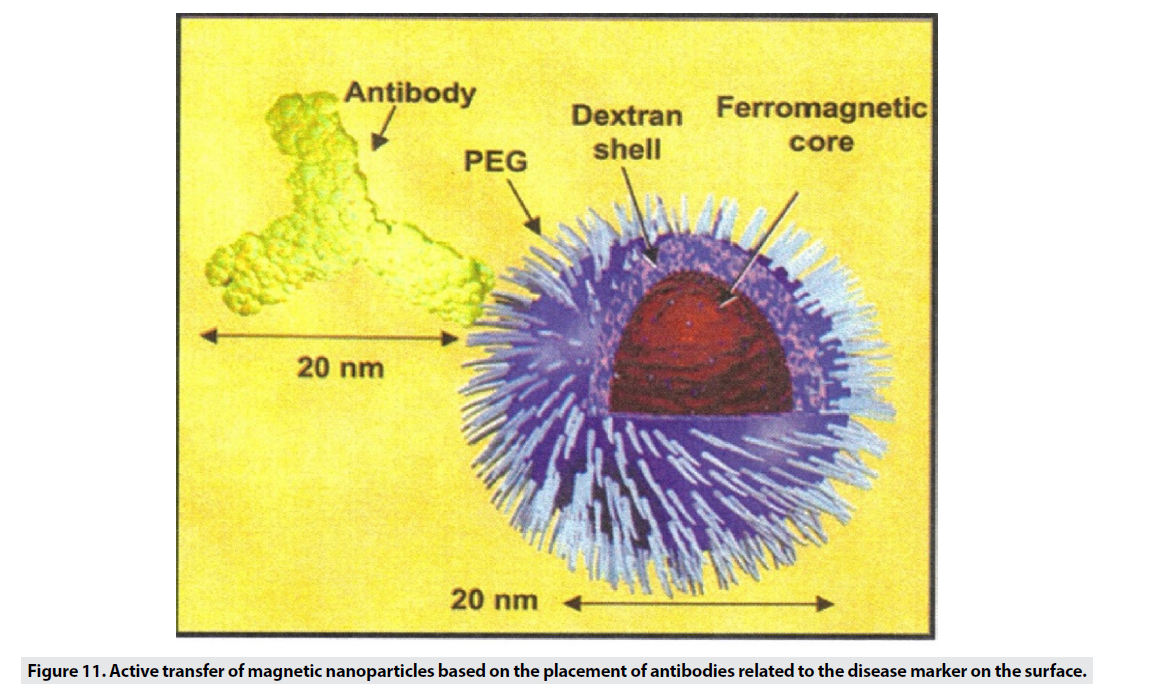
Active Transfer of Superparamagnetic Probes
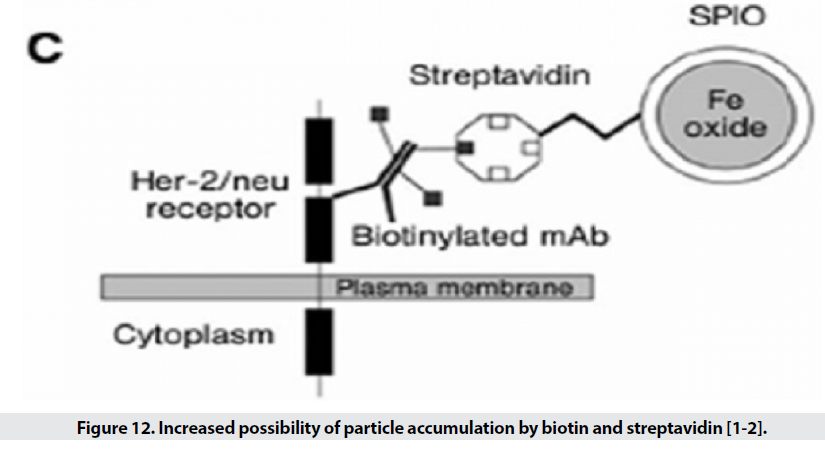
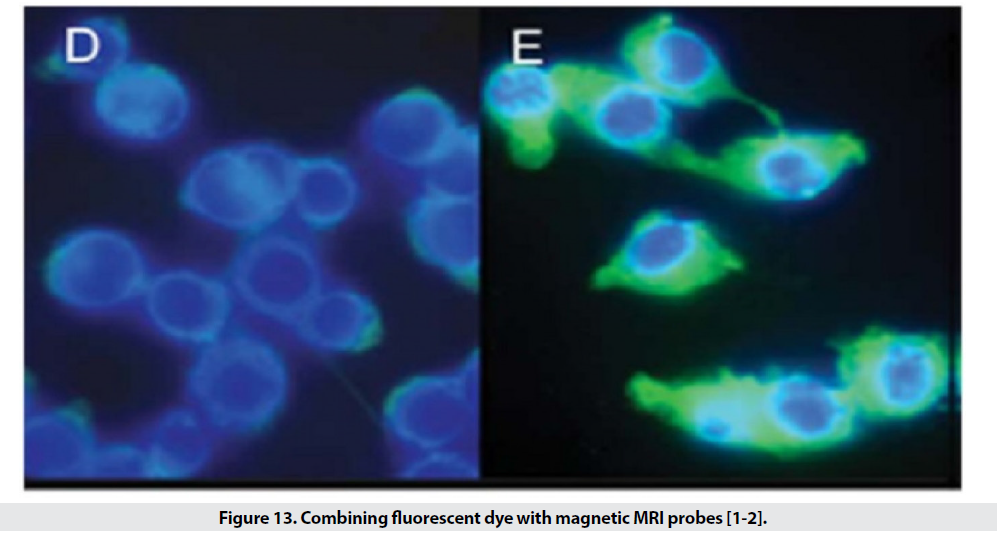
When the particle size is small, it escapes from phagocytic systems, which is why these particles, along with specific markers, are used for targeted transfer (Figure 11) [1]. Targeted transfer is superior to inactive mode because it also increases It contrasts and acquires the ability to detect molecules, which in addition to physiological information, provides insight into the molecular mechanism and leads to faster and more accurate detection. Imaging of inflammation, infarction, angiogenesis, apoptosis, gene expression and cancer has been performed with this method so far. In order to achieve targeted delivery of iron oxide superparamagnetic nanoparticles (SPIO), specific biomarkers of each tissue are required. In the first stage, the agent is attached directly to the hydrophilic coated surface. Polymer coatings have different active functional groups such as amines, sulfhydryl’s and carboxyl and accelerate the conjugation process. In the next step, sufficient accumulation of particles in the tissue is desired. Perhaps the biggest obstacle to targeted transfer is locating the right amount of SPIO at the site of injury to create enough contrast. For this purpose, various techniques are used to increase the accumulation of particles in a particular location. The most commonly used method is intracellular trapping, which is mediated by receptor-mediated harvesting and creates a high level of contrast in the damaged cell. Another method involves two-step amplification methods, such as the use of botanized antibodies that aggregate at disease sites and place the SPIO bound to streptavidin in the environment (Figure 12). Due to the high affinity of biotin and avid in, the particles aggregate at the desired site. MRI probes conjugate to cancer target antibodies and sometimes combine these magnetic probes with fluorescent dyes (Figure 13), which allows inbody and out-of-body studies [2].
Figure 11: Active transfer of magnetic nanoparticles based on the placement of antibodies related to the disease marker on the surface.
■ Cardiovascular imaging
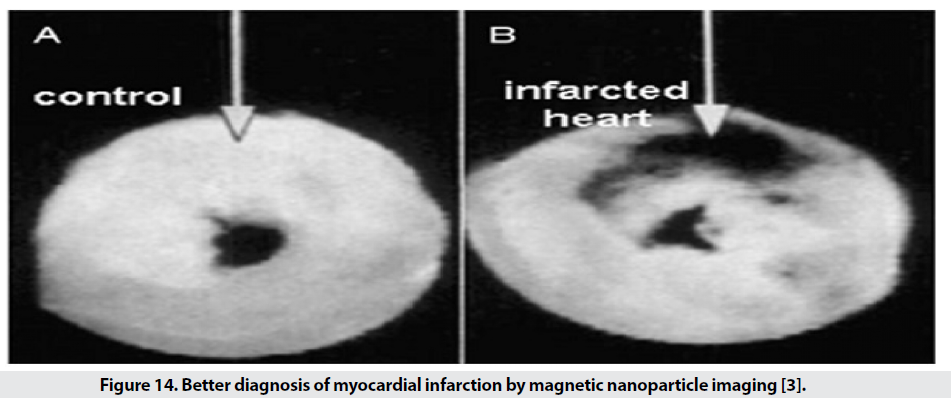
In addition to the possibility of diagnosing apoptosis (programmed cell death) and molecular imaging, it is possible to diagnose various cardiovascular diseases early, including atherosclerosis, thrombosis and myocardial defects. Early detection of atherosclerosis is performed with SPIO targeted with anti- VCAM-1 and with the specific peptide VCAM-1. Another factor used in these studies is E-selectin, a proinflammatory marker for endothelial cells used in atherosclerosis, tumor endothelial proliferation, and angiogenesis. Using SPIO conjugated with human anti-Eselectin, the amount of the mentioned factor in the in vitro culture medium can be displayed by MRI. The diagnosis of thrombosis is based on a specific diagnosis of αIIb-β3- released from activated platelets. Activated platelets are detected by RGD conjugated SPIO RGD, a ring acid peptide consisting of the three amino acids arginine, glycine, and separate, which specifically identify the αIIb-β3 integrin [31,32]. SPIO with RGD for better detection Compared to SPIO, it provides no marker and allows the observation of clots with a diameter of 0.2mm by 0.2mm. Single-crystalline iron oxide (MION) nanoparticles attached to antimyosine based on electrostatic or covalent attraction between antibody lysine groups and potassium periodideactivated surface hydroxyl groups are used to diagnose myocardial infarction (Figure 14). A myocardial infarction cell has a more porous membrane than a normal cell. SPIO conjugated with antimyosine Fab effectively enters the damaged cell and detects myosin and is seen in images of the affected area in black, while in the sample without marker, good contrast is not seen [3].
Figure 14: Better diagnosis of myocardial infarction by magnetic nanoparticle imaging [3].
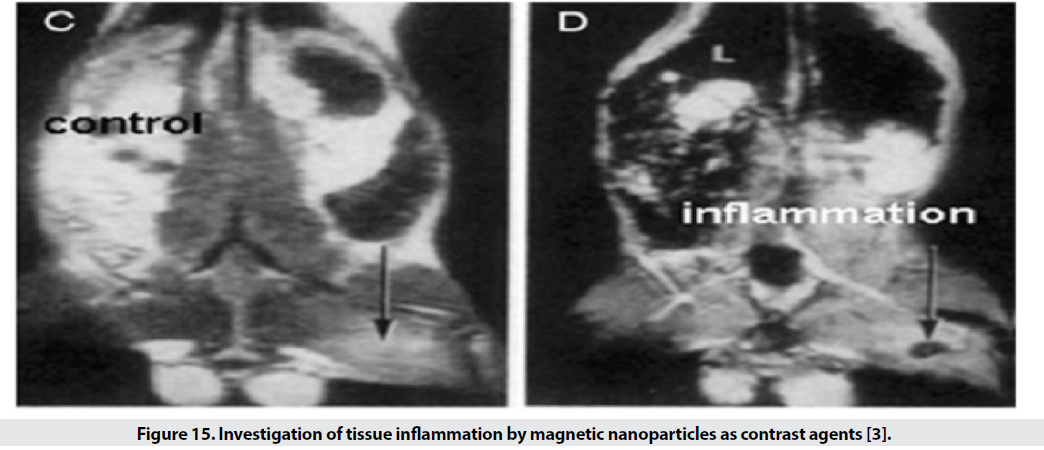
■ Inflammation
For the examination of inflamed tissues, by binding polyclonal human immunoglobulin antibody to the SPIO surface, it is possible to identify the tissue (Figure 15).
Figure 15: Investigation of tissue inflammation by magnetic nanoparticles as contrast agents [3].
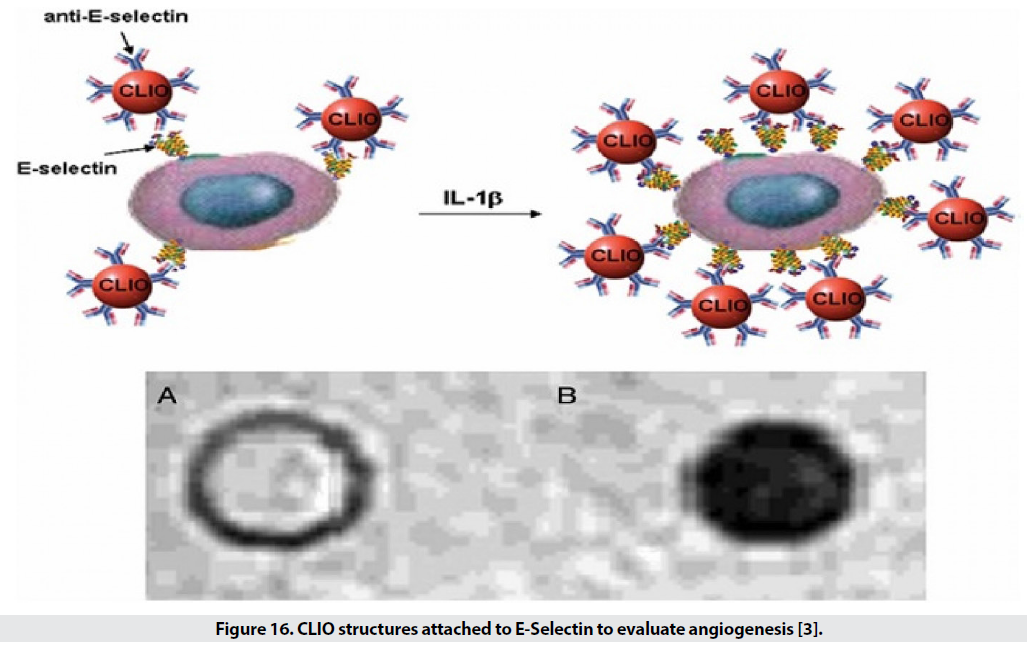
■ Angiogenesis
Angiogenesis is an essential process of growth of new blood vessels for the development, proliferation and repair of tissues, and of course it is an obvious sign of tumor growth. Therefore, the severity of angiogenesis is effective in diagnosing cancer and examining the stages of recovery. Several molecules are involved in angiogenesis including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived endothelial cell growth factor (PD-EDGF), Tie2 receptor, integrin, and E-selectin (Figure 16). Among these, VEGF receptors, Tie2, E-selectin and integrin have been further investigated [3].
■ Apoptosis
Figure 16: CLIO structures attached to E-Selectin to evaluate angiogenesis [3].
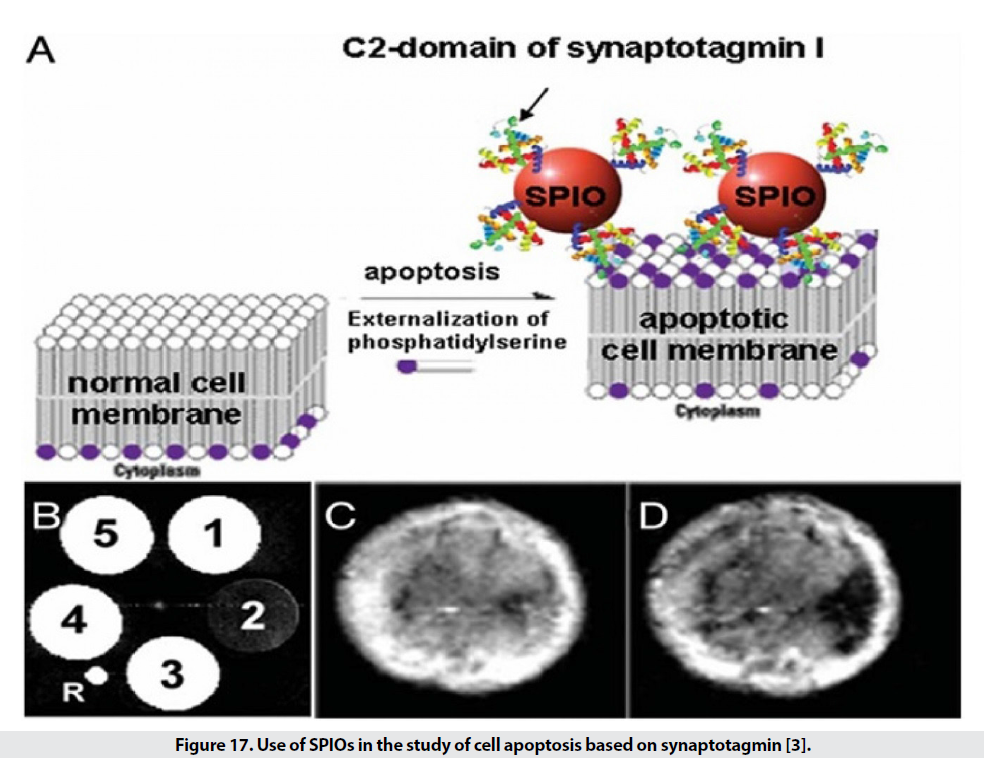
SPIO targeting is also discussed in the diagnosis of apoptosis. Apoptosis is a programmed self-destruction of cells and is seen in the pathogenesis of cancer, nerve tissue destruction, acute myocardial infarction and chronic inflammation, and is also used to demonstrate the effectiveness of the drug. [3]. One of the first steps in this process is the degradation of phosphatidylserine in the cell membrane. Synaptotagmin and annexing are used to identify this stage. The use of SPIOs attached to the C2 region of synaptotagmin detects apoptotic cells (Figure 17) and has a resolution of 0.1 nm, which is 3 times better than conventional methods. Other methods in this section include the use of phospholipids phosphatidylserine and Annexing V. The use of nanoparticles conjugated with Annexing V. detects a concentration of 0.1 gμ in extracorporeal conditions of apoptotic cells [3].
Figure 17: Use of SPIOs in the study of cell apoptosis based on synaptotagmin [3].
■ Gene expression
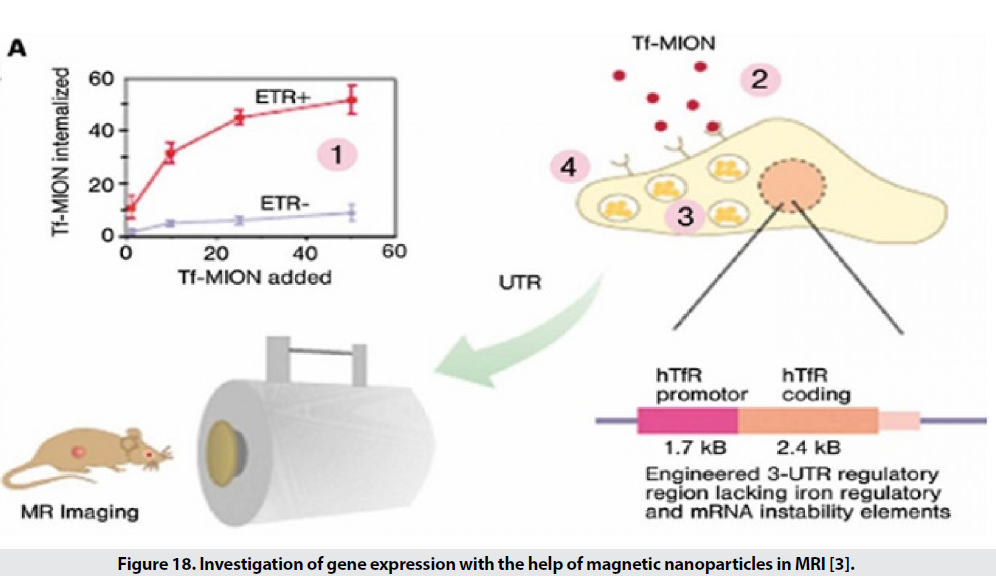
Examining the extent or manner of gene expression is one of the expanding disciplines in the biomedical sciences. There are several methods for studying gene expression, but they have limitations, including low penetration depth in optical imaging and low spatial resolution in radioisotope imaging. For example, they study the expression of transferrin receptor genes in tumor cells. For this purpose, transferrin is placed on the surface of MIONs. If there is a receptor in the environment, these particles are attached (Figure 18). The higher the expression, the greater the decreasing gradient in T2 [29-32].
Figure 18: Investigation of gene expression with the help of magnetic nanoparticles in MRI [3].
Cancer Imaging
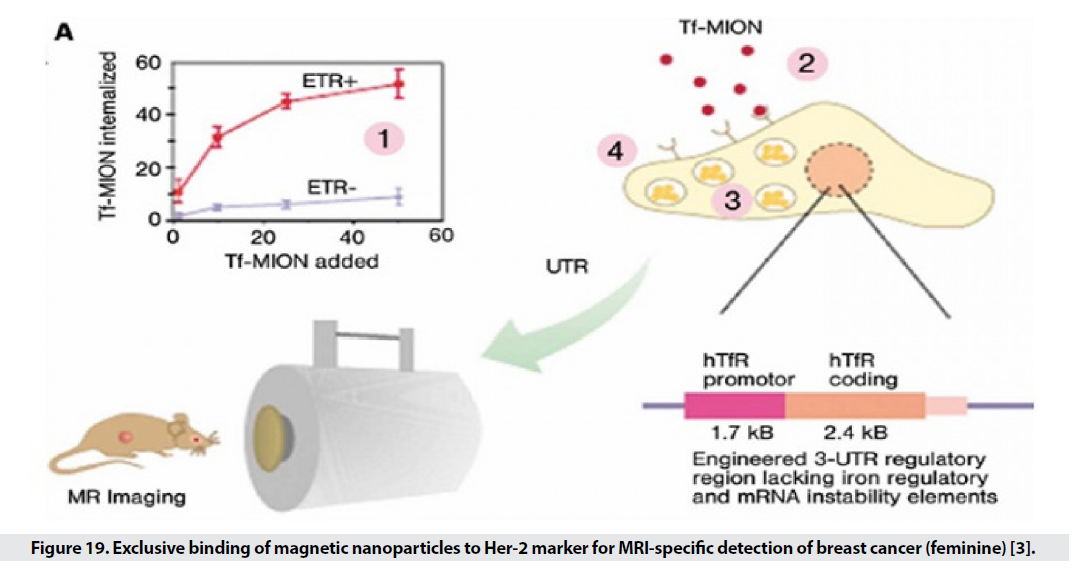
Non-invasive diagnosis of cancer is very significant that can increase the life expectancy of patients. Conventional MRI methods detect a mass of 1 cm, but with the help of nanoparticles, the range of detection can be introduced into molecular and molecular interactions, and very small masses of cancer can be detected from normal tissue. Many cancer markers are targeted by direct ligands of SPIOs. Markers selected for targeted transmission, in addition to having higher expression levels in cancer cells, should allow for higher accumulation within the cell by receptor-mediated endocytosis (Figure 19). One of these markers is transferrin receptors, which have a high expression on the surface of most cancer cells, especially breast cancer. On the other hand, placing folate (folic acid) on the SPIO surface allows rapid and effective removal by cancer cells that have a high level of platelet receptor and shows a 38% reduction in T2 intensity. Another marker identified by SPIOs is the MUC-1 tumor antigen, which is a common cause of many epithelial cell adenocarcinomas and is found in breast, pancreatic, choleric, lung, prostate, and gastric cancers. EPPT1 is tracked. Many markers have been used in this field, including matrix metalloproteinase 2, a membrane-bound endopeptidase that has a high expression level in glioma. Another way to identify cancerous tissue is to target the particles with specific receptors on the surface of the normal cell that are not expressed in the cancer cell. The decrease in T2 in healthy tissues can be seen in the range of tumor tissue. Liver cancer often follows metastases from cancers of the breast, lung, rectum, and clone. The researchers were looking for a specific marker for the contrast agent in MR imaging to characterize liver cancer. The receptor (ASG (asialoglycoprotein is present in normal hepatocytes and is killed in primary cells and the metastatic form of cancer cells. For this purpose, SPIOs bind to arabinogalactan AG), which is a polysaccharide ligand of ASG. MR images show that the use of AG-SPIO in targeted imaging creates a good contrast between normal and cancerous tissues [3].
Figure 19: Exclusive binding of magnetic nanoparticles to Her-2 marker for MRI-specific detection of breast cancer (feminine) [3].
■ Advantages of MRI
• Non-offensive.
• Has a high spatial resolution.
• Has the ability of multidimensional tomography.
• The problem is low sensitivity.
• The ability of modern MRI techniques to distinguish between diseased, tumor, and inflammatory tissues depends on the contrast agent used. Contrast agents that are often used include paramagnetic metal ions such as Mn2 +, Fe 3+, or rare earth chelates such as Gd3+, which have a number of disadvantages [1-3,28-32].
■ Disadvantages of MRI
• Toxicity.
• Free manganese causes problems in various systems of the body including cardiovascular, central nervous system, lung, liver, reproductive and fetal toxicity, and gadolinium is highly toxic to kidney function [5].
• They have a very short time in the body, which makes it difficult to examine.
• Does not have the ability to fully identify the inflamed tissue of atherosclerosis, cancer metastasis and show the healing stages of treatment.
• These agents have a single function and do not have other capabilities such as drug delivery [5].
Conclusion
1. MRI is based on the interaction of radio waves with the sample surface in the presence of a magnetic field, and by receiving and converting the emitted waves from tissue protons, accurate images of the tissue can be obtained. Commonly used contrast agents have a number of disadvantages such as toxicity, low half-life and impossibility of multiple operation. In contrast, magnetic nanoparticles with low toxicity, long half-life and multiple performance, and most importantly better contrast, overtake the ball. They have stolen other contrast factors. The structure of these particles consists of magnetite and marmite cores with a coating of polysaccharide, polymer or monomer. The use of these particles reduces the time T1 and T2 and increases the contrast of the images.
2. In this paper, it was observed that reducing the size of magnetic nanoparticles increases the contrast and half-life of particles, and particles were classified according to size. Preparation of nanoparticles in waterless and high temperature conditions has better control over particle size and crystallinity, but reduces the solubility of the particles. Is present and has led to diversity in nanoparticles. Coating is a very important and effective factor, especially in medical applications, and reduces particle toxicity, accelerates and facilitates particle excretion mechanisms, increases the possibility of functionalizing the particle surface and facilitates antibody binding, and increases the physicochemical stability of particles in the body. Types of nanoparticles that can be used include MION, CLIO, magneto ferritin, magneto dendrimer and magneto liposomes. These particles can be passively transported to tissues, enabling imaging of various tissues such as the liver and spleen, lymph nodes, cancer, and macrophages.
3. Transfer of particles to tissue is active and inactive, which is done according to the characteristics and methods of transfer in each tissue. Active transfer from passive causes more contrast and is more efficient in molecular methods and leads to faster and more accurate diagnosis of the disease. The discussion of hydrophilic coating of particles and how they accumulate in the target tissue is discussed in detail in each section. On the other hand, particle aggregation increases the signal and by providing particle aggregation conditions, a more desirable contrast can be achieved and the detection of oligonucleotides, proteins, enzymes and enantiomers is provided.
Funding Statement
This study did not receive any funding in any form.
Competing Interests
Conflict of interest the authors declare that they have no conflict of interest
Data Availability
Not applicable
References
- https://fa.wikipedia.org/wiki/
- Shi Y. Superparamagnetic Nanoparticle for magnetic Resonance Imaging (MRI) Diagnosis. School of Chemical Engineering. (2006).
- Varadan VK, Chen L, Xie J. Nanomedicine design applications of magnetic Nanomaterials, Nanosensors nanosystems. John Wiley & Sons. (2008).
- Stephen ZR, Kievit FM, Zhang M. Magnetite nanoparticles for medical MR imaging. Mater Today. 14, 330-338 (2011).
- Labhasetwar V, Leslie-Pelecky D, Jain T. Multifunctional Magnetic Nanoparticles for Imaging Drug Delivery. Sci Innov. (2007).
- https://www.rxlist.com/feridex-iv-drug.html
- Mirkin CA, Niemeyer CM. Nanobiotechnology II. Wiley-VCH, Weinheim, As I An J. 2, 1363 (2007).
- Thorek DL, Chen AK, Czupryna J et al. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 34, 23-38 (2006).
- Goodwill PW, Saritas EU, Croft LR et al. X-Space MPI: Magnetic Nanoparticles for Safe Medical Imaging. Adv Mater. 24, 3870-3877 (2012).
- He R, Wang H, Su Y et al. Incorporating 131I into a PAMAM (G5.0) dendrimer-conjugate: Design of a theranostic nanosensor for medullary thyroid carcinoma. RSC Adv. 7, 16181–16188 (2017).
- Mehta JP, Knappett BR, Divitini G et al. Advances in the Synthesis and Long-Term Protection of Zero-Valent Iron Nanoparticles. Part Syst Charact. 35, 1-8 (2018).
- Kalubowilage M, Covarrubias-Zambrano O, Malalasekera AP et al. Early detection of pancreatic cancers in liquid biopsies by ultrasensitive fluorescence nanobiosensors. Nanomedicine. 14, 1823–1832 (2018).
- Chen C, Bao S, Zhang B et al. Coupling Fe@Fe3O4 nanoparticles with multiple-walled carbon nanotubes with width band electromagnetic absorption performance. Appl Surf Sci. 467, 836–843 (2019).
- Bao Y, Sherwood JA, Sun Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J Mater Chem C. 6, 1280–1290 (2018).
- Baker I. Magnetic nanoparticle synthesis. Nanobiomaterials. pp: 197–229 (2018).
- Hajalilou A, Kianvash A, Lavvafi H et al. Nanostructured soft magnetic materials synthesized via mechanical alloying: A review. J Mater Sci Mater Electron. 29, 1690–17 (2018).
- Wang H, Shrestha TB, Basel MT et al. Hexagonal magnetite nanoprisms: Preparation, characterization and cellular uptake. J Mater Chem B. 3, 4647–4653 (2015).
- Konecny AP, Covarrubias J, Wang H. Magnetic Nanoparticle Design and Application in Magnetic Hyperthermia. In Magnetic Nanomaterials. pp: 25-58 (2017).
- Ahmad F, Zhou Y. Pitfalls and Challenges in Nanotoxicology: A Case of Cobalt Ferrite (CoFe2O4) Nanocomposites. Chem Res Toxicol. 30, 492–507 (2017).
- Odio OF, Reguera E. Nanostructured spinel ferrites: Synthesis, functionalization, nanomagnetism and environmental applications. Magnetic Spinels-Synthesis, Properties and Applications. pp: 185–216 (2017).
- Sharma VK, Mitra S, Mukhopadhyay R. Dynamic Landscape in Self-Assembled Surfactant Aggregates. Langmuir. 35, 14151-14172 (2019).
- Lee K, Lee S, Oh MC et al. Alkaline metal reagent-assisted synthesis of monodisperse iron oxide nanostructures. Metals. 8, 107 (2018).
- Ortiz-Quinonez JL, Pal U, Villanueva MS. Structural, Magnetic, and Catalytic Evaluation of Spinel Co, Ni, and Co-Ni Ferrite Nanoparticles Fabricated by Low-Temperature Solution Combustion Process. ACS Omega. 3, 14986–15001 (2018).
- Piche D, Tavernaro I, Fleddermann J et al. Targeted T1 Magnetic Resonance Imaging Contrast Enhancement with Extraordinarily Small CoFe2O4 Nanoparticles. ACS Appl Mater Interfaces. 11, 6724–6740 (2019).
- Sun Y, Zhang J, Zong Y et al. Crystalline-Amorphous Permalloy@Iron Oxide Core-Shell Nanoparticles Decorated on Graphene as High-Efficiency, Lightweight, and Hydrophobic Microwave Absorbents. ACS Appl Mater Interfaces. 11, 6374–6383 (2019).
- Voelz BE, Kalubowilage M, Bossmann SH et al. Associations between activity of arginase or matrix metalloproteinase-8 (MMP-8) and metritis in periparturient dairy cattle. Theriogenology. 97, 83–88 (2017).
- Podaru G, Chikan V. Magnetism in Nanomaterials: Heat and Force from Colloidal Magnetic Particles. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences. 26 (2017).
- Bai YY, Zheng S, Zhang L et al. Non-invasively evaluating therapeutic response of nanorod-mediated photothermal therapy on tumor angiogenesis. J Biomed Nanotechnol. 10, 3351–3360 (2014).
- Jaiswal MK, Pradhan A, Banerjee R et al. Dual pH and temperature stimuli-responsive magnetic nanohydrogels for thermo-chemotherapy. J Nanosci Nanotechnol. 14, 4082–4089 (2014).
- Kalubowilage M, Janik K, Bossmann SH. Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia. Applied Sciences. 9, 2927 (2019).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref