Research Article - Interventional Cardiology (2017) Volume 9, Issue 3
Magnetic resonance imaging in patients with atrial fibrillation before left atrial appendage closure after brain hemorrhage
- Corresponding Author:
- Nighoghossian N
Department of Neurology, Hospices Civils de Lyon
University Lyon 1, France
Tel: 330472357810
Fax: 330472357329
E-mail: norbert.nighoghossian@chu-lyon.fr
Submitted: 06 May 2017 Accepted: 02 June 2017 Published online: 07 June 2017
Abstract
Background: Brain MRI may be helpful for selecting patients at higher risk of further bleeding after a first hemorrhagic stroke in patients with atrial fibrillation treated by oral anticoagulants. MRI may detect imaging markers consistent with an increased risk of further bleeding that could improve patient selection for left atrial appendage closure (LAAC). Method: We studied clinical and imaging data of patients with atrial fibrillation treated by oral anticoagulants who experienced hemorrhagic stroke. MRI was performed for the detection of small vessels disease abnormalities (cerebral amyloid angiopathy (AA) or severe hypertensive microangiopathy) suggesting a higher hemorrhagic risk thus supporting the choice of LAAC closure instead of anticoagulants to prevent thromboembolic events. Results: Between December 2013 and February 2016, 37 patients were included. Among them 25 patients experienced brain hemorrhage and underwent a cerebral MRI before LAAC. 16% (4/25) had severe white matter damage, 24% (6/25) exhibit MRI features consistent with hypertensive microangiopathy/or multiple cortical microbleeds (CMBs) and cortical superficial siderosis (CSS) consistent with AA. 16% (4/25) with mixed anomalies and 11 (44%) had undetermined small vessel disease. Conclusion: Brain MRI after a first hemorrhagic stroke may provide helpful information on the risk of bleeding recurrence before LAAC.
Keywords
Magnetic resonance imaging; Atrial fibrillation; Left atrial appendage closure
Introduction
In atrial fibrillation (AF), anticoagulation by vitamin K antagonists (VKA) or direct oral anticoagulants (DOA) reduces by 65% the risk of cerebral embolic events [1,2]. Although DOA trials showed a decreased incidence of fatal brain hemorrhage, several conditions (e.g. history of cerebral hemorrhage, large infarct size, severe leucoencephalopathy (LA), suspicion of cerebral amyloid angiopathy (CAA) may limit the resumption of anticoagulant treatments [3].
Left atrial appendage closure (LAAC) could be an acceptable alternative to anticoagulation in the prevention of brain embolism in patients with non valvular AF as suggested by several studies [4-9] especially in patients with recent hemorrhagic stroke in whom anticoagulation use may raise concern. Systemic bleeding risk is commonly assessed by the HAS BLED score [10]. However, this scale does not take into account brain imaging. Brain magnetic resonance imaging (MRI) may detect small vessels disease thus providing additional information to assess the risk of bleeding.
Methods
Patients
Between December 2013 and February 2016, 37 patients with nonvalvular AF and a high cardioembolic risk (CHA2DS2- VASc scores >2) were included. Among them 25 patients underwent a cerebral MRI. Risk of hemorrhage was assessed by the HAS BLED score. Functional outcome was evaluated with the modified Rankin scale (mRS) at baseline and all follow-ups. Any serious adverse events (defined by: death, life-threatening event, requiring or prolonging hospitalization, or resulting in persistent or significant disability/incapacity) were recorded.
Imaging protocol
MRI was performed using 1.5T clinical whole body unit with standard head coils. All patients underwent diffusion-weighted imaging (DWI), fluid-attenuated-inversion- recovery (FLAIR), T2-weighted gradient echo (T2*) and time of flight magnetic resonance angiography. Imaging data were independently reviewed by two experienced readers who were blinded to clinical history. MRI markers of small vessel disease were assessed as follows:
1. leucoencephalopathy was defined on FLAIR (or DWI b=0 images whenever FLAIR was unavailable) as hyperintense supratentorial white matter lesions. Leukoariosis was graded according to the Fazekas scale, both in periventricular (0=absent, 1=caps or pencil lining, 2= smooth halo, and 3=irregular periventricular hyperintensities extending into deep white matter) and subcortical areas (0=absent, 1=punctuate foci, 2=beginning confluence of foci, and 3=large confluent areas). The total Fazekas score was calculated by adding the periventricular and subcortical scores [11].
2. Cerebral microbleeds (CMBs) were defined as small perivascular hemosiderin deposits. CMBs appear as areas of signal void on T2*-weighted sequences. The location of CMBs was specified as either deep or lobar.
3. Cortical superficial siderosis (CSS) was defined as hypointense rims on the cortex in T2* images.
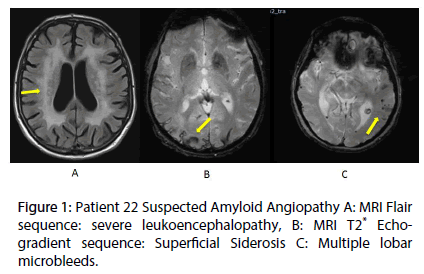
According to MRI findings, patients were divided into three categories: (Figure 1A) suspected AA according to the Boston criteria (Table 1, Figure 1B) [12] hypertensive microangiopathy and C) undetermined small vessel disease.
| Definite AA | Probable AA with supporting pathology | Probable AA | Possible AA |
|---|---|---|---|
| Full postmortem examination demonstrating : - Lobar, cortical, or corticosubcortical hemorrhage - Severe CAA with vasculopathy - Absence of other diagnostic lesion |
Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating: - Lobar, cortical, or corticosubcortical hemorrhage - Some degree of CAA in specimen - Absence of other diagnostic lesion |
Clinical data and MRI or CT demonstrating: - Multiple hemorrhages restricted to lobar, cortical, or corticosubcortical regions (cerebellar hemorrhage allowed) - Age ≥55 years - Absence of other cause of hemorrhage |
Clinical data and MRI or CT demonstrating: - Single lobar, cortical, or corticosubcortical hemorrhage - Age ≥55 years - Absence of other cause of hemorrhage |
Table 1: Boston Criteria for Diagnosis of Cerebral Amyloid Angiopathy-Related Hemorrhage
LAAC procedure and medications
Echocardiographic criteria for LAA closure were an ejection fraction ≥30% and the absence of severe mitral regurgitation, mitral stenosis, or thrombus in the left atrium and/or LAA. Patients with large patent foramen ovale (PFO), defined as PFO with atrial septal aneurysms (total excursion >15 mm or length ≥15 mm) or large shunts (substantial passage of bubbles within 3 beats) were excluded as well. Only patients whose LAA lengths were greater than their ostial diameters were closed with the Watchman LAA closure device. The procedure was performed under general anesthesia and three dimensional echocardiography guidance. Heparin was administered to a recommended active clotting time of 200s to 300s. The optimal compression grade was considered to range between 80% and 92%. A trans-esophageal echocardiography (TEE) was performed at 3 and 12 months to assess device position, peri-device LAA flow, and device-related thrombus. Aspirin (100 mg/day) was started on the day before implantation and was combined to clopidogrel during one month, then continued until 6-months. This work did not require an ethics committee as patient management was considered as routine clinical practice but was conducted according to the World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. All participants provided informed verbal consent or a legally authorized representative In the case of impaired comprehension of oral speech.
Results
The main indication of LAAC was a history of cerebral hemorrhage (20 patients: 6 patients had a lobar intracranial hemorrhage (ICH) and 12 patients had a deep ICH) and 2 had post-traumatic bleeding). MRI revealed that 16%: (4/25) patients had severe white matter damage, 24% (6/25) exhibit abnormalities consistent with hypertensive microangiopathy whereas 16% (4/25) had multiple cortical microbleeds (CMBs) and cortical superficial siderosis (CSS) consistent with AA and 11 (44%) had mixed anomalies.
The baseline clinical characteristics and follow up of these 25 patients are summarized in Table 2. The mean follow-up post-procedure was to 11.2 months ±6. Ischemic stroke recurrence occurred in 3 patients out of 37 patients who underwent LAA (8%).
| Before LAAC | After LAAC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age (y) | sex | CHA2DS2VASC score | HAS BLED score | stroke (nb) | TIA (nb) | Deep basal ganglion ICH (nb) | Lobar ICH (nb) | Other cerebral hemorrhage (nb) |
mRS | contraindication to anticoagulants |
Follow up (months) | Stroke (date after LAAC) |
HIC (date after LAAC) |
|||
| 1 | 60 | M | 4 | 4 | 3 | 1 | 0 | 0 | 1 | 3 | cerebral hemorrhage | 27 | 0 | 0 | ||
| 2 | 66 | M | 3 | 4 | 0 | 0 | 1 | 0 | 0 | 4 | ICH | 28 | 21 months | 0 | ||
| 3 | 79 | M | 5 | 4 | 2 | 0 | 1 | 1 | 0 | 1 | ICH | 24 | 14 months | 0 | ||
| 4 | 85 | M | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 4 | ICH | 10 | 0 | 0 | ||
| 5 | 76 | M | 8 | 4 | 2 | 0 | 0 | 0 | 0 | 4 | severe leucopathy | 13 | 0 | 0 | ||
| 6 | 67 | M | 4 | 4 | 1 | 0 | 1 | 0 | 0 | 1 | ICH | 24 | 0 | 24 months | ||
| 7 | 73 | F | 5 | 5 | 1 | 0 | 1 | 0 | 0 | 2 | ICH | 19 | 0 | 0 | ||
| 8 | 76 | M | 7 | 4 | 1 | 0 | 0 | 1 | 0 | 3 | ICH | 6 | 1 month | 0 | ||
| 9 | 60 | M | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 2 | ICH | 15 | 0 | 0 | ||
| 10 | 65 | M | 3 | 2 | 0 | 0 | 2 | 0 | 0 | 4 | ICH | 14 | 0 | 0 | ||
| 11 | 77 | M | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 1 | ICH | 7 | 0 | 0 | ||
| 12 | 84 | F | 4 | 3 | 0 | 0 | 1 | 0 | 0 | 2 | ICH | 13 | 0 | 0 | ||
| 13 | 77 | F | 5 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | cerebral hemorrhage | 12 | 0 | 0 | ||
| 14 | 57 | F | 4 | 4 | 1 | 2 | 1 | 0 | 0 | 4 | ICH | 11 | 0 | 0 | ||
| 15 | 76 | F | 4 | 4 | 0 | 0 | 1 | 0 | 0 | 2 | ICH | 7 | 0 | 0 | ||
| 16 | 82 | F | 6 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | ICH | 10 | 0 | 0 | ||
| 17 | 63 | M | 4 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | cerebral hemorrhage | 10 | 0 | 0 | ||
| 18 | 69 | M | 4 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | ICH | 6 | 0 | 0 | ||
| 19 | 81 | M | 7 | 4 | 2 | 0 | 1 | 0 | 0 | 3 | ICH | 6 | 0 | 0 | ||
| 20 | 82 | M | 6 | 3 | 2 | 0 | 0 | 0 | 0 | 2 | Suspected AA | 3 | 0 | 0 | ||
| 21 | 73 | F | 5 | 3 | 4 | 0 | 0 | 0 | 0 | 4 | Suspected AA | 3 | 0 | 0 | ||
| 22 | 70 | F | 5 | 3 | 1 | 0 | 0 | 0 | 0 | 4 | hemorrhagic transformation of infarct | 1 | 0 | 0 | ||
| 23 | 81 | F | 7 | 5 | 1 | 0 | 0 | 1 | 0 | 1 | cerebral hemorrhage | 3 | 0 | 0 | ||
| 24 | 83 | F | 6 | 4 | 0 | 0 | 0 | 2 | 0 | 1 | ICH | 3 | 0 | 0 | ||
| 25 | 88 | M | 7 | 4 | 2 | 0 | 0 | 0 | 0 | 2 | hemorrhagic transformation of infarct | 1 | 0 | 0 | ||
Table 2: Baseline clinical characteristics and follow up.
Discussion
Our study showed that MRI provides additional information on bleeding risk through the detection of markers of small vessels vulnerability which may further increase the risk of bleeding in patients treated with oral anticoagulants. Previous studies suggest that LAAC may be an alternative to anticoagulation among patients with AF [5-7]. However, most of these studies only included patients eligible for warfarin treatment, excluding patients with previous ICH. In this setting data are limited; a single study assessed the safety and feasibility of LAAC in patients with AF and previous ICH [13]. This study provided Class III evidence that in patients with a history of previous ICH and AF, percutaneous LAAO is safe and feasible. No ischemic or hemorrhagic stroke occurred during a mean followup of 13.6 months. Conversely, in our study, with a similar follow up of 11 months, ischemic stroke occurred in three cases (11%) and intracranial hemorrhage in one case. However due to the limited sample size no conclusion can be drawn concerning the risk/benefit of this procedure after brain hemorrhage.
There is a lack of evidence on the optimal prevention of ischemic stroke and other thrombo-embolic events in patients with non-valvular (AF) and recent ICH related to oral anticoagulants. However DOAs have not been tested in patients with AF and a recent ICH. An ongoing trial: “Apixaban Versus Antiplatelet Drugs or no Antithrombotic Drugs after anticoagulation-associated Intracerebral Hemorrhage in patients with atrial fibrillation (APACHE-AF) ClinicalTrials.gov NCT02565693 may provide helpful information in this setting.
Brain MRI before LAA may be helpful for selecting patients at higher risk of further bleeding after brain hemorrhage [14] as the presence of cerebral CMBs and severe LA are recognized as markers of bleeding risk [16-18].
The consensus view is that cortical CMBs tend to be manifestations of CAA, while deep hemispheric lesions are suggestive of hypertensive microangiopathy [18]. CAA-related ICH is classically diagnosed in adults over 55 years of age who had a lobar ICH and at least one other lobar CMBs or superficial siderosis and no other causative vascular or parenchymal pathologies. ICH related to hypertensive microangiopathy is seated in deep brain locations such as the basal ganglia, thalamus, or pons, mostly in patients who have a long-standing hypertension. The differentiation of these two most common types of ICH is very important in predicting the risk of recurrent ICH. Patients who suffer from CAA-related ICH have a 9-10% annual risk of ICH recurrence, compared to a ~2% risk per year in survivors of deep ICH likely related to hypertensive microangiopathy [18].
CAA may be considered as a contraindication to anticoagulation before or after the occurrence of a lobar ICH but without obvious evidence from clinical trials. However patients with suspected CAA on MRI are usually considered for LAAC. The situation is less clear in patients with hypertensive microangiopathy.
Although small vessels disease may promote further bleeding, additional prospective studies are required to delineate the role of MRI in guiding therapeutic decisions between oral anticoagulants and LAAC after brain hemorrhage.
References
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 7: 492–501 (1999).
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 9921: 955-962 (2014).
- O’Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am. Heart. J. 167: 601–609 (2014).
- Holmes DR. Atrial fibrillation and stroke management: present and future. Semin. Neurol.30: 528-536 (2010).
- Holmes DR, Reddy VY, Turi ZG, et al. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 9689:534-542 (2009).
- Belgaid DR, Khan Z, Zaidi M, Hobbs A. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. Int. J. Cardiol. 219:177-179 (2016).
- Holmes DR, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J. Am. Coll. Cardiol. 65: 2614–2623 (2015).
- Reddy VY, Sievert H, Halperin J, et al.Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 312: 1988–1998 (2014).
- Sharma D, Reddy VY, Sandri M, et al. Left Atrial Appendage Closure in Patients With Contraindications to Oral Anticoagulation. J. Am. Coll. Cardiol.18:2190-2192 (2016).
- Pisters R, Lane DA, Nieuwlaat R,et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest.5:1093-1100 (2010).
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR.Am. J. Roentgenol. 149:351–356 (1987).
- Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 56:537–539 (2001).
- Horstmann S, Zugck C, Krumsdorf U, et al. Left atrial appendage occlusion in atrial fibrillation after intracranial hemorrhage. Neurology. 82(2):135-138 (2014).
- Fisher M. MRI screening for chronic anticoagulation in atrial fibrillation. Front. Neurol. 4:137 (2013).
- Poels MM, Vernooij MW, Ikram MA,et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 41:S103–S106 (2010).
- Charidimou A, Boulouis G, Haley KE, et al. White matter hyperintensity pattern in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 6:505–511 (2016).
- Haley KE, Greenberg SM, Gurol ME. Cerebral microbleeds and macrobleeds: should they influence our recommendations for antithrombotic therapies? Curr. Cardiol. Rep. 12:425 (2013).
- Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? a decision analysis. Stroke. 7:1710–1716 (2003).