Review Article - Imaging in Medicine (2013) Volume 5, Issue 1
Mammography of breast calcifications
Malak Itani1, Aaron T Griffin2and Gary J Whitman*3
1Department of Diagnostic Radiology, American University of Beirut Medical Center, Beirut, Lebanon
2Texas A&M University, College Station, TX 77840, USA
3Department of Diagnostic Radiology and Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- *Corresponding Author:
- Gary J Whitman
Department of Diagnostic Radiology and Department of Radiation Oncology
The University of Texas MD Anderson Cancer Center
Houston, TX, USA
Tel: +1 713 745 3520
Fax:+1 713 563 9779
E-mail: gwhitman@mdanderson.org
Abstract
Keywords
benign breast calcification; BI-RADS®; breast biopsy; breast calcification; stereotactic breast biopsy
Breast imaging is one of the fastest growing fields in radiology. The number of mammograms has markedly increased over the last decade. The increase in the number of mammograms has led to an increase in the number of well-trained radiologists required to perform mammographic interpretations. Calcifications are identified in approximately half of all mammograms, and although most of the calcifications are benign, many cancers are only detected by the presence of calcifications. It is important for the radiologist to be aware of the different types of breast calcifications and to provide appropriate management recommendations. Breast calcifications can be produced from cell secretions or from necrotic cellular debris, and thus they may be intramammary, within or around ducts, within lobules, in vascular structures, in interlobular connective tissues or fat, or in the skin. An associated mass or an area of architectural distortion may or may not be detected on imaging studies.
Breast calcifications detected by mammography are classified as being benign, of intermediate concern or malignant. The classification depends on several characteristics of the calcifications, including morphology, distribution, size, number and stability. After the calcifications have been classified according to these characteristics, the radiologist’s decision regarding further management will be made according to the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS®). BI-RADS was established by the ACR in order to standardize mammography reporting, reduce confusion in breast imaging interpretations and facilitate outcome monitoring. The BI-RADS system assigns each breast imaging study an assessment category (Box 1) [1].

BI-RADS has specified selected terminology for different findings on breast imaging studies, including lexicons for mammography, breast ultrasound and MRI. In this article, the BI-RADS system is used as an outline as various types of breast calcifications are described [1]. This is followed by a discussion of management of breast calcifications.
Morphology of breast calcifications
Each characteristic of breast calcifications should be studied carefully. Special attention should be paid to the morphology of the calcifications. If the calcifications are not typically benign and they are not highly suspicious for malignancy, the calcifications can be placed in the intermediate concern category.
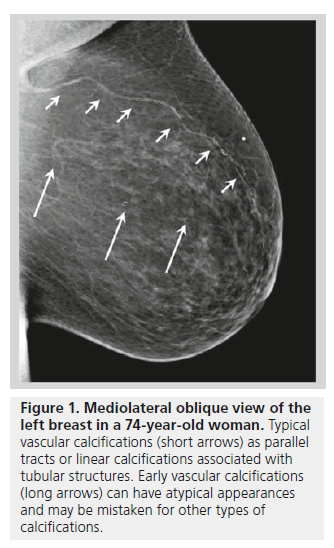
Benign calcifications frequently identified on mammograms include vascular and skin calcifications. Vascular calcifications typically appear as parallel or linear calcifications associated with tubular structures (Figure 1) [2]. Vascular calcifications in the breast affect the composition of the arterioles [3]. Arterial calcifications are present in 9.1% of women on screening mammograms, and arterial calcifications are rarely observed in patients younger than 50 years of age. The relationship between breast arterial calcifications and atherosclerosis risk remains debatable, although patients with chronic kidney disease have been found to have a higher incidence of arterial calcifications on mammography [4,5].
Figure 1. Mediolateral oblique view of the left breast in a 74-year-old woman. Typical vascular calcifications (short arrows) as parallel tracts or linear calcifications associated with tubular structures. Early vascular calcifications (long arrows) can have atypical appearances and may be mistaken for other types of calcifications.
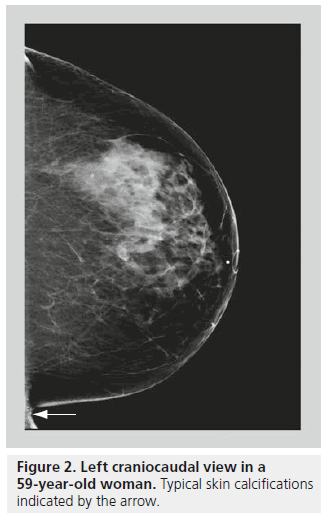
Skin calcifications are usually relatively large, round calcifications in the range of a few millimeters, with lucent centers [6]. Skin calcifications are most commonly seen along the inframammary folds, parasternally, or in the axillary or the areolar regions (Figure 2). If there is any suspicion regarding calcifications being in the skin, the location may be confirmed with tangential views or a skin localization procedure.
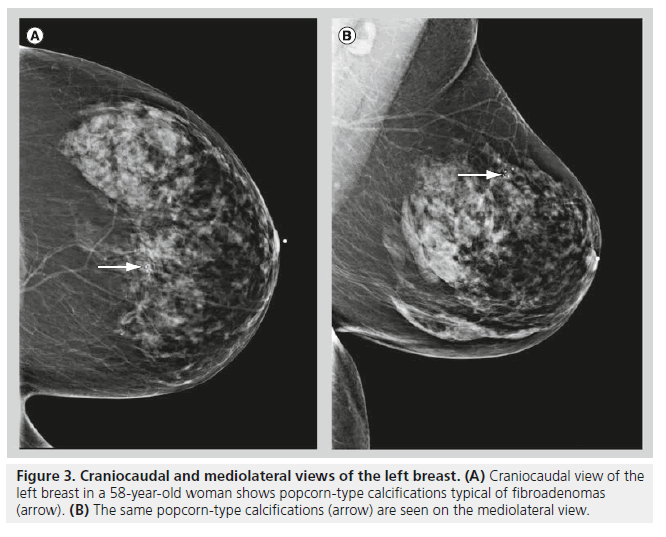
Although fibroadenomas are the most common breast lesions in adolescents and young women, fibroadenomas may also be seen in postmenopausal women. Calcifications initially begin in fibroadenomas as small, peripheral calcifications, they then coalesce over time to form typical coarse, popcorn-like calcifications (Figure 3). Fibroadenomas are often hormone responsive. Hormone responsiveness explains why fibroadenomas grow during pregnancy or with hormone replacement therapy, and involute after menopause. Involuting fibroadenomas typically produce large calcifications (>2–3 mm in diameter), and these calcifications often have a characteristic appearance on mammography. It is when fibroadenomas are in the early calcifying phase that their appearance on mammography becomes confusing, often leading to an indeterminate classification.
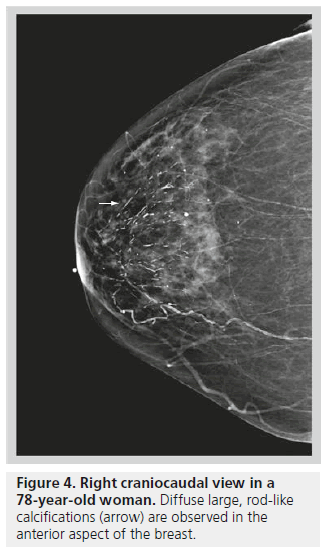
Large rod-like or secretory calcifications are benign. These are usually solid or discontinuous smooth linear rods that are ≥1 mm in diameter and secondary to ductal ectasia or plasma cell mastitis [7]. These calcifications can be intraductal but are more commonly periductal. Benign secretory calcifications follow a ductal distribution, tend to be retroareolar and branch out along the ductal system (Figure 4).
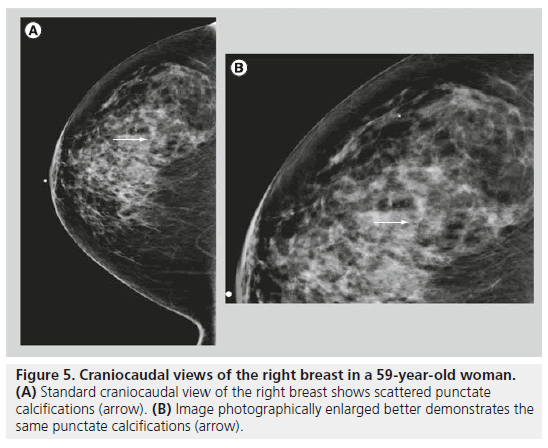
Round calcif ications are smooth, well circumscribed and occur within the lobules (Figure 5). Round calcifications are typically benign. They vary in size, and are called punctate when they are smaller than 0.5 mm. Punctate calcifications are distinct, with a round, pearl-like and uniform appearance. Punctate calcifications are often associated with fibrocystic changes, and they are often diffuse and scattered in both breasts.
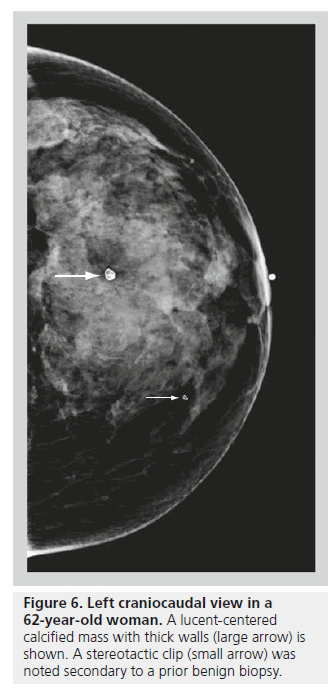
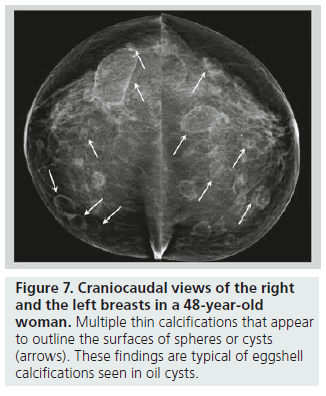
Lucent-centered calcifications have a wide variation in size, ranging from less than 1 mm to more than 1 cm. They typically have smooth, round surfaces, and are often observed secondary to fat necrosis or calcified debris in ducts. Lucentcentered calcifications have thicker walls than eggshell calcifications (Figure 6). Both types of calcifications are stromal in origin. Eggshell or rim calcifications are thin calcifications that surround all, or part, of the margin of a mass. They represent calcium deposited on the surface of a sphere, and are found in the walls of cysts. Eggshell calcifications are usually less than 1 mm in thickness (thinner than lucent-centered calcifications), and they can be observed with fat necrosis or oil cysts (Figure 7).
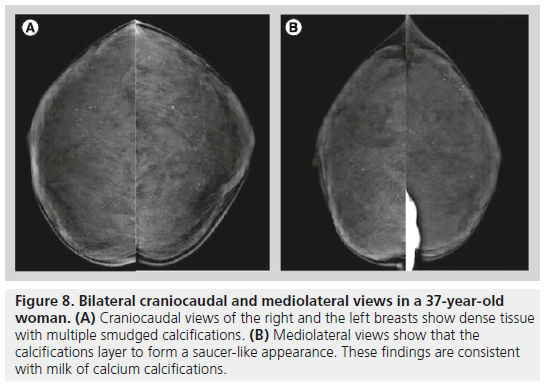
Milk of calcium has a specific morphology, enabling radiologists to determine its benign nature. Milk of calcium represents sedimental calcifications in small cysts. These calcifications are amorphous, fuzzy or smudgy on the craniocaudal view, and on the mediolateral or the lateromedial view, they layer in the dependent portions of cysts, appearing as semilunar, crescent-shaped or curvilinear calcifications (Figure 8). This change in shape on different mammographic projections is characteristic of milk of calcium.
Figure 8.Bilateral craniocaudal and mediolateral views in a 37-year-old woman. (A) Craniocaudal views of the right and the left breasts show dense tissue with multiple smudged calcifications. (B) Mediolateral views show that the calcifications layer to form a saucer-like appearance. These findings are consistent with milk of calcium calcifications.
Suture calcif ications represent calcium deposited on suture material, and suture calcifications are encountered in irradiated breasts and, occasionally, in postsurgical breasts [8]. Suture calcifications are linear, tubular, and/or knot-like. Dystrophic calcifications occasionally form following breast irradiation, surgery or trauma, and they are located in the stromal or fatty parts of the breast. Dystrophic calcifications are irregular in shape, coarse and large (usually larger than 0.5 mm in size). Dystrophic calcifications often have lucent centers and are typically benign. Unusual causes of non-malignant calcifications include calcifications due to silicone or paraffin injections, venous calcifications in Mondor disease, and parasitic calcifications as observed in individuals with filariasis, onchocerciasis, loiasis and trichinosis [9].
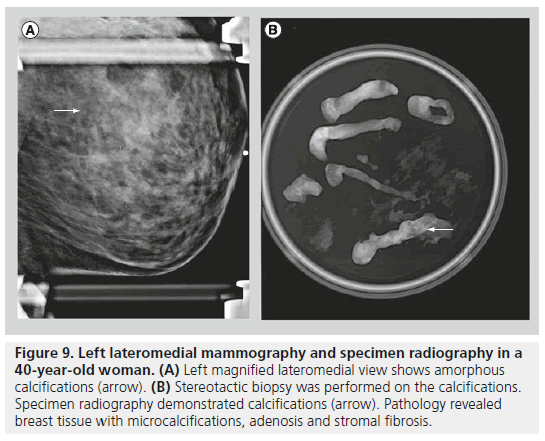
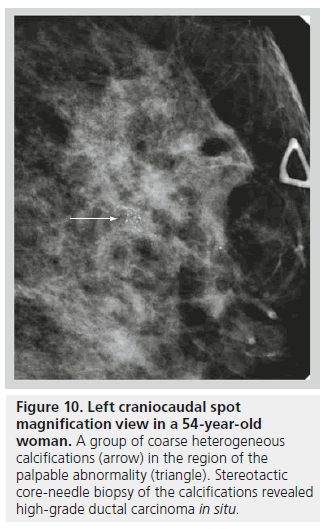
The second class defined under the BI-RADS lexicon describing calcifications is calcifications of intermediate concern. This category includes amorphous or indistinct calcifications and coarse heterogeneous calcifications. Amorphous or indistinct calcifications are sufficiently small or hazy in appearance, meaning that a specific morphology cannot be determined (Figure 9). Amorphous or indistinct calcifications account for the majority of stereotactic core needle biopsies generated due to suspicious calcifications on mammography. Amorphous or indistinct calcifications are usually associated with fibrocystic changes, but they can be seen with ductal carcinoma in situ (DCIS). When assessing amorphous calcifications, other features, most importantly distribution and stability, have substantial weight in guiding management decisions. Coarse heterogeneous calcifications are irregular, conspicuous calcifications, and are generally larger than 0.5 mm (Figure 10). Coarse heterogeneous calcifications are classified in the intermediate concern category since they can be seen with DCIS. These types of calcifications tend to coalesce. As with amorphous calcifications, distribution and stability play leading roles in classifying these calcifications.
Figure 9.Left lateromedial mammography and specimen radiography in a 40-year-old woman. (A) Left magnified lateromedial view shows amorphous calcifications (arrow). (B) Stereotactic biopsy was performed on the calcifications. Specimen radiography demonstrated calcifications (arrow). Pathology revealed breast tissue with microcalcifications, adenosis and stromal fibrosis
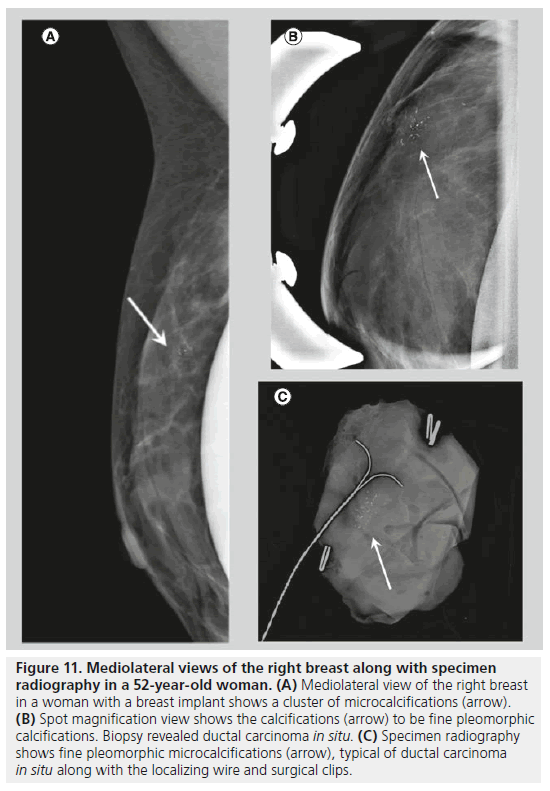
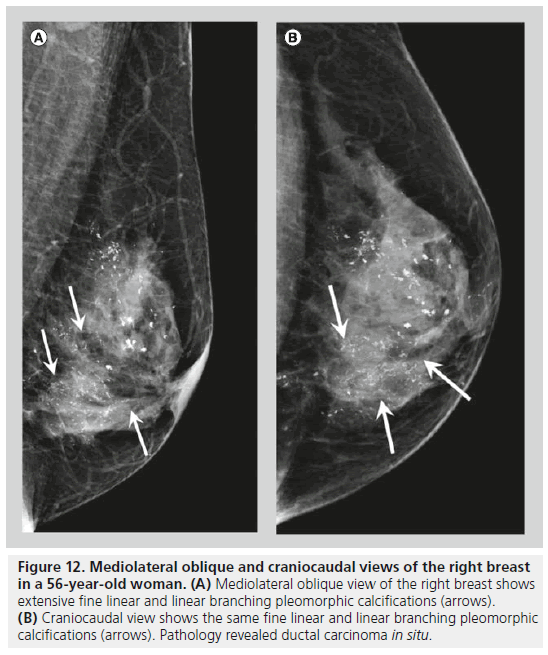
Two forms of calcifications, fine pleomorphic calcifications and fine linear or linear branching calcifications, are classified as calcifications with a high probability of malignancy. Mammographically-detected calcifications are the only presenting feature of DCIS in 90% of cases [10]. The presence of calcifications in DCIS is thought to be due to active secretion by epithelial cells and calcification of necrotic debris in comedocarcinoma. Comedocarcinoma is a highgrade DCIS and noncomedocarcinoma is a lowto intermediate-grade DCIS. Fine pleomorphic calcifications (Figure 11) are smaller than 0.5 mm in diameter and vary in size and shape; they are irregular and more conspicuous than amorphous calcifications. Pleomorphic calcifications can be seen with different grades of DCIS [11], with invasive ductal carcinoma [12], as well as with other benign conditions such as fibrocystic changes, fibroadenomas and papillomas. Fine linear or linear branching calcifications (Figure 12) are more specific for malignancy, as their appearance is suggestive of a duct lumen that is filled with calcified necrotic debris. Fine linear or linear branching calcifications are thin, linear or curvilinear irregular calcifications, which may be discontinuous and smaller than 0.5 mm in width. Fine linear and linear branching calcifications suggest irregular involvement of a duct by DCIS, usually comedocarcinoma.
Figure 11.Mediolateral views of the right breast along with specimen radiography in a 52-year-old woman. (A) Mediolateral view of the right breast in a woman with a breast implant shows a cluster of microcalcifications (arrow). (B) Spot magnification view shows the calcifications (arrow) to be fine pleomorphic calcifications. Biopsy revealed ductal carcinoma in situ. (C) Specimen radiography shows fine pleomorphic microcalcifications (arrow), typical of ductal carcinoma in situ along with the localizing wire and surgical clips.
Figure 12.Mediolateral oblique and craniocaudal views of the right breast in a 56-year-old woman. (A) Mediolateral oblique view of the right breast shows extensive fine linear and linear branching pleomorphic calcifications (arrows). (B) Craniocaudal view shows the same fine linear and linear branching pleomorphic calcifications (arrows). Pathology revealed ductal carcinoma in situ.
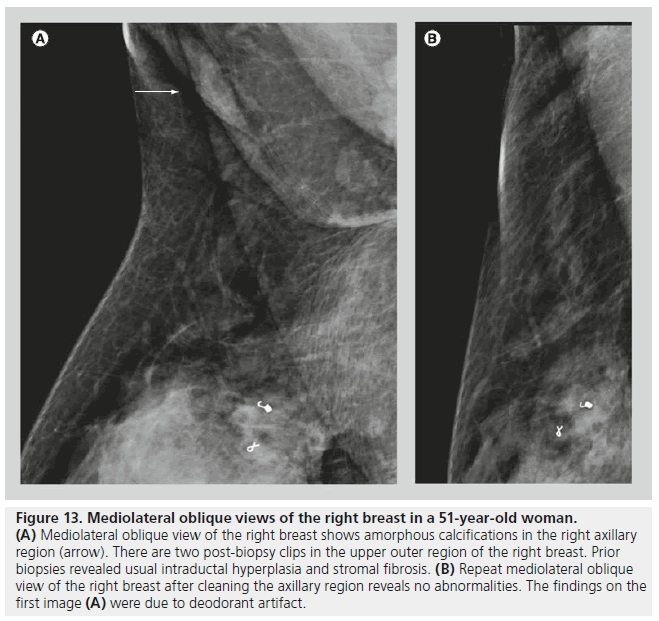
Artifacts or ‘pseudocalcif ications’ are occasionally encountered on mammograms. These findings can be related to technical factors, such as artifacts from the cassette, dust or dirt, or fingerprints. It is important to inform the patient to wipe off creams and deodorant from their skin before imaging (Figure 13). It is also important to note on the imaging request, or on any form of communication between the technologist and the radiologist, the presence of skin lesions, markings, scars or tattoos. Calcifications from a tattoo may appear as a cluster of calcifications that maintain a fixed and reproducible relationship to each other, while breast calcifications tend to change in relation to each other with different compression forces due to the compressibility of the breast parenchyma and the interspersed fatty tissue [13].
Figure 13.Mediolateral oblique views of the right breast in a 51-year-old woman. (A) Mediolateral oblique view of the right breast shows amorphous calcifications in the right axillary region (arrow). There are two post-biopsy clips in the upper outer region of the right breast. Prior biopsies revealed usual intraductal hyperplasia and stromal fibrosis. (B) Repeat mediolateral oblique view of the right breast after cleaning the axillary region reveals no abnormalities. The findings on the first image (A) were due to deodorant artifact.
Distribution of breast calcifications
Frequently, the morphology of breast calcifications is not conclusive for a BI-RADS category; in such cases, the distribution is a key factor in directing the radiologist’s decision towards a benign or a malignant classification and in guiding the management towards a conservative or an aggressive approach. The distribution of calcifications is particularly important with amorphous or indistinct calcifications.
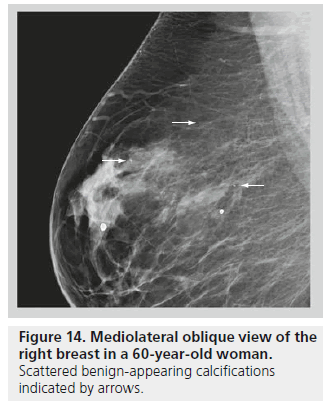
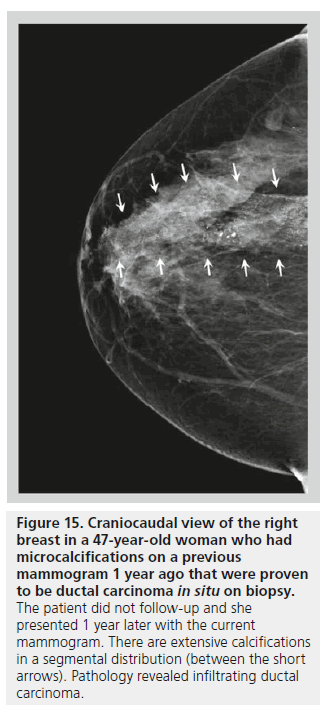
The distribution of breast calcifications can be diffuse or scattered, regional, clustered or grouped, linear, or segmental. Diffuse or scattered calcifications are seen throughout the breast parenchyma, and are indicative of a benign process. Calcifications may be diffuse or clustered in scattered foci in the breast (Figure 14). Diffuse calcifications are often round or punctate calcifications that are lobular in origin. Calcifications in a regional distribution are often benign. Regional calcifications are scattered in a volume of the breast (typically in a volume greater than 2 cc) that does not follow a ductal distribution. On the other hand, segmental calcifications are scattered in an area of the breast (also more than 2 cc in volume) that confines to the distribution of a duct, in keeping with calcium deposits in branches or segments of one segment or one lobe (Figure 15), and this distribution is suggestive of a malignant etiology. Likewise, a linear distribution is associated with a high probability of malignancy. Care should be taken to differentiate early benign vascular calcifications from linear ductal calcifications due to DCIS. When at least five calcifications are confined within 1 cm3 of tissue, they are described as clustered or grouped [14]. As mentioned earlier, if there are multiple foci of clustered calcifications, the calcifications are usually not worrisome; however, if there is a single area of clustered calcifications, then the calcifications may be malignant, and a biopsy may be required.
Figure 15.Craniocaudal view of the right breast in a 47-year-old woman who had microcalcifications on a previous mammogram 1 year ago that were proven to be ductal carcinoma in situ on biopsy. The patient did not follow-up and she presented 1 year later with the current mammogram. There are extensive calcifications in a segmental distribution (between the short arrows). Pathology revealed infiltrating ductal carcinoma.
Other characteristics of breast calcifications
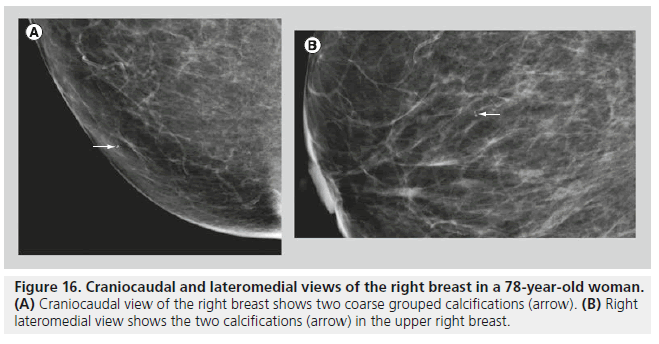
In most cases, the morphology and distribution of the calcifications are sufficient to guide the radiologist in making a management decision. When the morphology and the distribution are indeterminate, size, number (Figure 16) and stability of the calcifications may influence management decisions. Benign breast calcifications are typically larger than 1 mm, while calcifications that are associated with malignancy typically range between 50 and 500 microns (0.05–0.5 mm). With digital mammography (DM), the resolution is lower than with screen film mammography, and calcifications smaller than 100 microns may be difficult to detect and characterize. In malignant lesions, the two mechanisms responsible for calcification development are secretion and necrosis. Secretion is an active mechanism usually observed with low-grade DCIS. In comedocarcinoma, calcification occurs in necrotic debris, which is a passive mechanism [15]. The calcifications in necrotic debris are typically small. Benign calcifications, as observed with dystrophic calcifications, fat necrosis and fibroadenomas, are usually stromal, and they tend to be large. Calcifications associated with fibroadenomas may appear punctate or pleomorphic, and biopsy may be required in some cases.
As for the number of calcifications, a cluster is usually defined as five or more calcifications in a volume of less than 1 cc of breast tissue [16]. The vast majority of cases of DCIS are associated with ten calcifications or more [15]. However, early DCIS may present with less than five calcifications. If just a few calcifications are observed, but the morphology and the distribution are suspicious, then the number of calcifications is no longer of importance and biopsy should be suggested.
The presence of new or an increasing number of calcifications is associated with a higher probability for the presence of carcinoma. However, malignant microcalcif ications may be associated with stability. Lev-Toaff et al. identified three cases of invasive ductal carcinoma among 26 patients (12%) who had mammographic calcifications that were stable for a period of 8–63 months [17]. Thus, stability of indeterminate or suspicious calcifications is not reliable for the exclusion of malignancy.
Implementing overall findings
Detecting calcifications on mammography may be an easy task when compared to decision-making and issuing a final mammographic report. It is not acceptable to biopsy calcifications that are definitely benign. In addition, it is not acceptable to follow calcifications thay may be malignant. The negative biopsy rate for stereotactic biopsy of calcifications should be approximately 75%. In other words, approximately 25% of the biopsies should be positive for malignancy. If the positive biopsy rate falls below 25%, the breast imager is probably missing cancers, and if the rate of positive biopsies rises above 25%, the breast imager may be overly suspicious of some calcifications recommended for biopsy. The ACR calls this value the positive predictive value (PPV) 2 and defines it as the percentage of women with examinations recommending biopsy (BI-RADS category 4 or 5) with a tissue diagnosis of cancer within 1 year. The PPV 2 should be analyzed on a regular basis and included in the annual mammography audit.
A key point in the assessment of mammograms is for the radiologist to classify the calcifications as benign or malignant based on the most worrisome finding. As mentioned previously, morphology is usually the most characteristic descriptor, followed by distribution. This generalization, however, only applies if neither of the calcification descriptors is suggestive of malignancy. Punctate calcifications (which are usually benign) with a segmental distribution (which is often malignant) may be alarming despite being punctate, and linear branching calcifications (which are usually malignant) should be regarded as troublesome, even if they have a regional distribution (which is typically benign). In both cases, a biopsy should be performed as management should be based on the most worrisome characteristics.
In addition to calcifications, the breast imager should look for associated findings, such as masses, architectural distortions, lymphadenopathy, skin thickening and nipple retraction. The radiologist should go by the rule of classifying the case according to the most worrisome finding. If the calcifications have a benign morphology and a benign distribution, but there is an associated architectural distortion or an associated mass, then further work-up may be needed. In addition, if the patient is feeling a palpable abnormality or complaining of a thickening, nipple discharge, or nipple retraction, mammography alone may not be sufficient. A negative mammogram is not reassuring and additional testing, usually with ultrasound, may be needed.
Work-up
Many of the calcif ications detected on mammography can be classified as definitely benign, such as vascular, popcorn-like, skin, eggshell and lucent-centered calcifications. Other types of calcifications identified on screening mammography may require callback for diagnostic imaging with additional views. Skin calcifications can be confirmed by tangential views and/or skin localization procedures. Milk of calcium can be confirmed by true lateral (mediolateral or lateromedical) views. Indeterminate punctate or amorphous calcif ications will usually require spot magnification views in the craniocaudal and the true lateral projections. With spot magnification views, the radiologist can better delineate the morphology of the calcifications, and determine whether there is an associated abnormality, such as a mass or an area of architectural distortion. If there is an associated abnormality, the patient should undergo an ultrasound for further evaluation and possible ultrasound-guided biopsy.
The majority of microcalcif ications in the breast are too small to be detected by ultrasound. If ultrasound is negative and the calcifications are suspicious on mammography, stereotactic biopsy should be performed. If the calcifications are seen on ultrasound (often with an associated hypoechoic mass or a hypoechoic duct), then ultrasound-guided core biopsy may be appropriate. If the calcifications are biopsied with sonographic guidance, specimen radiography should be performed following the biopsy to document retrieval of calcifications.
If a mammogram is classified as BI-RADS category 3, the likelihood of malignancy should be 2% or less. When a mammogram is read as BI-RADS category 3, short-term follow-up is usually performed. In most cases, the short-term follow-up will be a follow-up mammogram in 6 months. If a mammogram is read as BI-RADS category 4, then the likelihood of malignancy is thought to be greater than 2% and a biopsy is warranted. BI-RADS category 4 has been further divided into 4A, 4B and 4C, for low, intermediate and high suspicions of malignancy, respectively [1].
Stereotactic biopsy of breast calcifications
Stereotactic guidance is the use of angled mammographic views to determine the 3D location of a lesion. Stereotactic guidance is commonly used to sample microcalcifications [18], and stereotactic biopsy is sometimes used to sample small masses and small areas of architectural distortion. In general, when stereotactic biopsy yields a diagnosis of DCIS or atypical ductal hyperplasia (ADH), the lesion should be surgically removed. There may be occasional cases of minimal focal ADH for which careful follow-up may be appropriate rather than excision [19,20]. At surgery, approximately 28% of DCIS cases will be upgraded to invasive ductal carcinoma [21]. Likewise, approximately 20% of cases yielding ADH on stereotactic biopsy will be upgraded to DCIS or invasive ductal carcinoma on excision [22].
Stereotactic biopsies are performed with specialized mammographic units called stereotactic breast biopsy systems, designed to image the breast with angled views and identify the biopsy site in the x, y and z planes. The biopsy device is mounted securely on a supporting device. During a stereotactic biopsy procedure, mammographic images are taken, usually in sets of three: one is taken as a straight-on image, and two angled views are acquired (typically ± 15° from the straight-on position). Lesions noted on the mammograms will shift in the opposite direction of the tube’s rotation. The degree of the shift will depend on the distance of the lesion relative to the tube. Lesions located closer to the tube will produce a greater shift in the offset images, while more distant lesions will only shift slightly. The distance that the lesion shifts is used to calculate its position relative to the detector using trigonometric relationships. This information is then used to calculate a trajectory and a depth for the biopsy needle, controlled by the computer. The radiologist then positions the needle device to sample tissue from the designated region of the breast. Additional insertions at different depths or positions may be performed as deemed necessary by the radiologist. Verification of removal of the targeted lesion is then achieved using specimen radiography. A clip marker is usually placed at the biopsy site immediately following the biopsy procedure. Clip markers are helpful for showing the biopsy site, especially when all or most of the targeted lesion was removed. The immediate postclip image may be obtained as a stereotactic image. Thereafter, a two-view mammogram should be obtained to document the position of the clip, and to note any evidence of clip migration or hematoma formation.
The pathology results from the stereotactic biopsy should be reviewed to establish imaging–pathology concordance. When the patient is referred with a mammogram classified as BI-RADS category 5, even if the stereotactic biopsy is negative, the lesion should be worked-up further, either with a repeat percutaneous biopsy or with a surgical excision. When the patient referred for biopsy had a mammogram with a BI-RADS category 4 assessment and a benign biopsy, the radiologist should then decide on further management. In most cases, the patient usually undergoes follow-up mammography, especially if specimen radiography following biopsy demonstrated that the biopsy specimen was truly representative of the suspicious calcifications.
Conclusion
Every time a radiologist reads a mammogram, the patient’s life may be affected; it can be challenging to differentiate malignant from benign calcifications. The radiologist has to keep in mind radiologic–pathologic correlation of breast calcifications in order to know what he or she is looking for. The BI-RADS lexicon and classification system has given radiologists a guide to follow when interpreting breast calcifications and deciding on further management. Nonetheless, it remains the job of the radiologist to detect breast calcifications carefully, to categorize them accurately and decide wisely on appropriate management [23].
Future perspective
Currently, 88.8% of mammography units in the USA are digital units and 87.3% of Mammography Quality Standards Act certified facilities have at least one DM unit [101]. DM has led to increased recalls for calcifications along with decreased PPVs following screening mammography and decreased PPVs following biopsy [24]. These findings are probably due to improved visualization of calcifications with DM and more patients with benign calcifications undergoing biopsy. As digital breast tomosynthesis gains momentum, attention will be focused on calcif ication detection and characterization. In 2010, a study by Spangler et al. noted that DM was slightly more sensitive than digital breast tomosynthesis for calcif ication detection [25]. The investigators found that diagnostic performance, as measured by the area under the curve using BI-RADS, was not significantly different [25]. In the future, with improvements in processing algorithms and display functions, it is likely that calcification detection and characterization will be improved with digital breast tomosynthesis.
Acknowledgement
The authors thank P Castro for assistance in manuscript preparation.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
* of interest
* of considerable interest
- BI-RADS® – American College of Radiology (ACR) Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas). In: Mammography (4th Edition). American College of Radiology, Reston, VA, USA (2003). & Describes the American College of Radiology BI-RADS® mammography lexicon.
- Cao MM, Hoyt AC, Bassett LW. Mammographic signs of systemic disease. Radiographics 31(4), 1085–1100 (2011).
- Kim H, Greenberg JS, Javitt MC. Breast calcifications due to Mönckeberg medial calcific sclerosis. Radiographics 19(5), 1401–1403 (1999).
- Sommer G, Kopssa H, Zazgornil J, Salomonowitz E. Breast calcification in renal hyperparathyroidism. Am. J. Roentgenol. 148(5), 855–857 (1987).
- Hassan NA, D’Orsi ET, D’Orsi CJ, O’Neill WC. The risk for medial arterial calcification in CKD. Clin. J. Am. Soc. Nephrol. 7(2), 275–279 (2012).
- Giess CS, Raza S, Birdwell RL. Distinguishing breast skin lesions from superficial breast parenchymal lesions: diagnostic criteria, imaging characteristics, and pitfalls. Radiographics 31(7), 1959–1972 (2011).
- Chen PH, Ghosh ET, Slanetz PJ, Eisenberg RL. Segmental breast calcifications. Am. J. Roentgenol. 199(5), W532–W542 (2012). & Focuses on benign and malignant segmental calcifications.
- Lai KC, Slanetz PJ, Eisenberg RL. Linear breast calcifications. Am. J. Roentgenol. 199(2), W151–W157 (2012). nn Focuses on benign and malignant linear calcifications.
- Friedman PD, Kalisher L. Case 43: filariasis. Radiology 222(2), 515–517 (2002).
- Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology 170(2), 411–415 (1989).
- Hofvind S, Iversen BF, Eriksen L, Styr BM, Kjellevold K, Kurz KD. Mammographic morphology and distribution of calcifications in ductal carcinoma in situ diagnosed in organized screening. Acta Radiol. 52(5), 481– 487 (2011). & Analyzed combinations of mammographic morphology and distribution of calcifications according to Van Nuys nuclear grade.
- Bargallo X, Santamaria G, Velasco M et al. Mammographic features of screening detected pT1 (a–b) invasive breast cancer using BI-RADS lexicon. Eur. J. Radiol. 81(10), 2620–2626 (2012).
- Loffman Felman RL. The tattoo sign. Radiology 223(2), 481–482 (2002).
- Demetri-Lewis A, Slanetz PJ, Eisenberg RL. Breast calcifications: the focal group. Am. J. Roentgenol. 198(4), W325–W343 (2012). & Reviews grouped distribution of calcifications.
- Tse GM, Tan PH, Pang ALM, Tang APY, Cheung HS. Calcification in breast lesions: pathologists’ perspective. J. Clin. Pathol. 61(2), 145–151 (2008).
- Fondrinier E, Lorimier G, Guerin-Boblet V, Bertrand AF, Mayras C, Dauver N. Breast microcalcifications: multivariate analysis of radiologic and clinical factors for carcinoma. World J. Surg. 26(3), 290–296 (2002).
- Lev-Toaff AS, Feig SA, Saitas VL, Finkel GC, Schwartz GF. Stability of malignant breast microcalcifications. Radiology 192(1), 153–156 (1994).
- O’Flynn EA, Wilson AR, Michell MJ. Image-guided breast biopsy: state-of-the-art. Clin. Radiol. 65(4), 259–270 (2010).
- de Mascarel I, Brouste V, Asad-Syed M, Hurtevent G, MacGrogan G. All atypia diagnosed at stereotactic vacuum-assisted breast biopsy do not need surgical excision. Mod. Pathol. 24(9), 1198–1206 (2011).
- McGhan LJ, Pockaj BA, Wasif N, Giurescu ME, McCullough AE, Gray RJ. Atypical ductal hyperplasia on core biopsy: an automatic trigger for excisional biopsy? Ann. Surg. Oncol. 19(10), 3264–3269 (2012).
- Koskela AK, Sudah M, Berg MH et al. Addon device for stereotactic core-needle breast biopsy: how many biopsy specimens are needed for a reliable diagnosis? Radiology 236(3), 801–809 (2005).
- Lomoschitz FM, Helbich TH, Rudas M et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: influence of number of specimens on diagnostic accuracy. Radiology 232(3), 897–903 (2005).
- Onega T, Smith M, Miglioretti DL et al. Radiologist agreement for mammographic recall by case difficulty and finding type. J. Am. Coll. Radiol. 9(11), 788–794 (2012).
- Glynn CG, Farria DM, Monsees BS, Salcman JT, Wiele KN, Hildebolt CF. Effect of transition to digital mammography on clinical outcomes. Radiology 260(3), 664–670 (2011). & Evaluates the effect of the transition to digital mammography on clinical measures, including the recall rate, the cancer detection rate and the positive predictive value.
- Spangler ML, Zuley ML, Sumkin JH et al. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. Am. J. Roentgenol. 196(2), 320-324 (2011).