Review Article - Imaging in Medicine (2013) Volume 5, Issue 2
Managing incidental cystic lesions in pancreatic imaging: challenges for the radiologist
Charikleia Triantopoulou*1 & Christos Dervenis21Radiology department, Konstantopouleio General Hospital, 3–5, Agias Olgas Street, 14233 N Ionia, Athens, Greece
2First Surgery Department, Konstantopouleio General Hospital, 3–5, Agias Olgas Street, 14233 N Ionia, Athens, Greece
- Corresponding Author:
- Charikleia Triantopoulou
Radiology department
Konstantopouleio General Hospital
3–5, Agias Olgas Street, 14233 N Ionia, Athens, Greece
Tel: +30 213 2057 926
E-mail: ctriantopoulou@gmail.com
Abstract
Keywords
CT ▪ cyst n imaging ▪ management ▪ MRI ▪ pancreatic lesions ▪ ultrasound
Cystic pancreatic lesions are generally divided into mucinous (30%) and nonmucinous (70%) lesions [1]. This distinction is very important as mucinous neoplasms require resection because they present malignant potential, while nonmucinous neoplasms that present low or no malignant potential and pseudocysts usually do not require surgical intervention. Half of cystic pancreatic lesions are asymptomatic and incidentally discovered at the time of imaging for other reasons. Despite advances in imaging, it seems that accurate differential diagnosis is not always feasible and guidelines for their management are still unclear. The problem is that pancreatic surgery carries significant morbidity. If resection is performed for all incidentally discovered cystic pancreatic lesions, there is the potential to harm patients for whom the malignant risk is negligible [1].
In this article, the advances of different imaging modalities and their accuracy in the evaluation and management of cystic pancreatic lesions are discussed, guidelines and recommendations are given, and future perspectives are also described in detail.
Terminology: classification
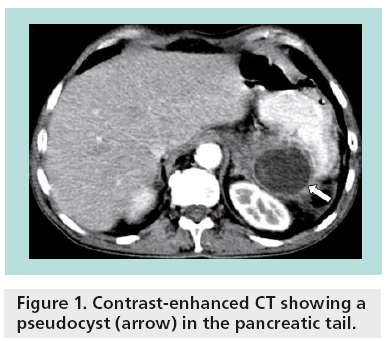
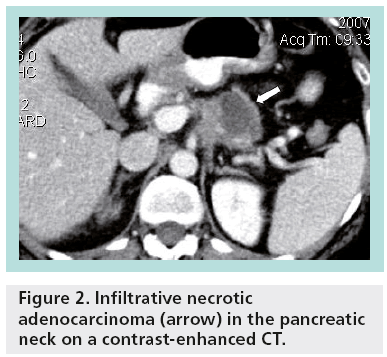
Unlike most hepatic and renal cysts, pancreatic cystic lesions raise clinical concern because of their potential for malignancy. Pseudocysts account for almost one-third of pancreatic cystic lesions (Figure 1), while approximately 50–60% of pancreatic cystic lesions are cystic neoplasms. Cystic degeneration of solid neoplasms and retention cysts represent the remaining 10% (Figure 2). The differential diagnosis for incidental pancreatic cystic lesions include benign serous cystadenoma or premalignant mucinous cystic lesions, which are classified into mucinous cystic neoplasms (MCNs), branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), main duct-intraductal papillary mucinous neoplasms (MD-IPMNs) or mixed intraductal papillary mucinous neoplasms (IPMNs) [2].
The true incidence of malignancy in MCNs is unknown, although recent studies suggest rates of invasive cancer as 12–29% and carcinoma in situ as 5.5% [2]. Approximately 40% of MD-IPMNs are malignant at the time of diagnosis. Although main pancreatic duct dilation greater than 15 mm and the presence of mural nodules have been associated with malignancy, malignancy is also present in up to 30% of patients with MD-IPMNs without symptoms, mural nodules or marked duct dilation. Approximately 15% of BD-IPMNs can also undergo malignant transformation [3]. The rate of high-grade dysplasia and/or invasive IPMNs is low for gastric subtype (25%), higher for intestinal subtype (85%) and reaching almost 100% for both pancreatobiliary and oncocytic subtypes.
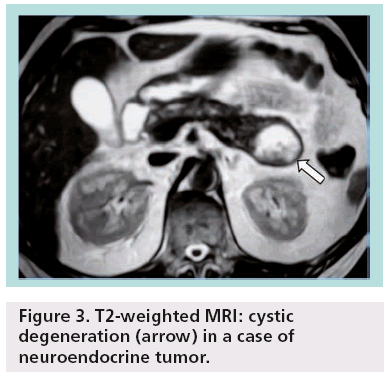
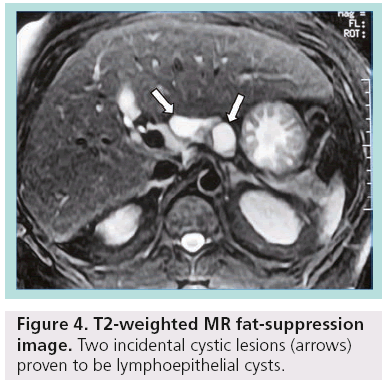
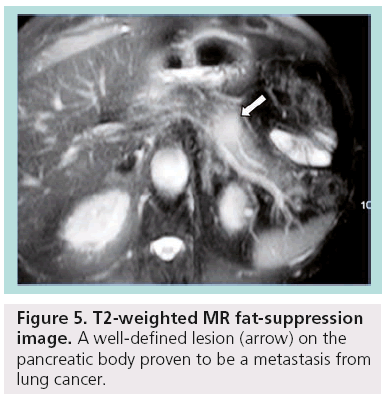
Other less common pancreatic cystic neoplasms include solid pseudopapillary neoplasms (SPENs), which occur almost exclusively in young women. Approximately 10–15% of SPENs are malignant, and to date no predictors of aggressive behavior have been identified. In addition, less commonly observed lesions such as neuroendocrine or acinar cell tumors can sometimes undergo cystic degeneration (Figure 3). Finally, in the differential diagnosis of incidentally discovered cystic pancreatic lesions, lymphoepithelial cyst, echinococcal (hydatid) cyst, cystic lymphangioma, as well as cystic metastasis, including renal cell carcinoma, melanoma, lung tumors, breast carcinoma and ovarian tumors, should also be included ( Figures 4 & 5) [4–7].
Figure 3: T2-weighted MRI: cystic degeneration (arrow) in a case of neuroendocrine tumor.
Figure 5: T2-weighted MR fat-suppression image. A well-defined lesion (arrow) on the pancreatic body proven to be a metastasis from lung cancer.
Imaging modalities
■Ultrasound
Endoscopic ultrasound (EUS) and cystic fluid analysis have become powerful tools in the differential diagnosis of incidental cystic pancreatic lesions [8]. EUS has many features that make it the ideal tool for evaluating pancreatic cysts. The strict proximity between the transducer and the lesions allows for a very precise definition of the structural component of the cysts and some components of pancreatic cysts, such as thin septa or small mural nodules, are better visualized with EUS than with other modalities.
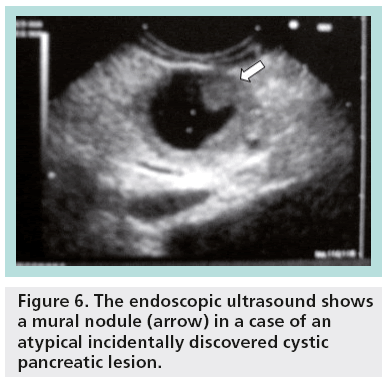
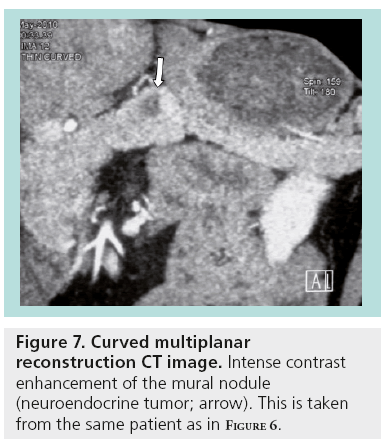
With EUS, it is possible to define cystic localization, size, locularity, internal structural features, mural nodules, contours, cystic wall, pancreatic duct involvement and calcification. EUS characteristics of the cystic lesions (microcystic, macrocystic, ‘honeycomb’ appearance, presence of stellate scar, associated mural nodule or solid component and presence of pancreatic duct dilatation) can provide additional information important for the differential diagnosis and patient management (Figures 6 & 7).
Figure 6: The endoscopic ultrasound shows a mural nodule (arrow) in a case of an atypical incidentally discovered cystic pancreatic lesion.
The diagnostic accuracy of EUS morphology in differentiating cystic lesions of the pancreas varies in different studies and reported results are between 51 and 90% [8,9]. In general, it is believed that morphological criteria are not usually enough to permit differential diagnosis among benign, premalignant and malignant cystic pancreatic lesions. Therefore, in most of the cases fine-needle aspiration (FNA) is needed.
EUS-guided FNA allows for the differentiation between neoplastic and non-neoplastic cysts, if the amount of aspirated fluid is sufficient for proper analysis. Carcinoembryonic antigen (CEA) was proven to be one of the most powerful markers, presenting a pooled sensitivity of 75% and specificity of 84% if a level of >192 ng/ml is used as a cutoff value for the detection of mucinous neoplasms [9]. Levels <5 ng/ml suggest a nonmucinous cyst (serous or pseudocyst). It should be noted that high levels of CEA do not necessarily associate with malignancy. In general, a wide overlap exists and even benign lymphoepithelial cysts have been found to express high levels of CEA.
Cytology is specific but has a low sensitivity. The presence of mucin, cystic fluid CA19-9 and CEA levels, KRAS mutation and DNA analysis could enhance the diagnostic value of the pancreatic cystic lesion aspirate, but is not available at all institutions [10,11]. If KRAS mutation is present in a cyst fluid, the cyst is probably mucinous. In the absence of KRAS mutation (sensitivity of ~50%), cyst fluid CEA is the most accurate marker of a mucinous cyst. Positive staining for mucin indicates the presence of IPMN (40% specific; 80% sensitive). Furthermore, MUC1 is related to invasive lesions, while MUC2 and MUC5A to noninvasive.
Another promising application of ultrasound (US) is the contrast-enhanced technique. Contrast-enhanced US (CEUS) involves the use of microbubble contrast agents and specialized imaging techniques to demonstrate sensitive blood flow and tissue perfusion information. A qualitative and quantitative CEUS analysis should be performed in order to differentiate pancreatic cystic lesions. Several parameters are important such as peak intensity, time to peak, maximum ascending gradient and time to maximum gradient. There is a significant difference of normalized time to peak ratio and normalized time to maximum gradient ratio between the benign and malignant lesions. Normalized time to peak ratio of <9 s and normalized time to maximum gradient ratio of <8.5 s rules out malignant cysts in almost 90% of cases.
In centers where CEUS is used for the evaluation of cystic pancreatic lesions in daily practice, it is demonstrated that this technique compares favorably with MRI in displaying the anatomic features of cystic pancreatic masses seen on transabdominal sonography. The difference between the diagnostic accuracy of CEUS and that of MRI in the identification of septa and nodules was not significant in one study [12].
■CT
With advancements in CT technology and improved spatial resolution, unsuspected small pancreatic cysts are being detected with increased frequency. In a large study conducted on the outpatient population, the prevalence of unsuspected, asymptomatic cysts identified on 16-multidetector CT (MDCT) was 2.6%. The presence of a cyst showed a strong correlation with increasing age, while multiple cysts were also revealed in asymptomatic patients [13].
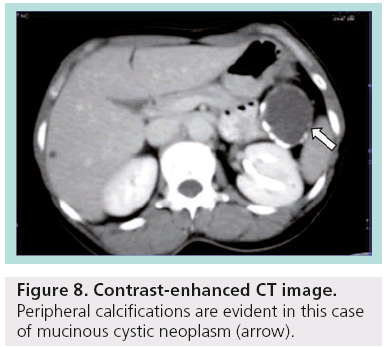
Despite advances and technological development in MDCT, differential diagnosis between serous and mucinous cystic lesions, which is the critical point for the proper management, is not feasible in many cases. There is significant variability in the CT appearance of serous and mucinous neoplasms of the pancreas, making CT an insensitive tool for differentiating these tumors. The dimension of the largest cyst (<2 cm in serous; >2 cm in mucinous) as well as the site of calcifications (central in serous; peripheral in mucinous) are the commonest criteria used (Figure 8) [14].
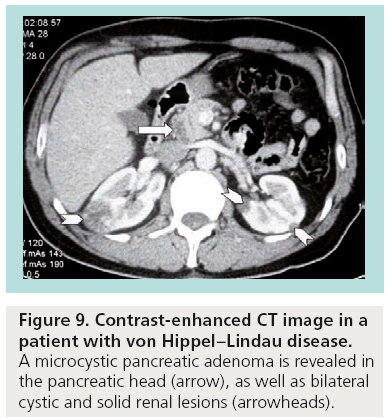
Typically, benign microcystic adenomas are described as multilocular well-circumscribed masses that often contain a central stellate scar and calcifications. Innumerable small cysts ranging in size from 1 to 20 mm are filled with clear fluid and have a honeycombed appearance on CT as a result of intervening connective tissue septa (Figure 9). Mucinous cystadenomas and cystadenocarcinomas can appear as unilocular or multilocular masses filled with mucin, hemorrhage and debris. Although CT continues to be the method of choice for the evaluation of pancreatic lesions, it is important to identify when the imaging findings are not specific enough to confidently exclude a mucinous lesion.
Another important issue that has also been discussed and verified in a previous study is that the combined opinion of more radiologists improves accuracy [15]. It seems that diagnosis in consensus, when the final opinion for each tumor is determined by agreement between at least two of three reviewers, increases specificity.
■MRI
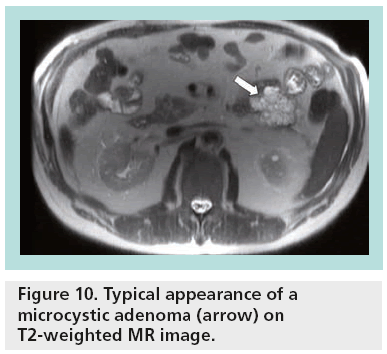
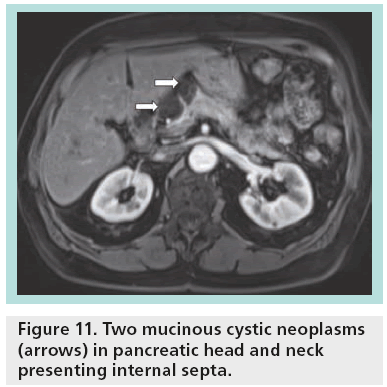
The great sensitivity of MR to static fluids with highly T2-weighted sequences permits demonstration of the fluid content in the cases of equivocal microcystic adenoma in other imaging modalities (Figure 10). In the cases of mucinous tumors, heavily T2-weighted sequences permit better identification of the thin septa than CT, while MR cholangiopancreatography is a valuable technique permitting differentiation between mucinous cystadenoma (lack of communication between the cystic tumor and the main pancreatic duct) and IPMNs (Figure 11). After contrast injection, there is variable enhancement of the wall, the septa or the solid component. Signs considered indicative of potential malignancy are papillary proliferations; thick septa; parietal nodules; and thick walls.
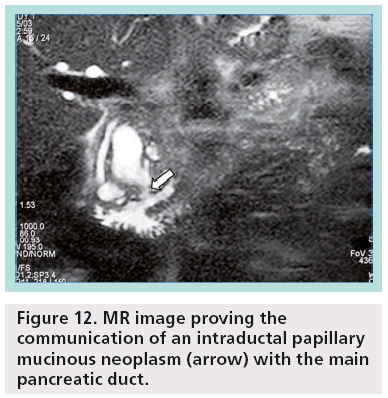
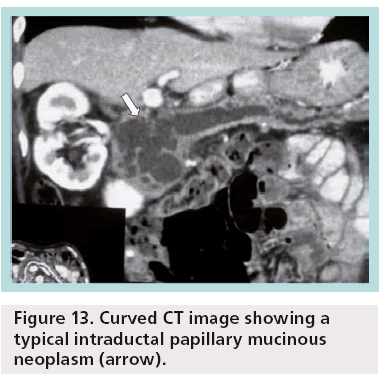
Pancreatic MRI shows better diagnostic performance than MDCT for differentiating IPMN from other cystic pancreatic lesions, as it is accurate in the prediction of possible communication between a pancreatic cyst and the pancreatic duct (Figure 12) [16]. Other researchers have shown that MDCT with curved 2D reformations can provide imaging details similar to MR cholangiopancreatography (Figure 13) [17].
Figure 12: MR image proving the communication of an intraductal papillary mucinous neoplasm (arrow) with the main pancreatic duct.
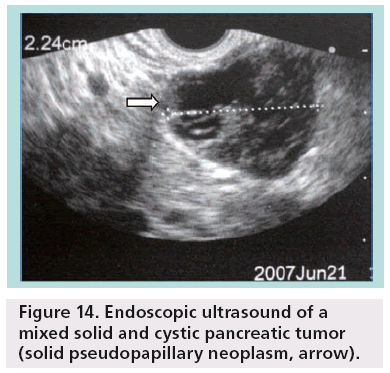
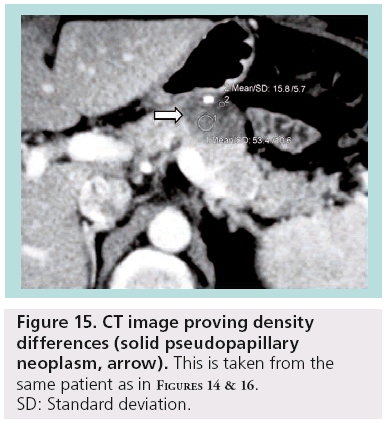
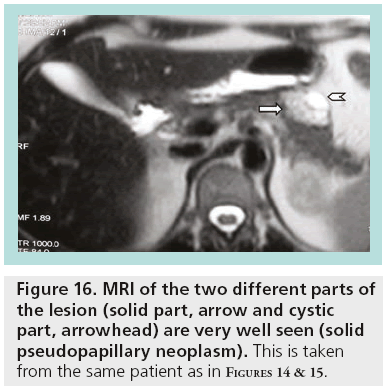
SPENs usually present combined solid and cystic features, but they can also be totally solid or cystic (figures 14–16). They show areas of high signal intensity (SI) on T1-weighted images and low or inhomogeneous SI on T2-weighted and they do not demonstrate the hypervascularity typically seen in islet cell tumors.
Diffusion-weighted MRI (DWI) is becoming an important noninvasive technique in the characterization of biologic tissues based on their water diffusion properties. There are data suggesting that DWI may be helpful in the differential diagnosis of cystic pancreatic lesions, thus affecting proper management. In a recent study, it has been shown that on DWI with a b factor of 1000 s/mm, all abscesses, hydatid and neoplastic cysts were hyperintense, whereas most of the simple cysts and pseudocysts were isointense. With a b factor of 1000 s/mm, the cystto- pancreas SI ratios of the abscesses, the hydatid and neoplastic cysts were significantly higher than those of the simple cysts and pseudocysts [18].
In another interesting study, the authors demonstrated that DWI may differentiate MCNs from IPMNs. The higher apparent diffusion coefficient (ADC) of IPMNs has been attributed to their connection with the pancreatic ductal system, presumably allowing for cyst contents to move more freely [19].
Other researchers have moved one step forward, proving that significant differences in the mean ADC exist between neoplastic versus non-neoplastic and mucinous versus nonmucinous lesions in a study conducted on 3.0-T MRI. However, despite their findings, they concluded that although mean ADC values and focal cystic pancreatic lesions-topancreas SI and ADC ratios may be helpful in differentiating focal cystic pancreatic lesions, the characterization of individual focal cystic pancreatic lesions by means of 3.0-T DWI appears limited [20].
■18-fluorodeoxyglucose PET
18-fluorodeoxyglucose PET (F18-FDG PET) is a noninvasive imaging technique based on tissue metabolism, owing to selective F18- FDG uptake and retention by malignant or inflammatory cells. PET has been proposed as a valuable technique for diagnosing and staging different malignancies, including pancreatic adenocarcinoma and borderline or malignant pancreatic cystic lesions.
A study conducted in 56 patients with a suspected cystic tumor of the pancreas who underwent PET–CT in addition to CT showed a sensitivity of 94% and a specificity of 95%. The single false-negative result occurred in a patient with insulin-dependent diabetes, while the only one false-positive result occurred in a patient with a mucinous cystadenoma, which is considered as a premalignant lesion that requires resection [21]. According to a recent review article, the sensitivity of F18-FDG PET or PET–CT in the differential diagnosis between benign and malignant IPMN calculated on a per-patient-based analysis ranged from 78 to 100%, while the pooled specificity of F18-FDG PET or PET–CT ranged from 87 to 100% [22].
It should be noted that the standard uptake value is not a specific marker of malignancy. In many studies, a statistically significant difference between the standard uptake value of benign and malignant cystic pancreatic lesions has been demonstrated. After receiver operating characteristic curve analysis, a standard uptake value cutoff value of 2.5 was identified as the optimum in terms of the best compromise between sensitivity and specificity [22].
A limitation of F18-FDG PET is that, as a functional imaging modality, it cannot replace anatomic imaging (CT or MRI) in the assessment of lesion morphology and local tumor resectability. Despite this, F18-FDG PET could be considered a sensitive and specific adjunct to CT when applied in the preoperative differential diagnosis and staging of pancreatic cystic tumors. Positive F18-FDG PET findings may change surgical decision-making for 16% of patients, either suggesting surgical resection for asymptomatic patients without signs of malignancy on conventional imaging, or avoiding laparotomy for patients with hepatic metastases not seen on CT. On the other hand, negative F18-FDG PET enables planning of a follow-up strategy for patients with a positive CT or one suspicious for malignancy cystic lesion. The relatively high cost of PET scanning is balanced by the number of unexpected malignancies detected in addition to CT and by avoiding other unnecessary, invasive and expensive procedures.
Accuracy of imaging diagnosis
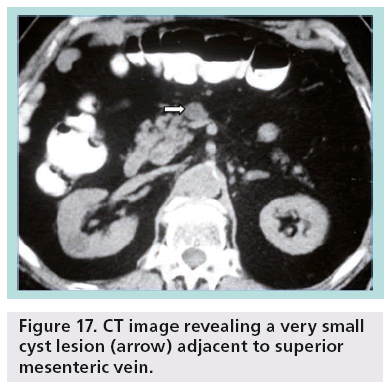
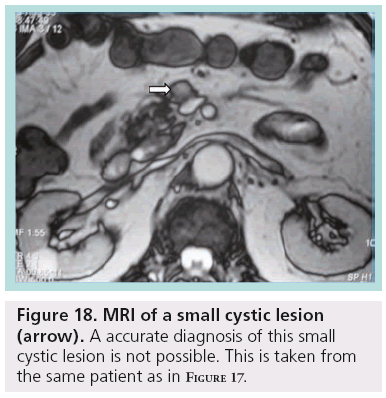
Despite the advances and applications of modern imaging techniques in the differential diagnosis of incidentally discovered cystic pancreatic lesions, their management continues to be debated and preoperative diagnosis is often inaccurate. While pancreatic cystic lesions may occasionally have characteristic imaging features, there is extensive overlap in imaging appearance, and all of them may appear as a relatively simple cyst. Small lesions are more susceptible to misdiagnosis (Figures 17 & 18).
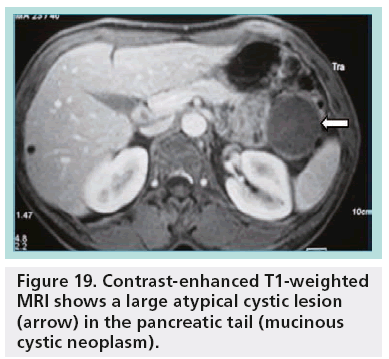
In fact, only microcystic adenoma and main duct IPMN can be diagnosed with almost complete certainty. In most cases, diagnosis of mucinous cystic lesions is hypothetical. A unilocular cystic lesion with a thin wall could be similar to many other cystic lesions (pseudocyst, serous adenoma; Figure 19). On the other hand, pseudocysts with necrotic debris may simulate parietal nodules of a MCN. In general, the ability of imaging to enable a specific diagnosis of an individual pancreatic cyst is limited (40–60%) [23,24].
CT and MRI are reasonably and similarly accurate in the characterization of cystic pancreatic masses as benign or malignant. Both have limitations, mainly in the small morphologically benign-appearing cysts, that may finally show moderate frequency of malignancy. The level of diagnostic certainty depends on experience, while the examination protocol and the equipment play a crucial role [23].
Nowadays, most ‘authorities’ agree that no imaging modality is sufficiently accurate to differentiate among the multiple benign, premalignant and malignant cystic lesions. Considerable controversy presently exists with respect to the imaging evaluation of small, asymptomatic pancreatic ‘cysts’ [24].
In a retrospective study reviewing 330 patients with incidentally discovered cystic pancreatic neoplasms in an experienced high-volume center, preoperative diagnosis was incorrect in one-third of the patients who underwent resection [25]. Of presumed BD-IPMN, 20% had a main duct component, while 5% of resected cysts were not even neoplastic. The diagnosis was correctly predicted in 63% of cases when cross-sectional (CT or MRI) imaging was used alone; when both studies were performed, the accuracy was also 63%. Interestingly, it was not improved in patients who had EUS in addition to either CT or MRI.
In another large study where CEUS was also used, 476 patients were analyzed and the final pathologic diagnosis matched the preoperative diagnosis in 78% of cases. The highest accuracy was reached for SPENs (95%) and for mixed duct IPMNs or MD-IPMNs (81%). Surprisingly, 23 cysts (5%) were found to be ductal adenocarcinoma, whereas 45 patients (9%) underwent a pancreatic resection for a nonneoplastic condition. The accuracy in diagnosing rare entities such as cystic neuroendocrine neoplasms was only 50% [26].
Although the discrepancy between the preoperative and final diagnosis is not that important in some cases, in other patients the alternative diagnoses might influence the clinician towards either more aggressive or more conservative management. It should be kept in mind that in patients with asymptomatic cysts being followed with the presumptive diagnosis of BD-IPMN, it is quite probable that this diagnosis is incorrect in approximately onethird. Prospective studies, technology advances and new biochemical and genetic techniques are obviously necessary to overcome current diagnostic pitfalls by imaging.
Consensus guidelines & management recommendations
The decision on whether to observe or to operate on a pancreatic cystic lesion depends on the presenting symptoms, patient age, comorbidities, resectability and morphologic features [27,28]. The size of the lesion is not always a valid criterion in decision-making because even smaller lesions can have malignant potential [29].
The discovery of a pancreatic cyst in a asymptomatic patient is even more challenging and there are many questions raised concerning appropriate management, the best method for imaging follow-up and optimal frequency of follow-up [30].
A variation exists in radiologists’ or other clinicians’ recommendations. In fact, 83% of recommendation variation is the result of the preference or opinion of the individual radiologist. It should be noted that variation in management recommendations can lead to under- or over-treatment, while variation in practice can lead to underuse, misuse and overuse of care [31].
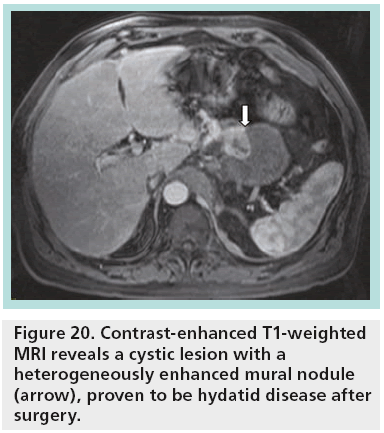
When resection is indicated, parenchymasparing pancreatectomies are proposed, being safe and technically feasible surgical options for treating benign, borderline or low-grade malignant tumors of the pancreas. In experienced hands, these procedures are performed with low mortality and low recurrence rate but a significantly high complication rate. The main indications for parenchyma-sparing pancreatectomies are cystic endocrine neoplasms, serous and mucinous cystadenomas, noninvasive branch-type IPMNs, small SPENs and nonneoplastic cysts (simple, lymphoepithelial, hydatid cyst) not suitable for enucleation (Figure 20) [32].
Figure 20: Contrast-enhanced T1-weighted MRI reveals a cystic lesion with a heterogeneously enhanced mural nodule (arrow), proven to be hydatid disease after surgery.
The Sendai consensus proposed guidelines for the management of IPMNs and MCNs have been widely adopted in the surgical decision-making and management process [33]. However, they are based on the premise that we can accurately classify these lesions on the basis of imaging characteristics, an assumption yet to be proven. Current recommendations are to resect all MCNs, whereas observation is considered appropriate for BD-IPMN <3 cm. Concerning follow-up frequency, lesions <1 cm should be followed each year; lesions measuring 1–2 cm for 24 months at 6-month intervals and then annually for a second 24 months declaring stability after 4 years; and finally lesions 2–3 cm every 3–6 months.
Current treatment guidelines from several major gastrointestinal societies recommend surgical resection for all definite MCNs and MD-IPMNs. Since the occurrence of malignancy is much lower in BD-IPMNs, resection is reserved for those patients with pancreatitis symptoms, a main pancreatic duct dilation >10 mm, presence of mural nodules, cytology suspicious or positive for malignancy and/or cysts >3 cm, particularly in younger patients. Nonmucinous pancreatic lesions (small asymptomatic pseudocysts or serous cystadenomas) do not require surgery, but further follow-up and evaluation by imaging is needed, as serous cystadenomas may also increase in diameter with time and become symptomatic; therefore, surgery could be indicated.
There are also other guidelines published by societies involved in the management of cystic pancreatic lesions [34] and by the American College of Radiology (ACR) in a white paper of the ACR Incidental Findings Committee [35]. According to the ACR guidelines, lesions <2 cm require a single follow-up in 1 year; lesions measuring 2–3 cm a follow-up of every 6 months for 2 years and then yearly; while lesions >3 cm should be resected unless they are serous cystadenoma or proven to be pseudocyst through aspiration. In general, one should consider decreasing the follow-up interval if the patient is younger or omitting follow-up if the patient has a limited life expectancy.
The American College of Gastroenterology guidelines are based on published data and are designed to address the most frequent and important clinical scenarios. In addition to providing a summary of the diagnostic data, they offer diagnostic and management suggestions based on 13 common clinical problems. Core principles are provided based on a balance between the risk of malignancy and the benefit of pancreatic resection [36].
During the follow-up, some important issues need to also be taken into consideration. According to the ACR, MRI is the preferred follow-up procedure because of the superior contrast resolution and the lack of radiation. It should be noted that for patients older than 60 years, ionizing radiation issues may not be as significant. In any case, care must be taken to ensure that measurements are made carefully and consistently.
A recent study provides the results of clinical outcomes of cystic pancreatic lesions after more than 5 years of follow-up. This study confirms the indolent nature and favorable prognosis of most of these lesions as a result of long-term follow-up. However, it also shows the potential for growth and malignant transformation of cystic lesions of the pancreas even after 5 years (development of mural nodules or septations), which provides evidence to support long-term surveillance despite initial features of a benign lesion [37].
Radiologists need to be familiar with published guidelines for the management of pancreatic cysts and be a part of the multidisciplinary group in the decision-making process with the gastroenterologists and surgeons. A recent Markov model incorporates these features to guide therapy [38]. It is important to remember that it is unlikely that any combination of clinical and radiological parameters can accurately make the important distinction between mucinous and nonmucinous cystic pancreatic lesions and thus specific care should be taken in equivocal or unclassified lesions.
Future directions in differential diagnosis
There is a lot of research concerning the use of new methods in the differential diagnosis of pancreatic cystic lesions. In a recent ex vivo pilot study the authors used optical coherence tomography (OCT) to differentiate between low- and highrisk pancreatic cysts [39]. OCT was able to reveal specific morphologic features of pancreatic cysts and presented over 95% sensitivity and specificity. This pilot study suggests that OCT could be used by clinicians in the future to more reliably differentiate between benign and potentially malignant pancreatic cysts.
OCT, a high-resolution structural imaging technology based on low coherence interferometry, has shown great promise in disease diagnosis, including differentiation between benign and malignant lesions [40]. Ovarian cystic masses can be differentiated through laparoscopic OTC, while there are also many applications in laryngology, in the investigation of lymph nodes, lung cancer, intestinal metaplasia and Barret’s eosophagus, as well as main pancreatic duct strictures and pancreatic cystic lesions [41–47].
As it was shown in the ex vivo study of tissue specimens, serous adenomas showed a clear lack of OCT signal in the lumen. On the other hand, MCNs and IPMNs presented moderate-to-high scattering in the lumen. Although the results are promising, further investigation is needed to explore the potential of OCT imaging for aiding EUS-FNA in differentiating between various cystic lesions of the pancreas.
Proteomics is a powerful method used to identify, characterize and quantify proteins within biologic samples. Emerging data suggests that the use of protein-based biomarkers in aspirated pancreatic cystic fluid may have a great impact in the diagnosis and management of cystic neoplasms by identifying which are malignant [48].
There is considerable interest in genetic material within cyst fluid and its potential to serve as biomarkers. DNA mutations, such as KRAS, and allelic loss amplitude of a proprietary list of specific pancreatic cancer-related genes within cyst fluid have been studied as surrogate markers for mucinous and malignant cysts [49].
miRNAs are small, approixmately 22-nucleotide, noncoding RNAs that regulate the stability and translation of mRNA transcripts. Deregulation of miRNA expression has been identified in several human cancers, including pancreatic adenocarcinoma [50]. Habbe et al. described the identification of abnormal miRNA expression in surgical histology from 15 noninvasive IPMNs compared with normal pancreatic tissue [51]. Further studies will be required to validate these findings and to define the true utility of using miRNAs as biomarkers in pancreatic cyst fluid.
In addition, there is extensive research needed in order to identify the role of specific proteins already known to be involved in pancreatic cancer and there are many working groups involved [52]. Another group examined the role of PGE2, in distinguishing different types of pancreatic mucinous cysts [53]. A recent study has also demonstrated differential mucin expression in cyst fluid from 40 surgically resected IPMNs using ELISA [54]. Patients with high-grade dysplasia or carcinoma were categorized as ‘high risk’. Cyst fluids MUC2 and MUC4 were elevated in high-risk patients compared with low-risk patients.
The interest in using proteomics in pancreatic cyst fluid analysis is growing. The feasibility of proteomic analysis of pancreatic cyst fluid was established by Scarlett et al. [55]. Glycoproteomics specifically examines the carbohydrate modification or glycosylation of proteins. Aberrant glycosylation is a hallmark for tumor genesis and progression and not surprisingly, many previously identified biomarkers are glycoproteins.
In conclusion, pancreatic cyst fluid provides an appealing source for improved biomarker development, particularly by proteomic analysis. Preliminary work with cyst fluid glycosylated mucins demonstrates promising results in distinguishing mucinous from nonmucinous cysts and differentiating types of mucinous cysts.
The possibility of measuring markers of dysplasia preoperatively had also attracted the interest of many researchers. This is quite important specifically for IPMNs, as the level of CEA in the aspirated fluid does not correlate with the presence or absence of malignancy. At the current time, there is not a reliable marker in IPMN cyst fluid to aid the surgeon in clinical decision-making, or to inform the patient that they may have a high-risk IPMN that should be resected.
Dysplasia is known to arise in a background of chronic inf lammation. IL-1b expression in tumor specimens has been found to be a determinant of cancer risk and survival in many other neoplasms including ovarian, cervical and breast cancer, and is a mediator of pancreatic cancer cell invasion [56]. In a recent study, it has been shown that high-risk IPMN were associated with elevated levels of cytokines ref lective of a Th1 and Th2 immunologic response. The presence of IL-1b in particular was highly predictive of malignancy [57]. IL-1b levels correlated with the degree of cyst dysplasia and were highly predictive of high-risk lesions. These findings definitely require further validation studies that may help towards proper patient selection for surgery.
Conclusion
Cross-sectional imaging is of key importance for the diagnostic evaluation of patients with a cystic pancreatic lesion affecting management. Cyst fluid examination (cytology, biochemical/genetic analysis) is also possible by using EUS-guided FNA and may provide useful information for the differential diagnosis [58]. Advances in imaging modalities, specifically MRI and CEUS, may enhance diagnostic accuracy in the important discrimination between benign and malignant pancreatic cystic lesions and in the evaluation of possible aggressiveness [59].
Although many guidelines have been published, the management of asymptomatic pancreatic cysts is still controversial and indications for excision are based on imaging, pathology and natural history [60].
Future perspective
It seems that, in the future, pancreatic cyst fluid analysis will be the most important component of the work up of pancreatic cysts. Recently, GNAS mutations have been implicated in the development of IPMNs. The use of cytokines as a measurement of antitumor response will also be further evaluated in patients with IPMNs, as well as the expression of hTERT, which seems to be strongly associated with malignant transformation in these tumors. Newer markers to improve diagnostic accuracy are on the horizon, but clinical application of these exciting potentially predictive biomarkers requires further studies. Finally, confocal laser endomicroscopic examination of pancreatic cysts is a novel technique also currently being studied to better characterize cystic pancreatic lesions.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• • of considerable interest
- Spinelli KS, Fromwiller TE, Daniel RA et al. Cystic pancreatic neoplasms: observe or operate? Ann. Surg. 239(5), 651–659 (2004).
- Lee LS, Clancy T, Kadiyala V, Suleiman S, Conwell DL. Interdisciplinary management of cystic neoplasms of the pancreas. Gastroenterol. Res. Prac. 2012, 513163 (2012).
- Demos TC, Posniak HV, Harmath C, Olson MC, Aranha G. Cystic lesions of the pancreas. AJR Am. J. Roentgenol. 179(6), 1375–1388 (2002).
- Adsay NV, Hasteh F, Cheng JD, Bejarano PA, Lauwers GY, Batts KP. Lymphoepithelial cysts of the pancreas: a report of 12 cases and a review of the literature. Mod. Pathol. 15(5), 492–501 (2002).
- Haddad MC. Hydatid cyst of the pancreas as a cause of pancreatic cystic lesion. AJR Am. J. Roentgenol. 181(3), 885–886 (2003).
- Koenig TR, Loyer EM, Whitman GJ, Raymond AK, Charnsangavej C. Cystic lymphangioma of the pancreas. AJR Am. J. Roentgenol. 177(5), 1090 (2001).
- Triantopoulou C, Kolliakou E, Karoumpalis I, Yarmenitis S, Dervenis C. Metastatic disease to the pancreas: an imaging challenge. Insights Imaging 3(2), 165–172 (2012).
- Cocieru A, Brandwein S, Saldinger PF. The role of endoscopic ultrasound and cyst fluid analysis in the initial evaluation and follow-up of incidental pancreatic cystic lesions. HPB (Oxford) 13, 459–462 (2011).
- Brugge WR, Lewandrowski K, Lee-Lewandrowski E et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 126, 1330–1336 (2004).
- Roggin KK, Chennat J, Oto A, Noffsinger A, Briggs A, Matthews JB. Pancreatic cystic neoplasm. Curr. Probl. Surg. 47, 459–510 (2010).
- Huang ES, Turner BG, Fernandez-Del-Castillo C, Brugge WR, Hur C. Pancreatic cystic lesions: clinical predictors of malignancy in patients undergoing surgery. Aliment. Pharmacol. Ther. 31, 285–294 (2010).
- D’Onofrio M, Megibow AJ, Faccioli N et al. Comparison of contrast-enhanced sonography and MRI in displaying anatomic features of cystic pancreatic masses. AJR Am. J. Roentgenol. 189(6), 1435–1442 (2007).
- Laffan TA, Horton KM, Klein AP et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am. J. Roentgenol. 191(3), 802–807 (2008).
- Curry CA, Eng J, Horton KM et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am. J. Roentgenol. 175(1), 99–103 (2000).
- Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am. J. Roentgenol. 151(6), 1133–1138 (1998).
- Song SJ, Min Lee J, Kim YJ et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirowdetector CT and MR imaging using ROC analysis. J. Magn. Reson. Imaging 26(1), 86–93 (2007).
- Sahani DV, Kadavigere R, Blake M, Fernandez-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations – correlation with MRCP. Radiology 238(2), 560–569 (2006).
- Inan N, Arslan A, Akansel G, Anik Y, Demirci A. Diffusion-weighted imaging in the differential diagnosis of cystic lesions of the pancreas. AJR Am. J. Roentgenol. 191(4), 1115–1121 (2008).
- Fatima Z, Ishikawa T, Motosugi U, Muhi A, Sano S. Magnetic resonance diffusionweighted imaging in characterization of pancreatic mucinous cystic lesions. Clin. Radiol. 66(2), 108–111 (2011).
- Mottola JC, Sahni VA, Erturk SM, Swanson R, Banks PA, Mortele KJ. Diffusion-weighted MRI of focal cystic pancreatic lesions at 3.0-Tesla: preliminary results. Abdom. Imaging 37(1), 110–117 (2012).
- Sperti C, Pasquali C, Chierichetti F, Liessi G, Ferlin G, Pedrazzoli S. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann. Surg. 234(5), 675–680 (2001).
- Bertagna F, Treglia G, Baiocchi GL, Giubbini R. F18-FDG-PET/CT for evaluation of intraductal papillary mucinous neoplasms (IPMN): a review of the literature. Jpn J. Radiol. doi:10.1007/s11604-012-0176-0172 (2013) (Epub ahead of print).
- Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am. J. Roentgenol. 189(3), 657–661 (2007).
- Visser BC, Muthusamy VR, Yeh BM, Coakley FV, Way LW. Diagnostic evaluation of cystic pancreatic lesions. HPB (Oxford) 10(1), 63–69 (2008).
- Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernández-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology 10(2–3), 144–150 (2010).
- Salvia R, Malleo G, Marchegiani G et al. Pancreatic resections for cystic neoplasms: from the surgeon’s presumption to the pathologist’s reality. Surgery 152(3 Suppl. 1), S135–S142 (2012).
- Salvia R, Crippa S, Partelli S et al. Pancreatic cystic tumours: when to resect, when to observe. Eur. Rev. Med. Pharmacol. Sci. 14, 395–406 (2010).
- Ferrone CR, Correa-Gallego C, Warshaw AL et al. Current trends in pancreatic cystic neoplasms. Arch. Surg. 144, 448–454 (2009).
- Walsh RM, Vogt DP, Henderson JM et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery 144, 677–684 (2008).
- Megibow AJ, Baker ME, Gore RM, Taylor A. The incidental pancreatic cyst. Radiol. Clin. N. Am. 49, 349–359 (2011).
- Ip IK, Mortele KJ, Prevedello LM, Khorasani R. Focal cystic pancreatic lesions: assessing variation in radiologists’ management recommendations. Radiology 259(1), 136–141 (2011).
- Sperti C, Beltrame V, Milanetto AC, Moro M, Pedrazzoli S. Parenchyma-sparing pancreatectomies for benign or border-line tumors of the pancreas. World J. Gastrointest. Oncol. 2(6), 272–281 (2010).
- Tanaka M, Chari S, Adsay V et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6, 17–32 (2006).
- Simeone DM. SSAT/AGA/ASGE state of the art conference on cystic neoplasms of the pancreas. J. Gastrointest. Surg. 12(8), 1475–1477 (2008).
- Berland LL, Silverman SG, Gore RM et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J. Am. Coll. Radiol. 7(10), 754–773 (2010).
- Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. AJR Am. J. Gastroenterol. 102(10), 2339–2349 (2007).
- Ahn DW, Lee SH, Kim J et al. Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 6(4), 493–500 (2012).
- Weinberg BM, Spiegel BM, Tomlinson JS, Farrell JJ. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology 138(2), 531–540 (2010).
- Iftimia N, Cizginer S, Deshpande V et al. Differentiation of pancreatic cysts with optical coherence tomography (OCT) imaging: an ex vivo pilot study. Biomed. Opt. Express 2(8), 2372–2382 (2011).
- Huang D, Swanson EA, Lin CP et al. Optical coherence tomography. Science 254(5035), 1178–1181 (1991).
- Hariri LP, Bonnema GT, Schmidt K et al. Laparoscopic optical coherence tomography imaging of human ovarian cancer. Gynecol. Oncol. 114(2), 188–194 (2009).
- Kraft M, Glanz H, von Gerlach S, Wisweh H, Lubatschowski H, Arens C. Clinical value of optical coherence tomography in laryngology. Head Neck 30(12), 1628–1635 (2008).
- McLaughlin RA, Scolaro L, Robbins P, Hamza S, Saunders C, Sampson DD. Imaging of human lymph nodes using optical coherence tomography: potential for staging cancer. Cancer Res. 70(7), 2579–2584 (2010).
- Michel RG, Kinasewitz GT, Fung KM, Keddissi JI. Optical coherence tomography as an adjunct to flexible bronchoscopy in the diagnosis of lung cancer: a pilot study. Chest 138(4), 984–988 (2010).
- Evans JA, Bouma BE, Bressner J et al. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest. Endosc. 65(1), 50–56 (2007).
- Testoni PA, Mariani A, Mangiavillano B, Arcidiacono PG, Di Pietro S, Masci E. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. AJR Am. J. Gastroenterol. 102(2), 269–274 (2007).
- Cizginer S, Deshpande V, Iftimia N, Karaca C, Brugge WR. Optical coherence tomography (OCT) imaging can detect fine morphologic features of pancreatic cystic neoplasms and differentiate between mucinous and non-mucinous cysts. Gastroenterology 136(5), A–45 (2009).
- Kwon RS, Simeone DM. The use of proteinbased biomarkers for the diagnosis of cystic tumors of the pancreas. Int. J. Proteomics 2011, 413646 (2011).
- Khalid A, Zahid M, Finkelstein SD et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest. Endosc. 69(6), 1095–1102 (2009).
- Zhang XJ, Ye H, Zeng CW, He B, Zhang H, Chen YQ. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J. Hematol. Oncol. 3, 46 (2010).
- Habbe N, Koorstra JB, Mendell JT et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol. Ther. 8(4), 340–346 (2009).
- Allen PJ, Qin LX, Tang L, Klimstra D, Brennan MF, Lokshin A. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann. Surg. 250(5), 754–760, (2009).
- Crowell PL, Schmidt CM, Yip-Schneider MT, Savage JJ, Hertzler DA, Cummings WO. Cyclooxygenase-2 expression in hamster and human pancreatic neoplasia. Neoplasia 8(6), 437–445 (2006).
- Maker AV, Katabi N, Gonen M et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg. Oncol. 18(1), 199–206 (2010).
- Scarlett CJ, Samra JS, Xue A, Baxter RC, Smith RC. Classification of pancreatic cystic lesions using SELDI-TOF mass spectrometry. ANZ J. Surg. 77(8), 648–653 (2007).
- Greco E, Basso D, Fogar P et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1beta not by transforming growth factor-beta1. Int. J. Biol. Markers 20, 235–241 (2005).
- Maker AV, Katabi N, Qin LX et al. Cyst fluid interleukin-1b (IL1b) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin. Cancer Res. 17(6), 1502–1508 (2011).
- Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: part II. Mucinous cystic neoplasms. Surg. Oncol. 20(2), 93–101 (2011).
- Kurihara N, Kawamoto H, Kobayashi Y et al. Vascular patterns in nodules of intraductal papillary mucinous neoplasms depicted under contrast-enhanced ultrasonography are helpful for evaluating malignant potential. Eur. J. Radiol. 81(1), 66–70 (2012).
- Morris-Stiff G, Falk GA, Chalikonda S, Walsh RM. Natural history of asymptomatic pancreatic cystic neoplasms. HPB (Oxford) 15(3), 175–181 (2013).
• • Provides a brief overview of the clinical problem followed by interdisciplinary management algorithms based on the current literature, including recent guidelines.
• • Shows that even in an experienced, high-volume center, preoperative diagnosis was incorrect in one-third of incidentally discovered cystic pancreatic lesions.
• • Shows the diagnostic accuracy by comparing the preoperative and final pathologic diagnoses in 476 patients who underwent resections for pancreatic cystic neoplasms.
• • Presents the Sendai consensus guidelines that have been widely adopted in the management process of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms.
•• Presents in detail all scenarios that one may encounter during the evaluation of different asymptomatic cystic pancreatic lesions.