Special Report - Imaging in Medicine (2013) Volume 5, Issue 6
Measuring patient satisfaction with the CT consent process: the COMRADE outcome measure
Patrick Rogers*1, Simon Lloyd2, Dushyant Shetty3, Paul Newell4 and David Gay5
1Peninsula Radiology Academy,Plymouth, Devon, UK
4Centre for Medical statistics and Bioinformatics, Plymouth, UK
5Plymouth Hospitals NHS Trust, Devon, UK
Abstract
Keywords
communication; informed consent; outcome assessment (healthcare); radiation; risk assessment; x-ray computed tomography
Background
There are increasingly frequent calls for some patients to be involved in a more formal consent process for high-dose CT scans. A BMJ article published in 2011 suggests that: “Patients should be provided with the information needed to understand the potential benefits and risks of the intervention and assign subjective weight to these factors, in order to make an informed choice. Provision of this data is often overlooked" [1]. More recently three ‘active’ methods of communicating information about the risks have been encouraged: first, provision of written material; second, verbal communication (verbal informed consent); and lastly, verbal communication documented in writing with consent affirmed with the subject’s signature (written informed consent) [2]. It has been commented that at higher doses and risks the need for more formal and explicit consent becomes essential [3]. There are also calls to change the consent process for susceptible patients, particularly female and pediatric patients [4].
While there are different ways of measuring the effectiveness of consent, demanding that patients are specifically involved implies that patient satisfaction is one metric by which consent could be measured. This is controversial, not least because there are inherent uncertainties involved with estimating dose for radiation exposure, which could potentially be exacerbated by patient involvement rather than resolved. After discussing these difficulties, this article then considers how we might measure the success of a consent process for CT from the patient’s perspective. We describe the combined outcome measure for risk communication and treatment decision making effectiveness (COMRADE) score, an outcome measure that could be used to assess levels of patient perception of effective risk communication and confidence in their decision to undergo CT. While not a direct measure of patient satisfaction, COMRADE could be viewed as one simple, practical means of measuring the success of a consent process. We then evaluate the COMRADE outcome measure by using it to assess patients attending our center’s imaging department for outpatient CT.
Hot topics when considering consent for CT
Immediate benefit of investigations versus delayed consequences of radiation
In medicine we should strive to provide patients with a risk–benefit choice regarding their decision to undergo an intervention or not. The benefit of an intervention is often experienced immediately and the risk is usually related to the intervention – and is therefore likely to have immediate consequences, which could be shortor long-term. However, even with this immediacy of effect, a patient’s ability to understand and retain concepts of risk is often poor and there are several reasons why patients get confused with quantitative assessments of risk and end up using heuristic approaches instead [5,6].
With risk relating to radiation in imaging the dilemma is complex. In the case of stochastic radiation-induced cancer, the harm to the patient may come after a latency period of 5 to 15 years for leukaemia and 10 to 60 years for solid tumors [7]. On the other hand, the benefit, that is correct diagnosis and appropriate management, may be perceived as instantaneous. The perception of risk is strongly affected by whether the effect of the risk is perceived to be immediate or in the future [8].
Communicating and understanding delayed stochastic effects is very poorly researched, but experience of less complicated risk–benefit models suggest that patients will struggle to contextualize and understand the full ramifications of these delayed effects. Understanding of risk is important, but it has been shown that too much attention to radiogenic risk may distract attention from other risks and potential benefits [9]. To add to this problem, “the units used to quantify radiation exposure, effective dose and risk are specialised, complex and have an arcane quality that renders them unsuitable to effective communication with the public and health professionals” [3].
The difficulty of understanding the risk of radiation leads us to consider the question of who will consent the patient. Given the complexity of the topic consent could be extremely time-consuming in certain circumstances [10]; therefore, clinical time needs to be set aside to consent patients in an appropriate manner. It has been established that some non-radiologist clinicians are inaccurate in their estimation of radiation [8,11,12]. If junior doctors are expected to consent patients then we must learn from past mistakes in surgery where juniors used to consent patients for procedures they knew little about [13]. On the other hand if radiologists are to consent the patient then when would they do this? Radiologists suffer from frequent interruptions already [14] and may not have the time.
The justification principle
Currently we justify the risks of CT by trying to establish whether the risks are outweighed by the potential benefits of undergoing the investigation. This justification is the fundamental principle that any future consent process needs to honour. However, some proposed means of obtaining consent include non-trained or nonclinical staff providing patients with written information on CT and consent forms to sign if they are happy with this information. Doctors may then think that, as the patient has consented, the CT request is valid, despite the availability of other modalities with a lower risk profile. We must learn from surgical consent processes of the myth that a signed form is synonymous with valid consent [15]. By suggesting this kind of systematic informed consent in radiology, we must realise that we are potentially introducing a means of bypassing the justification principle.
Dose variation
If we consent the patient for CT we must ask how informative the consent process will be. How accurately can we answer the question: “How much dose will the patient receive”? The answer is complex, and there are a myriad of variables that affect the end dose provided by the CT scanner – and even this figure is not necessarily an accurate assessment. Experienced CT radiologists cannot reliably use scan parameters to predict whether individual examinations will exceed the weighted CT dose index (CTDIw) and volume CT dose index (CTDIvol) diagnostic reference levels (DRLs) [16]. There is quite a large variation in the dose received from the same type of CT examination. Doses within the same institution can vary by up to 100% [17]. Across a region doses may vary by a mean of 13-fold [18]. Additionally, the recorded effective dose for a CT examination may be very different to the actual dose received by the patient. The value calculated for effective dose is subject to a great many uncertainties. It is computed through multiple steps and depends on a number of approximations. Depending on which set of tissue-weighting coefficients are used, effective dose values can vary by 100% or more [19]. For example, an extremely simple ‘shortcut’ method of calculating effective dose in CT requires knowledge only of the scanner output (CTDIvol) and irradiated scan length, the product of which is referred to as the dose length product (DLP). The DLP method uses coefficients that are averaged over a large number of CT systems, and so provides only a quick, broad estimate of effective dose for a ‘standard’ (relatively thin) patient size, which is independent of scanner model, age or sex. The relatively recent application of the concept of effective dose to medical procedures allows comparison between examinations of different techniques. We are warned however of the following: “the medical community must use this information wisely … it in no way represents a risk to any one individual. Effective dose should not be used for epidemiologic studies or the estimation of population risks due to the inherent uncertainties and oversimplifications involved" [20].
At what equivalent radiation dose (millisievert, mSv) should consent occur?
The law does not directly prescribe a threshold above which a formal conversation between doctor and patient concerning consent should occur [21].
Uncertainty in predicting dose means that it is difficult to follow advice such as: “Mandatory use of such forms for higher dose investigations (e.g., >10 mSv) is suggested” [3,22,23].
What is the current risk of radiationrelated cancer?
This is perhaps the most pertinent question and one of the hardest to answer. Recent studies suggest that there is no strong agreement on whether there is a linear no threshold (LNT) model, which is favoured by the National Research Council of the National Academies [24]. It certainly has strong opposition [20] and even the BEIR VII report concedes that, “at doses less than 40-times the average yearly background exposure (100 mSv), statistical limitations make it difficult to evaluate cancer risk in humans” [24]. Recent advances in radiation dose reduction mean that cardiac CT examinations, once one of the most burdensome examinations, with a dose of 12 mSv as recently as 2009 [25], can now be performed at ∼2mSv with the benefit of prospective imaging with iterative reconstruction [26]. No doubt this dose will look outdated in a few years time (although perhaps not to such a significant degree). Therefore, a patient would now need to have 50 cardiac CTs before they reached a level beyond which the National Research Council of the National Academies feels it difficult to evaluate cancer risk.
Interestingly, despite these cautions and uncertainties, raised by both pro-LNT supporters and sceptics, there are regular publications that discuss the risks of cancer from ionizing radiation doses of <20 mSv, for instance a 1 in 8100 risk of cancer has been reported for a 40 year old woman who was a undergoing a routine head CT scan [18]. Furthermore, even if there was a clear association between cancer and CT doses, we do not know what cancer will mean for patients decades from now. With cancer survival rates set to increase further, the risks associated with cancer – morbidity and mortality – are set to fall markedly [27].
The COMRADE outcome measure
The recent change of emphasis in the USA from ‘informed consent’ obtained by the physician to an ‘informed request’ given from the patient, with the use of shared decision making strategies, is another means of introducing greater patient involvement in the consent process [28]. However, given the outlined controversies and difficulties associated with involving patients in the consent process, it is important that we find ways of objectively assessing any changes to the current status quo so that improvements can be established and ineffective alterations ignored. While there are many metrics that can be measured in the consent process (e.g., time, quality of information, etc.), given the drive for greater patient involvement, patient perception of effective risk communication and confidence in decision making should be measures of the success of any consent process.
COMRADE is an outcome measure that can be used to assess levels of patient satisfaction in the consent process. The measure is designed to make “policy implications regarding greater patient involvement … clearer” [29]. It combines items from some existing scales in the field of risk communication and treatment decision making with further items and constructs identified by qualitative research with consumer groups. It has been used by various clinical groups to assess patient satisfaction with the consent process for various interventions [30–32].
COMRADE is a scale consisting of a total of 20 statements that assess two patient-based factors of risk communication and treatment decision- making effectiveness. The 20 statements are split into these two factors, with statements A to J under the first factor, and statements 1 to 10 under the second factor. We decided to adapt the COMRADE outcome measure for the purpose of assessing the effective risk communication and confidence in decision making in patients undergoing high-dose CT. We felt that patient perceptions were important as risk assessments based solely on the accuracy of the knowledge and information exchange may merely expose the established limitation of the patients’ understanding of radiation [33]. The outcome measure was adapted such that the ‘intervention’ was high-dose CT, rather than a medical or surgical treatment. We evaluated this measure by using it to assess patients attending our center’s imaging department for outpatient CT. The hypothesis was that this adapted outcome measure can be used to measure patient satisfaction with the consent process for CT such that any future attempts to improve the consent process via patient involvement can be monitored.
Materials & methods
The COMRADE outcome measure questionnaire was adapted for the purposes of consenting patients for CT (Figure 1). The questionnaire is a series of 20 statements that the patients can agree or disagree with using a five-point scale, with 5 signifying strong agreement and 1 indicating strong disagreement. This gives a score out of 100 for each patient. The adaptation was submitted to the lead author of the original COMRADE paper and further modifications were made in view of suggestions. The setting for the questionnaire was two large National Health Service (NHS) general hospitals in the UK. The NHS runs a tax-funded, free at the point-of-care health system. Administration staff were briefed to ask outpatients attending a CT scan to fill out the questionnaire. Only those patients undergoing abdominal/thoracic or pelvic CT scans were asked to complete the questionnaire as these CTs were more likely to be of a relatively higher dose (which we defined as an effective dose of more than 2 mSv). While policymakers may use a greater dose at which to implement a consent process, choosing a lower dose increased our yield of patients. Although future consent processes may be limited to certain demographic criteria (e.g., young, female patients), for the purposes of this study there was no age limit on participating patients. Enrolment in the study was on a voluntary basis, and the number of patients who declined to fill out a form was not recorded. The patients were guaranteed anonymity. The patient’s gender, age and type of CT examination were recorded where possible. Patients were not exposed to any additional consent process other than that which they would have ordinarily received from their referring doctor, either a general practitioner or a specialist doctor. This was in order to achieve a baseline for current patient satisfaction within our region. The data will serve as a marker for any subsequent change to the consent process within the department. The idea is that this scale could be handed to patients for them to complete with minimum disruption to the department or the patient. This was a pragmatic study, aiming to be economically useful and widely reproducible and therefore no specific funding was requested. The study was an evaluation of routine service delivery, and therefore ethical approval was not sought.
Results
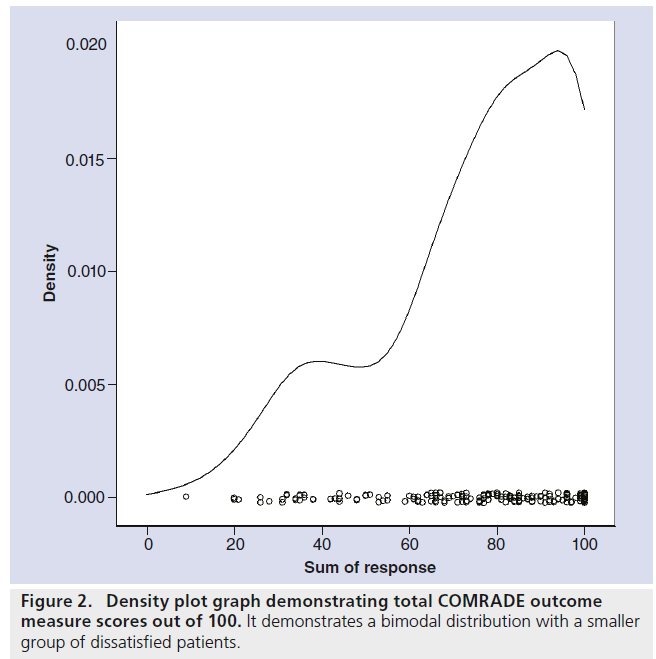
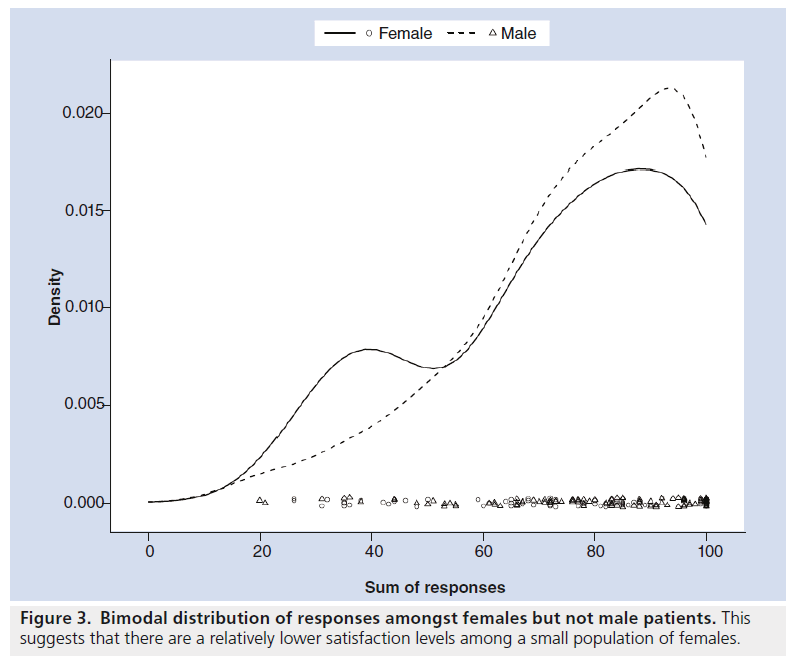
192 questionnaires were completed. The average age of the patients was 65.1 years. There were 71 female patients, 98 male patients and 23 of undisclosed gender. There was a bimodal distribution of total scores out of 100 (Figure 2), suggesting that there is a small group of patients who are generally less satisfied with the communication of risks and the confidence in their decision making. The 41 less satisfied patients (with a total average score of less than 60/100) were generally slightly younger (average age 62 years), and were more likely to be female (19/71 female vs 16/98 male) (Figure 3).
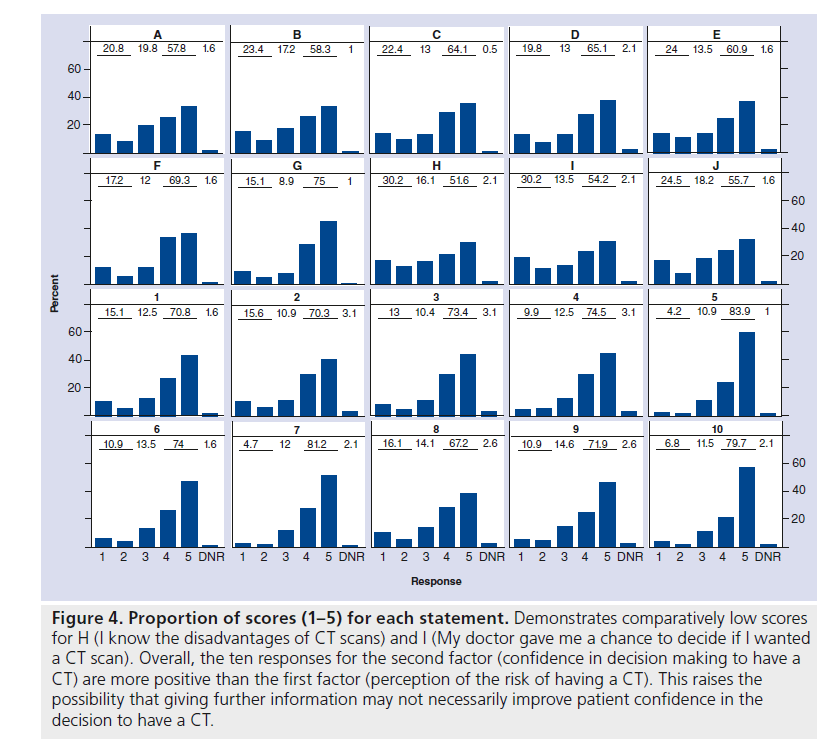
Using the COMRADE outcome measure, we observed a general tendency for patients to give high responses. For each statement, more than half of respondents agreed or strongly agreed, the percentages ranging from 51.6% for statement H to 83.9% for statement 5. For instance, this indicates that patients tended to be less confident about knowing the disadvantages of a CT scan (H), but they tended to be sure that it was the right decision for them (5). This apparent conflict between not knowing the disadvantages of CT – an information deficit – but nevertheless being confident in their decision to have a CT is reflected throughout the results of the two factors: perception in risk communication and confidence in the decision. The average overall score for the ten communication of risk items is 3.6, whereas the average for the ten confidence in decision making items is 4.1. The scores for the each individual question are displayed diagrammatically in bar charts, which highlights this trend (Figure 4).
Figure 4.Proportion of scores (1–5) for each statement. Demonstrates comparatively low scores for H (I know the disadvantages of CT scans) and I (My doctor gave me a chance to decide if I wanted a CT scan). Overall, the ten responses for the second factor (confidence in decision making to have a CT) are more positive than the first factor (perception of the risk of having a CT). This raises the possibility that giving further information may not necessarily improve patient confidence in the decision to have a CT.
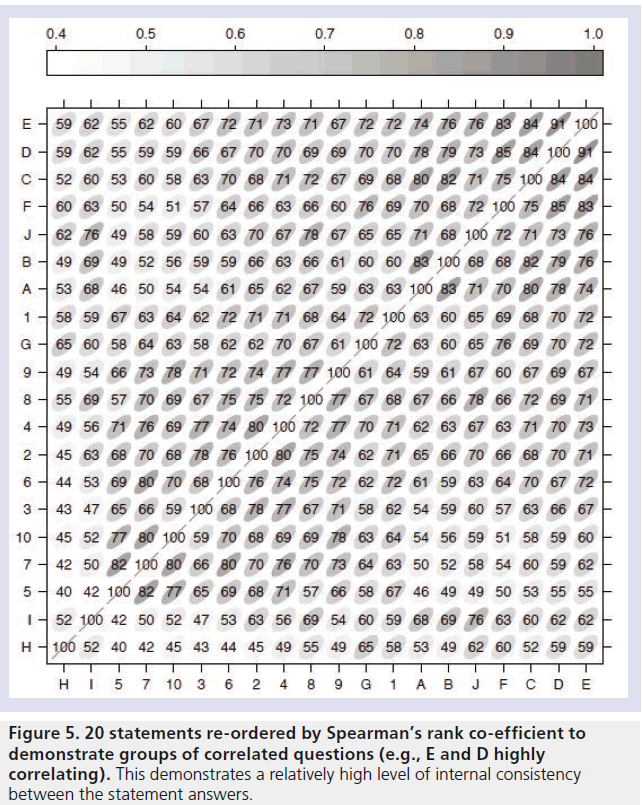
Correlation between the statements was estimated using Spearman’s rank correlation coefficient (Figure 5). All the correlation coefficients are greater than 0.4, indicating that high responses to one statement tend to be related to high responses to other statements. This demonstrates a high degree of internal consistency within the questioning, meaning that patients overall experiences were well represented by the individual statements on the questionnaire. We calculated Cronbach’s a to assess internal consistency between items on the questionnaire. We found an estimate of a = 0.982, with a 95% bootstrap CI of (0.974, 0.989).
Discussion
Principal findings
Recent calls for patient involvement in the consent process for CT are controversial. The potential obstacles and problems to involving patients in more informed consent for CT examinations are formidable. If we are to incorporate patients more in the consent process then we need to be able to demonstrate improvement otherwise changes may be costly, unproductive and time-consuming.
These results demonstrate that the adapted COMRADE outcome measure can be successfully adapted to evaluate patient perception with risk communication and confidence in decision making when attending for a CT scan. It can be used as an inexpensive and practical way to build a profile of satisfaction amongst patients, so that radiology departments can monitor any suggested changes to the risk communication and consent process if they are introduced in the future.
The results demonstrate that patients have a reasonably high level of satisfaction with communication of risks regarding their CT. It highlights a difference between the level of perception of communication of information, and the level of confidence in the patient’s decision to have a CT. This raises the possibility that despite feeling that the information regarding their CT is less than optimal, patients still feel confident with the decision to have a CT. Perhaps patients are content with a paternalistic approach to decision making regarding CT. Thus, it is possible that providing more information to the patient may not necessarily improve their confidence in the decision making process, as they do not appear inextricably linked.
The fact that younger women are more likely to score poorly with their perception of risk communication suggests that females may be more prone to worry about the risk implications of radiation. More research needs to be done to investigate whether young females are in need of more information around the consent process for CT, and whether this group needs to be specifically targeted.
Strengths & weaknesses
This is a proof-of-concept study to establish that assessing patient perception of effective risk communication and confidence over decision making for CT is possible. The numbers compare reasonably well with other studies of this type, however only provisional statistical conclusions can be made from the results. Only two similar healthcare settings were used, which could lead to a population bias. Patients were guaranteed anonymity, but patients may have felt pressured to provide positive answers just prior to the CT examination to ensure that their care was not negatively affected, leading to a reporting bias. This paper did not explore the other metrics through which consent can be assessed – for instance the patients accuracy in understanding their risk. This paper has quite a low cut-off for ‘higher dose’ examination (2 mSv) whereas other papers have suggested 10 mSv – this could lead to a bias in the results, which may be falsely reassuring.
Implications for policy & practice
Involving patients in consent for CT is a controversial move in an area of medical practice that is already limited by problems we have highlighted: variable doses of radiation, uncertain risk of cancer, limited patient understanding, limited clinical opportunity for achieving consent, and so on. Policymakers need to establish whether patient involvement is required and whether it will be beneficial. While opinions on this are varied and different metrics can be used to assess its success, the COMRADE outcome measure offers policymakers a means of quantifying whether there is any improvement to patient perception of risk communication and confidence in decision making with different consent processes. Our outcome measure can also help policymakers answer questions that others in the field have raised, such as: how effective are strategies (e.g., dialogue, visual aids, risk scales) for helping patients understand benefit and risk [34]?
Further research
A more rigorous and larger trial needs to question whether there is a need to increase patient involvement with CT, and if so which groups specifically need extra support. Additionally, more studies need to assess a variety of techniques to establish the most economical, ethical and practical solution. The COMRADE outcome measure can be used as one of a range of assessment tools to assess whether more direct patient involvement in the consent process results in any significant improvement. There are many different aspects to the consent process, however, given recent calls for greater patient involvement, analysis of how patients actually feel about consent for CT is increasingly viewed as important.
Conclusion
The debate on consenting for high-dose CT examinations needs to be explored more credibly. There are significant legal, practical, financial and ethical obstacles that need to be overcome before implementing any consent process that aims to promote better informed consent. Patient satisfaction, perception of effective risk communication and confidence in decision making are important measures of the success of any consent process. If we are to implement and try to improve the consent process for CT, then we need to have a means of measuring its success or failure. The COMRADE outcome measure described in this paper is a useful tool for assessing patient satisfaction with a consent process and can be used to assess the success of any changes. However, patient satisfaction is not the only aspect that needs to be considered and without an efficient, practical and economic alternative for improving the consent process that is evidence-based, the debate around obtaining consent for CT is destined to continue without resolution.
Future perspective
The proposed change from presumed or ‘passive’ consent for CT to informed consent would radically alter the current practice in clinical radiology worldwide. We discuss some of the significant technical, financial and practical implications policymakers need to be aware of before such a change in practice. The aims and objectives of such a change would need to be set out before embarking on what many radiologists see as an unnecessary approach to CT imaging. As a tool for assessing levels of patient perception of risk communication and confidence in their decision to undergo CT, the COMRADE measure may be of use, alongside other metrics, in assessing whether a consent process is achieving its objectives and aims.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
- Davies HE, Wathen CG, Gleeson FV. The risks of radiation exposure related to diagnostic imaging and how to minimise them. BMJ 342, d947 (2011).
- Semelka RC, Armao DM, Elias J, Picano E. The information imperative: is it time for an informed consent process explaining the risks of medical radiation? Radiology 262, 15– 18 (2012).
- Malone J, Guleria R, Craven C et al. Justification of diagnostic medical exposures: some practical issues. Report of an International Atomic Energy Agency Consultation. BJR 85(1013), 523– 538 (2012).
- Pearce MS, Salotti JA, Little MP. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380(9840), 499– 505 (2012).
- Ellis DA, Hopkin JM, Leitch AG et al. ‘Doctors’ orders’: controlled trial of supplementary, written information for patients. BMJ 1(6161), 456 (1979).
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. Qual. Health Care. 10(Suppl. 1), i14– i18 (2001).
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist (7thEdition). Lippincott Williams & Wilkins, PA, USA (2011).
- Borgen L, Stranden E, Espeland A. Clinicians’ justification of imaging: do radiation issues play a role? Insights Imaging 1, 193– 200 (2010).
- Balter S, Zanzonico P, Reiss GR et al. Radiation is not the only risk. AJR 196( 4), 762– 767 (2011).
- Ekdahl AW, Hellström I, Andersson L, Friedrichsen M. Too complex and timeconsuming to fit in! Physicians’ experiences of elderly patients and their participation in medical decision making: a grounded theory study. BMJ Open. 2(3), e001063(2012).
- Merzenich H, Krille L, Hammer G. Paediatric CT scan usage and referrals of children to computed tomography in Germany – a cross-sectional survey of medical practice and awareness of radiation related health risks among physicians. BMC Health Serv. Res. 12, 47 (2012).
- Shiralkar S, Rennie A, Snow M. Doctors’ knowledge of radiation exposure: questionnaire study. BMJ 327(7411), 371– 372 (2003).
- Houghton DJ, Williams S, Bennett JD et al. Informed consent: patients’ and junior doctors’ perceptions of the consent procedure. Clin. Otolaryngol. Allied. Sci. 22(6), 515– 518 (1997).
- Halsted MJ, Froehle CM. Design, implementation, and assessment of a radiology workflow management system. Am. J. Rheum. 191(2), 321– 327 (2008).
- Wheeler R. Consent in surgery. Ann. R. Coll. Surg. Engl. 88(3), 261– 264 (2006).
- Zeman RK, Herlihy V, Branham TA et al. Can experienced CT radiologists use technique parameters to predict excessive patient dose? An analysis of the ACR CT accreditation database. J. Am. Coll. Radiol. 8(4), 275– 280 (2011).
- Jaffe TA, Yoshizumi TT, Toncheva G. Radiation dose for body CT protocols: variability of scanners at one institution. Am. J. Rheum. 193, 1141– 1147(2009).
- Smith-Bindman R, Lipson J, Marcus R et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 169(22), 2078– 2086 (2009).
- McCollough CH, Christner JA, Kofler JM. How effective is effective dose as a predictor of radiation risk? Am. J. Rheum. 194(4), 890– 896 (2010).
- Tubiana M, Feinendegen LE, Yang C et al. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 251(1), 13– 22 (2009).
- Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J. Pediatr. Surg. 47(6), 1167– 1169(2012).
- McCollough CH. CT dose: how to measure, how to reduce. Health Phys. 95(5), 508– 517 (2008).
- Singh S, Kalra MK, Shenoy-Bhangle AS. Radiation dose reduction with hybrid iterative reconstruction for pediatric CT. Radiology 263(2), 537– 546(2012).
- National Research Council of The National Acadamies. Heritable genetic effects of radiation in human populations. In: Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. The National Academies Press, Washington, DC, USA, 7 (2006).
- Hausleiter J, Meyer T, Hermann F. Estimated radiation dose associated with cardiac CT angiography. JAMA 301(5), 500– 507(2009).
- McKavanagh P, Lusk L, Ball P et al. A comparative study of standard filtered back projection with novel iterative reconstruction techniques in cardiac CT. Heart 98(S1), A55 (2012).
- Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br. J. Cancer 107(7), 1195– 1202 (2012).
- Moulton B, Collins PA, Burns-Cox N, Coulter A. From informed consent to informed request: do we need a new gold standard? J. R. Soc. Med. 106(10), 391– 394 (2013).
- Edwards A, Elwyn G, Hood K et al. The development of COMRADE – a patient-based outcome measure to evaluate the effectiveness of risk communication and treatment decision making in consultations. Patient Educ. Couns. 50(3), 311– 322 (2003).
- Harmsen CG, Jarbol DE, Nexoe J et al. Impact of effectiveness information format on patient choice of therapy and satisfaction with decisions about chronic disease medication: the ‘Influence of intervention Methodologies on Patient Choice of Therapy (IMPACT)’ cluster-randomised trial in general practice. BMC Health Serv. Res. 13, 76 (2013).
- Powers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. Am. Heart J. 161(4), 673– 680 (2011).
- Kirkegaard P, Edwards A, Hansen B. The RISAP-study: a complex intervention in risk communication and shared decision-making in general practice. BMC Family Practice 11( 1), 70( 2010).
- Chesson RA, McKenzie GA, Mathers SA. What do patients know about ultrasound, CT and MRI? Clin. Radiol. 57( 6), 477– 482 (2002).
- Dauer LT, Thornton RH, Hay JL et al. Feelings, and facts: interactively communicating benefits and risks of medical radiation with patients. Am. J. Rheum. 196(4), 756–761 (2011).