Review Article - Interventional Cardiology (2023) Volume 15, Issue 3
Mechanisms of ischemic heart disease development in diabetes mellitus
- Corresponding Author:
- Alexander V. Blagov
Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia, E-mail: al.blagov2014@gmail.com; - Alexander N. Orekhov
Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia, E-mail: a.h.opexob@gmail.com
Received date: 10-Mar-2023, Manuscript No. FMIC-23-91323; Editor assigned: 13-Mar-2023, PreQC No. FMIC-23-91323 (PQ); Reviewed date: 27-Mar-2023, QC No. FMIC-23-91323;Revised date: 03-Apr-2023, Manuscript No. FMIC-23-91323 (R);Published date: 11-Apr-2023, DOI: 10.37532/1755-5310.2023.15(3).697
Abstract
Coronary Artery Disease (CAD) or Ischemic Heart Disease (IHD) has several risk factors, among which diabetes mellitus is one of the most important. In diabetic patients, the pathophysiology of myocardial ischemia is still unclear: Some have atherosclerotic plaques that impede coronary blood flow, while others have myocardial ischemia due to coronary microvascular dysfunction in the absence of plaques in the epicardial vessels. In the relationship between myocardial metabolism and coronary blood flow, ion channels play a major role, and in patients with diabetes mellitus, they are involved in the pathophysiology of coronary artery disease. Exposure to various cardiovascular risk factors and ischemic state determine the imbalance of the redox state, defined as oxidative stress, as well as advanced glycation end products and their receptors.
Keywords
Diabetes; Coronary Artery Disease; Ischemic Heart Disease; Glucose
Introduction
Diabetes Mellitus (DM) is a complex and heterogeneous chronic metabolic disease caused by elevated blood glucose levels. Diabetes is classified into four different etiological categories: Type 1, type 2, “other specific types” and “gestational diabetes”. Type 1 Diabetes Mellitus (DM1) occurs due to T-cell mediated autoimmune destruction of pancreatic β-cells, leading to insulin deficiency [1]. Type 1 diabetes is more common in young adults, usually under 30 years of age. Type 2 Diabetes (DM2) is characterized by both insulin resistance and deficiency of pancreatic β-cells. Other specific types of diabetes are caused either by single genetic mutations, by other pathological diseases of the pancreas, or by medication. Gestational diabetes develops during pregnancy [2].
Globally, 1 among 11 adults (90%-T2DM) has DM. The onset of T1DM increases gradually from birth and peaks between 4 and 6 years of age and then again between 10 and 14 years of age. Approximately 45% of children seek medical care before the age of ten. The prevalence among people under the age of 20 is about 2.3 per 1000 people. Although most autoimmune diseases are more common in women, there are no clear gender differences in the incidence of type 1 diabetes in children [3]. The onset of type 2 diabetes usually occurs at a later age, although obesity in adolescents leads to an increase in the incidence of type 2 diabetes at a younger age. T2DM has a prevalence of about 9% in the general population of the United States, but about 25% in those over 65 years of age. The International Diabetes Federation estimates that 1 in 11 adults aged 20 to 79 years worldwide had DM [4].
Coronary artery disease is a condition in which there is an insufficient supply of blood and oxygen to the myocardium. This occurs as a result of occlusion of the coronary arteries and leads to a mismatch in oxygen demand. From an epidemiological point of view, mortality from coronary heart disease (CHD) is about 12% of all causes of death, and in the population aged 35 to 74 years, myocardial infarction is the main cause of mortality and morbidity [5]. Ischemic heart disease is a multifactorial phenomenon. Etiological factors can be divided into non-modifiable and modifiable factors. Non-modifiable factors include gender, age, family history, and genetics. Modifiable risk factors include smoking, obesity, lipid levels, and psychosocial variables. One of the significant risk factors involved in the pathogenesis of CHD is diabetes mellitus. IHD is the main long-term complication and cause of death in diabetic patients [6].
Literature Review
The mechanisms of coronary flow regulation in normal conditions
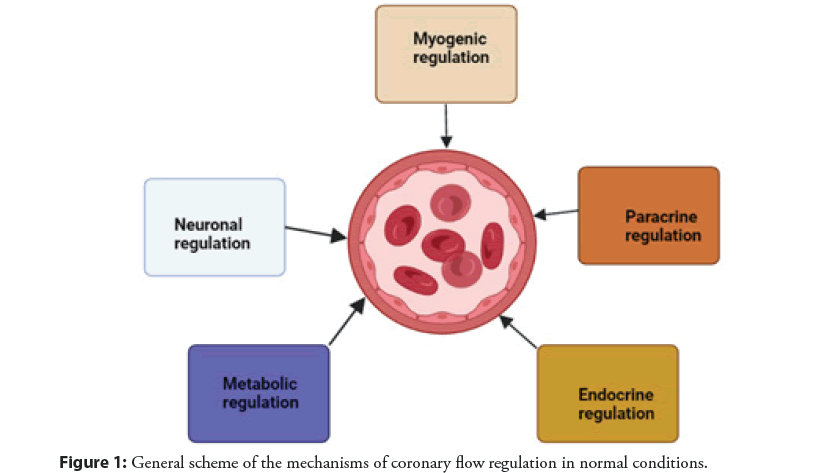
The regulation of coronary blood flow is determined by several mechanisms, including extravascular compressive forces (tissue pressure), coronary perfusion pressure, myogenic, local metabolic, endothelial, and neural and hormonal influences. Together, these mechanisms regulate coronary blood flow and provide an overall balance between oxygen delivery to the myocardium (supply) and metabolism (consumption) [7]. Microcirculation, characterized by small arteries and arterioles with a diameter of 50 to 200 μm, is the most important site for the regulation of total coronary resistance [8]. In the “interaction” between the myocardium and the coronary artery, several mechanisms of regulation of vascular tone operate to ensure adequate coronary blood flow to the myocardium and their contribution varies depending on the area under consideration [7]. The microcirculation, which is the distal part of the coronary arterial circulation, is the main place where metabolic and myogenic regulatory mechanisms operate, while the epicardial part of the artery, which is the proximal part of the coronary arterial circulation, is the main place where neurohumoral and shear stresses act as corresponding mechanisms of regulation [8]. At rest, the myocardium extracts about 80% of the coronary circulation, and the oxygen consumption is 10 ml of oxygen per minute per gram of myocardial tissue [7]. When myocardial oxygen consumption increases, coronary blood flow must modulate its vascular tone to ensure adequate supply to the myocardium. For these reasons, there are several mechanisms for regulating vascular tone. Neurohumoral regulation is carried out through sympathetic and parasympathetic innervation, which is expressed in the coronary arteries and their tonic activity determines the basal vascular tone at rest [9]. The endothelium is involved in the regulation of coronary blood flow by producing several molecules with paracrine effects such as the metabolites of arachidonic acid and NO which promotes vasodilation and endothelin which promotes vasoconstriction. Auto regulation acts myogenically, which, by reducing the tension of the vessel wall, guarantees a constant and sufficient coronary flow to the myocardium [7]. The myogenic response is mediated by a change in the level of calcium in smooth muscle cells, which modulates the state of their contraction [10]. Coronary blood flow is also modulated by several hormones such as progesterone, testosterone, histamine, and Antidiuretic Hormone (ADH), which are vasodilators, and angiotensin II, which is a vasoconstrictor. Insulin mediates both vasoconstrictions by activating sympathetic fibers and vasodilation by stimulating NO production [8]. Metabolic regulation acts mainly on microcirculation and is important for the rapid adaptation of coronary blood flow to the metabolic needs of the myocardium. The metabolic regulation effect is mediated by several molecules produced by cardiomyocytes that target specific receptors and ion channels. These molecules include Carbon Dioxide (CO2), adenosine, oxygen, H2O2, superoxide, and other reactive oxygen species [11]. The general scheme of the mechanisms of coronary flow regulation in normal conditions is shown in Figure 1.
General pathogenesis of IHD
Ischemic heart disease is a pathophysiological condition caused by a disproportion between myocardial oxygen demand and its supply. Myocardial nutrition depends on the oxygen capacity of the blood and the volume of coronary blood flow [12]. Ischemia is caused by myocardial oxygen demand at the time of occurrence of coronary artery spasm or intravascular coagulation at the site of atherosclerotic plaque rupture. This leads to restriction of coronary blood flow. It is possible to combine all these mechanisms at the same time. In general, the pathology refers to large coronary arteries, the stenosis of which reduces the coronary reserve in proportion to the degree of vasoconstriction. Stenosis may be accompanied by a spasm increasing its size. A ruptured atherosclerotic plaque often becomes a substrate for intravascular coagulation, leading to acute coronary events [13].
Atherosclerotic plaque is an accumulation of fatty material that narrows the lumen of the vessel and impedes blood flow. The first step in this process is the formation of a “fat streak”. The adipose streak is formed by sub endothelial deposition of lipid-rich macrophages, also called foam cells. In vascular stroke, the intimal layer ruptures, and monocytes migrate to the sub endothelial space, where they become macrophages. These macrophages engulf oxidized Low-Density Lipoprotein (LDL) particles and foam cells form. T-cells are activated, which release cytokines only to help in the pathological process. The released growth factors activate smooth muscles, which also take up oxidized LDL particles and collagen and are deposited along with activated macrophages and increase the foam cell population. This process leads to the formation of sub endothelial plaque [14].
Over time, this plaque may increase in size or become stable if no further damage to the endothelium occurs. If it becomes stable, a fibrous cap will form, and the lesion will calcify over time. Over time, the lesion may become hemodynamically significant enough that at the time of increased demands on the myocardial tissue, sufficient blood does not flow, and symptoms of angina pectoris occur. However, symptoms decrease at rest as oxygen demand decreases. For a lesion to cause angina at rest, it must be at least 90% stenotic [12]. Some plaques can rupture and become exposed to tissue factors, leading to thrombosis. This thrombosis can cause subtotal or complete lumen occlusion and can lead to the development of Acute Coronary Syndrome (ACS) in the form of unstable angina [15].
In acute ischemia, oxygen deficiency disrupts the oxidation of glucose and Free Fatty Acids (FFA), so enzymatic cytoplasmic glycolysis becomes the main source of energy. Secreted catecholamines (epinephrine and norepinephrine) increase the hydrolysis of fats reaching the heart. As a result, a reduced glucose supply promotes free fatty acid oxidation, thus becoming the only source of energy in which oxygen consumption increases and the supply decreases rapidly, thereby forcing the cell to switch to anaerobic glycolysis. This causes the accumulation of lactates and hydrogen ions. A few seconds of ischemia impair myocardial contractility and relaxation. Lack of return of myocardial reperfusion within 45-60 minutes leads to necrosis of cardiac cells, i.e. to infarction [16].
Transition factors from diabetes mellitus to coronary artery disease
Advanced Glycation End products (AGEs) production: Advanced Glycation End products (AGEs) are a heterogeneous class of endogenously produced or exogenously produced glycated proteins and lipoproteins. Endogenous formation of AGEs occurs through a complex Maillard reaction in which reducing sugars undergo a series of non-enzymatic reactions leading to the formation of reactive carbonyl compounds and subsequent glycoxidation of proteins, lipids, and nucleic acids. The metabolism of glucose during glycolysis leads to the formation of methylglyoxal, a carbonyl intermediate in the production of some AGEs. Under conditions of oxidative stress, reducing sugars, amino acids, and lipids undergo autoxidation with the formation of additional reactive carbonyl compounds, which leads to the accumulation of AGEs in tissues [17].
AGEs can accumulate in almost all tissues, including the eyes, kidneys, liver, vasculature, reproductive tissues, muscles, bones, and brain. Elevated AGEs in patients with DM may be the result of a cyclic process in which glycated albumin disrupts normal glucose metabolism in muscle and adipocytes, leading to reduced insulin-mediated glucose uptake and hyperglycemia [18]. Many studies have demonstrated an association between elevated levels of AGEs and cardiovascular disease in diabetic patients. Elevated AGEs have been associated with both systolic and diastolic dysfunction in patients with DM. AGEs levels in patients with DM have been shown to correlate with the degree of systolic dysfunction as well as measures of diastolic dysfunction such as delayed relaxation time and end-diastolic diameter [19].
Much evidence suggests that levels of AGEs may be useful as biomarkers for the presence and severity of CAD. In a Japanese study, circulating AGEs levels were higher in patients with type 2 diabetes and obstructive CAD than in patients with non-obstructive CAD [20]. This association was independent of other risk factors for CAD, including smoking, hypertension, hyperlipidemia, and hyperuricemia. In a large study of 1320 patients with type 2 diabetes, elevated levels of glycated albumin correlated with the severity of coronary artery disease as measured by quantitative coronary angiography. Circulating AGE levels have also been associated with the risk of stent restenosis in patients with DM [21].
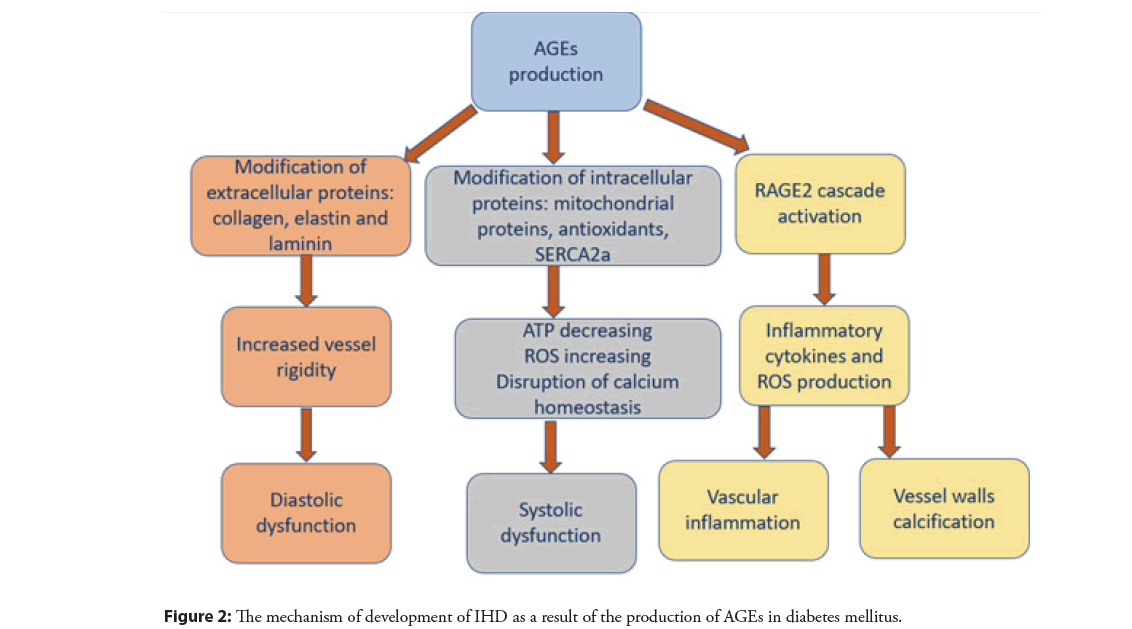
AGEs exert their pathogenic effects through three main molecular mechanisms: Modification of extracellular proteins, modification of intracellular proteins, and activation of signaling cascades through binding to the Receptor for Advanced Glycation End products (RAGE) on the cell surface. All three of these mechanisms may contribute to the development and progression of cardiovascular disease [18].
Modification of extracellular proteins by AGEs can alter the structure, function, and properties of normal tissue, as well as provoke an inflammatory response. Collagen, elastin, and laminin are key structural proteins in the basement membrane and connective tissue. Given their long half-life and amino acid composition, these molecules are highly susceptible to modification by AGEs. Glycosylated collagen molecules are resistant to proteolytic cleavage and cross-link with other extracellular proteins. This leads to a decrease in the flexibility of the vascular walls and the rigidity of the vessels. Glycation of structural extracellular proteins in the myocardial matrix similarly increases myocardial stiffness, contributing to impaired relaxation and diastolic dysfunction [22]. In addition to glycated collagen, the glycation of elastin and laminin in the basement membrane has been shown to impair the adhesion and migration of endothelial cells by destroying cell attachment sites. These changes in cell-matrix interactions are associated with a decrease in stress-induced production of nitric oxide by endothelial cells and impaired vasodilation [23].
Intracellular accumulation of AGEs occurs in the endoplasmic reticulum, leading to stress that can disrupt normal protein folding processes. There are cellular mechanisms to detect misfolded proteins and to activate the response of unfolded proteins, leading to cell apoptosis. Intracellular AGEs can bind to mitochondrial proteins involved in electron transport, decreasing ATP synthesis and increasing superoxide and reactive oxygen species production. In addition, glutathione peroxidase and glutathione reductase, enzymes of the antioxidant system, can be modified by AGEs, resulting in decreased enzymatic activity. In cardiomyocytes, cross-linking of intracellular glycosylated ryanodine receptors and SERCA2a alters calcium homeostasis, reducing tissue contractility and contributing to the development of systolic heart failure [24].
The binding of AGE to full-length RAGE activates many signaling cascades, which ultimately leads to the formation of pro-inflammatory mediators and reactive oxygen species, as well as stimulation of proliferative, fibrous, and thrombotic pathways leading to vascular inflammation [25]. AGE/RAGE interactions lead to NADPH and nitric oxide synthase activation (via NFκβ mediated activation), maintaining the reactive oxygen species production cycle, ongoing enzyme activation, and NFκβ stimulation. In addition, AGEs can directly inactivate nitric oxide, which at normal low intracellular concentrations acts as an antioxidant, antiproliferative, and antithrombotic agent and is an important mediator of vasodilation [17]. Reduced nitric oxide concentrations increase the formation of reactive oxygen and reactive nitrogen species, stimulating the cellular oxidative stress response. Oxidative stress caused by the AGE/RAGE interaction can also lead to apoptosis of vascular smooth muscle, which promotes the calcification of the vessel walls [18]. The mechanism of development of IHD as a result of the production of AGEs is illustrated in Figure 2.
Figure 2: The mechanism of development of IHD as a result of the production of AGEs in diabetes mellitus.
Disruption of ion channels work
Ion channels are end-effectors of various mechanisms of coronary blood flow regulation [7]. The influence of various cardiovascular risk factors and genetic predisposition can lead to impaired function and expression. These conditions may be critical in the determinism of CAD. In particular, altered ion channels can lead to CHD, being involved both in the pathogenesis of CHD and in coronary microvascular dysfunction [8].
Studies have shown that the disruption of several ion channels is associated with a predisposition to diabetes mellitus. AGEs can cause vascular damage both directly and indirectly [26]. First, AGEs detect disruption of the activity of voltage-gated potassium channels (Kv-channels) in Vascular Smooth Muscle Cells (VSMCs) via a glycation reaction. Second, the interaction between AGEs and their receptors is a stimulus for oxidative stress, which is associated with the suppression of the expression of Kv 1.2 and Kv 1.5 proteins and mRNA [27]. These mechanisms cause a change in the regulation of coronary blood flow due to disruption of both non-endothelial and endothelial-dependent mechanisms of vasodilation [26].
Moreover, the activity-of calcium-dependent potassium channel a (KCa channel) was disturbed in both DM2 and DM1 [28]. The studies performed have shown that the molecular basis for the disruption of the KCa channel activity is higher ubiquitination of the BK-β1 subunit. However, Nuclear factor E2-related factor-2 (Nrf2) regulates the redox state of cells, and its expression is reduced due to oxidative stress associated with T2D. Lower Nrf2 expression is associated with lower inhibition of Murf1 enzyme activity. Considering that Murf1 is an E-3 ligase involved in the mechanisms of ubiquitination, it determines the increase in BK-β1 ubiquitination and, as a result, the disruption of the KCa channel [29].
As for triphosphate-adenosine-sensitive potassium channels (K-AT P-channels), they are also highly expressed in α and β pancreatic is let cells. K-ATP pancreatic channels modulate the production of insulin and glucagon by pancreatic islets, playing a major role in the regulation of glucose homeostasis [30]. K-AT P-channels are normally open and determine the outward flow of potassium, which keeps the β-cell in a hyperpolarized state. When the glycemic level increases, glucose is transported into the β-cell by the GLUT2 transporter. An increase in glucose concentration by β-cells stimulates the production of ATP through aerobic glycolysis and other metabolic pathways. An increase in the level of intracellular A TF causes inhibition of K-ATP channels, a decrease in the outflow of potassium to the outside, and the subsequent depolarization of β-cells. Depolarization determines the activation of voltage-dependent calcium channels, which causes an increase in the level of intracellular calcium and subsequent exocytosis of insulin granules [31]. The K-AT F-channels also seem to play a major role in the simultaneous regulation of α-cell activity. As for α-cells, the closure of K-AT P is paradoxically associated with cell hyperpolarization and inhibition of glucagon secretion [32]. The function of the K-AT P channel is strictly related to the secretion of insulin and glucagon and, consequently, to the level of glucose in the blood. There have been several studies that have demonstrated how mutations and Single Nucleotide Polymorphisms (SNPs) of genes encoding the K-ATP subunit can cause dysregulation of channel activity, determining a higher or lower risk of developing type 2 diabetes mellitus and therefore cardiovascular disease diseases in humans [9].
The KCa 3.1 channel is expressed by GMCS and regulates their cell cycle and proliferation. In conditions such as DM, this channel becomes activated and stimulates the proliferation and migration of GMCS, causing the onset and growth of atherosclerotic lesions. The same KCa 3.1 channel is also expressed by macrophages, lymphocytes, and platelets [33]. Its activation is associated with a long-term increase in the level of intracellular calcium, which stimulates cell recruitment and proliferation, the secretion of cytokines, and, consequently, an increase in the inflammatory cascade [34]. In the arterial wall, all these biological processes contribute to the onset and progression of atherosclerotic lesions. The KCa 3.1 channel is not expressed in monocytes but is highly expressed in macrophages that make up atherosclerotic plaques. The same channel plays a major role in the polarization of M1 macrophages and increases the instability of atherosclerotic plaques [34].
Kv channel 1.3 is expressed both by vascular smooth muscle cells, where it stimulates their proliferation and migration and by lymphocytes, where it promotes the proliferation and production of cytokines [35]. The study showed a high expression of the Kv 1.3 channel in macrophages of patients with the acute coronary syndrome, suggesting the role of this channel in the initiation and progression of atherosclerosis and plaque instability. Upregulation of the expression and function of the Kv1.3 channel can stimulate greater activation of Extracellular signal-Regulated Kinase (ERK) through its phosphorylation. ERK belongs to the family of Mitogen-Activated Protein Kinases (MAPKs) and mainly promotes the recruitment of macrophages to the arterial intima [36].
It has also been demonstrated that activation of the K-AT P channels expressed by macrophages plays a major role in the instability and vulnerability of atherosclerotic plaques. Indeed, it may enhance the inflammatory cascade through continuous stimulation of the MAPK/NF-κB pathways [9].
Development of oxidative stress
Oxidative stress is defined as a state of cellular excess of oxidant molecules compared to antioxidant ones. The presence of oxidants is usually neutralized by the presence of antioxidant cellular systems, which include both enzymatic molecules such as Superoxide Dismutase (SOD) and catalase and non-enzymatic molecules such as trans-retinol 2 and ascorbic acid. The activity of these systems and the regulation of the redox state of cells are critical for the functioning and survival of cells. When ROS are produced in small amounts, they are involved in several physiological mechanisms related to the cardiovascular system [6]. They stimulate angiogenesis through Vascular Endothelial Growth Factor (VEGF) and are involved in the regeneration, proliferation, and migration of endothelial cells. Hydrogen peroxide is critical for post ischemic neovascularization and is also involved in the regulation of coronary endothelial-dependent and independent vasodilation [9]. Under pathological conditions, damage and/or overload of antioxidant systems renders them unable to counteract the production of oxidants. Reactive Oxygen Species (ROS) play a central role as mediators of oxidative stress and its complications. This term defines several agents, including oxygen radicals such as Hydroxyl (OH-), Superoxide (O2-), and peroxyl (RO2-), as well as several non-radical oxygen species such as hydrogen peroxide (H2O2). However, ROS are highly reactive molecules and, if accumulated, can cause several modifications in the structure and function of DNA, proteins, and lipids [6]. Metabolic cell activity and environmental factors such as malnutrition and smoking contribute to ROS production and hence oxidative stress, which may predispose to several pathological conditions such as neurological disease, cancer, atherosclerosis, hypertension, diabetes mellitus, and cardiovascular diseases. There is an important relationship between oxidative stress and the development of diabetes mellitus and its complications [37]. Indeed, in patients with diabetes mellitus, not only excessive production of ROS is observed, but also a violation of the function of the antioxidant mechanism, as well as a stronger and more prolonged inflammatory response. In diabetes mellitus, ROS, together with the inflammatory response and hyperglycemia, play a central role in the initiation and progression of vascular damage, supporting the process of atherosclerosis and microvascular dysfunction. SD is a stimulus for ROS production [38]. Hyperglycemia and an excess of intravascular ROS can cause not only an increase in Low-Density Lipoproteins (LDL), chylomicrons, and total cholesterol, but also oxidation and glycation of lipoproteins, increasing their atherogenicity and accelerating the atherosclerotic process [6]. Hyperglycemia increases the production of AGEs, which together with LDL can contribute to the activation of both the inflammatory response and vascular damage through the expression of IL-1β and TNF-α. Also, AGEs and AFK_ stimulate LDL oxidation. O c-LDL causes a decrease in the production of nitric oxide in the endothelium and, through the activation of caspases-3 and 9, stimulates apoptosis of endothelial cells [39]. ROS promote the development of atherosclerosis by also causing worsening of endothelial dysfunction, by increasing the expression of adhesion molecules such as Intercellular Adhesion Molecule-1 (ICAM-1) and Vascular Adhesion Molecule-1 (VCAM-1), and by modulating the expression of various growth factors, which is important for proliferation of GMCS. In diabetic patients, the main source of intravascular ROS is NADPH oxidase, the expression of which is greatly increased compared to patients without diabetes [6]. There are 4 isoforms of nitric oxide, NOx1, NOx2, NOx4, and NOx5, which are overexpressed and play a critical role in the progression of atherosclerosis in diabetic patients. NOx1 is expressed by endothelial cells. A decrease in NOx1 expression is associated with a decrease in the adhesion of leukocytes to the vascular wall and the recruitment of macrophages [40]. NOx4 is expressed by endothelial and muscle cells and plays a protective role in the vessel wall. A decrease in its expression supports an increase in the production of inflammatory markers such as IL-1 and MCP-1 and the progression of atherosclerosis [41]. Moreover, reduced expression of NOx4 on smooth muscle cells is associated with reduced expression of contractile genes and higher production and deposition of collagen [42]. NOx5 can alter the activity of endothelial Nitric Oxide Synthase (eNOS), contributing to endothelial dysfunction. Within the cell, the most important site for the formation of free radicals is in the mitochondria, as they are the energy center of the cell. Glucose from the bloodstream enters the cell and is used to produce ATP. Glycolysis produces pyruvate, ATP, NADH, and FADH2. NADH and FADH2 are transported into mitochondria and play the role of electron donors during oxidative phosphorylation. In the state of hyperglycemia, many electrons are lost in the mitochondrial respiratory chain, which becomes the most important source of O2- overproduction [43]. Moreover, an association has been found between ROS production in human atherosclerotic coronary arteries and the p22phox subunit of NADPH oxidase. Hyperglycemia increases Diacylglycerol (DAG) by activating phospholipase C or D, which activates Protein Kinase C (PKC). PKC activates NADPH oxidase. Activation of the NADPH oxidase complex requires the movement of cytosolic components to the plasma membrane, and their association with NOx2 leads to the formation of ROS [6].
MicroRNAs may also play a role in regulating the expression of proteins such as ion channels and their subunits. Several stimuli such as H2O2, Ultraviolet (UV), and ionizing radiation can induce both ROS production and modification of miRNA expression [44]. The results of the study demonstrated a strong activation of the miR-200 family in endothelial cells subjected to oxidative stress caused by hyperglycemia and hyperlipidemia. Considering that microRNAs are involved in several biological mechanisms, such as apoptosis, proliferation, and differentiation, it becomes clear that they can also be involved in the pathological processes of IHD [6].
Discussion
It has long been known that age, gender, hyperlipidemia, hypertension, and smoking contribute to the risk of developing coronary heart disease. It is known that concomitant diabetes mellitus poses an additional risk. Moreover, the risk of developing cardiovascular diseases is the same for patients with type 2 diabetes mellitus and for patients with type 1 diabetes mellitus, even if there are gender and age differences between these two types [9]. The underlying mechanism of the pathophysiology of diabetes mellitus is a state of long-term insulin resistance that is strongly associated with hyperglycemia followed by compensatory hyperinsulinemia. Hyperglycemia, insulin resistance, and excess fatty acid production lead to increased systemic oxidative stress, an inflammatory response, and increased production of glycation products. All these mechanisms contribute both to the onset and progression of coronary atherosclerosis and coronary microvascular dysfunction [9].
AGEs are currently an attractive therapeutic target. Numerous in vitro and animal studies have demonstrated the positive effect of lowering AGEs levels and activating the AGE/RAGE pathway in preventing and arresting complications of DM, including cardiovascular disease [45]. Several compounds have been shown to reduce the accumulation of AGEs, either by blocking the formation or by increasing their removal. Aminoguanidine and pyridoxamine have antioxidant properties that inhibit the formation of AGEs [46].
Among potassium channels, K-ATP is the main pharmacological target in the treatment of diabetes mellitus and cardiovascular diseases. In this context, it is involved in several pathophysiological processes and for this reason, shows remarkable therapeutic potential. In diabetic patients, pancreatic K-ATP β-cells, in particular SUR subunits, are a target for sulfonylurea, which act as antagonists of these channels, causing them to close [47]. Sulfonylureas promote the depolarization of β-cells and increase insulin secretion. Diazoxide is an agent that opens K-ATP channels Kir6.2/SUR1 and is used in hypertensive crises. Pinacidil and cromakalim are openers of the Kir6.2/SUR2A K-AT F and Kir6.2/SUR2B K-AT F channels and determine a decrease in arteriole resistance, a decrease in blood pressure, and vasodilation [47]. Moreover, some antioxidants can oppose the effect of ROS and preserve ion channel function. Several studies have described the protective role of some NOx inhibitors against the complications of DM. They can reduce the production of ROS, and among them are probucol, apocynin, plumbagin, and GLX351322 [48].
The Sirtuin 1 gene, called anti-aging, is responsible for a number of important processes in the body, including cell proliferation, apoptosis, lipid and glucose metabolism, and aging [49,50]. Not surprisingly, Sirtuin 1 activity or expression is modulated in a number of diseases, including cardiovascular diseases. In the study in patients with diabetes mellitus [51], accumulation of AGEs was shown to be associated with reduced expression of Sirtuin 1. Thus, Sirtuin 1 may be a potential therapeutic target and a prognostic marker for the development of coronary artery disease in patients with diabetes mellitus.
Conclusion
The regulation of coronary blood flow is heterogeneous and complex. Several ion channels play a key role in this regulation and in its homeostasis. Changing these mechanisms can lead to coronary artery disease, which is one of the most common causes of death in the world. Oxidative stress can also be a dangerous condition for the functioning of organs and systems and plays an important role in the progression of coronary artery disease, especially in patients with diabetes mellitus. Enhanced glycation end products and their receptors have also been shown to play a role in the development and severity of CAD in DM, however, to date, the literature evaluating these issues has been limited by differences in the quantification of AGE and RAGE as well as inconsistent measurements of specific molecules. Therefore, more research is needed in this area.
Author Contributions
Writing-original draft preparation, AVB, SGK; writing-review and editing, VNS, MAP, AVG, ANO.
Funding
This work was supported by the Russian Science Foundation (Grant#22-15-00134).
References
- Banday MZ, Sameer AS, Nissar S, et al. Pathophysiology of diabetes: An overview. Avicenna J Med. 10(4): 174-188 (2020).
- Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 6(6): 850-867 (2015).
- Mobasseri M, Shirmohammadi M, Amiri T, et al. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot Perspect. 10(2): 98-115 (2020).
- Khan MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes-global burden of disease and forecasted trends. J Epidemiol Glob Health. 10 (1): 107-111 (2020).
- Bauersachs R, Zeymer U, Briere JB, et al. Burden of coronary artery disease and peripheral artery disease: A literature review. Cardiovasc Ther. 1-9 (2019).
- Severino P, D'Amato A, Netti L, et al. Myocardial ischemia and diabetes mellitus: Role of oxidative stress in the connection between cardiac metabolism and coronary blood flow. J Diabetes Res. 1-16 (2019).
- Goodwill AG, Dick GM, Kiel AM, et al. Regulation of coronary blood flow. Compr Physiol. 7(2): 321-382 (2017).
- Fedele F, Severino P, Bruno N, et al. Role of ion channels in coronary microcirculation: A review of the literature. Future Cardiol. 9(6): 897-905 (2013).
- Severino P, D'Amato A, Netti L, et al. Diabetes mellitus and ischemic heart disease: The role of ion channels. Int J Mol Sci. 19(3): 802 (2018).
- Xie X, Wang Y. Flow regulation in coronary vascular tree: A model study. PLoS One. 10(4): e0125778 (2015).
- Ohanyan V, Yin L, Bardakjian R, et al. Kv1.3 channels facilitate the connection between metabolism and blood flow in the heart. Microcirculation. 24(4): e12334 (2017).
- Conna U, Cvejic E, Granville Smith I, et al. Characterizing acute coronary syndrome-associated depression: Let the data speak. Brain Behav Immun. 48: 19-28 (2015).
- Pajak A. A new model of secondary prevention of cardiovascular disease in patients after acute coronary syndrome. Kardiologia polska. 74(4): 399-402 (2016).
- Nakahara T, Dweck MR, Narula N, et al. Coronary artery calcification. JACC Cardiovasc Imaging. 10(5): 582-593 (2017).
- Dalen JE, Alpert JS, Goldberg RJ, et al. The epidemic of the 20th century: Coronary heart disease. Am J Med. 127(9): 807-812 (2014).
- Jankowski P, Czarnecka D, Badacz L, et al. (2018). Practice setting and secondary prevention of coronary artery disease. Arch Med Sci. 14(5): 979-987 (2018).
- Ott C, Jacobs K, Haucke E, et al. (2014). Role of advanced glycation end products in cellular signaling. Redox Biol. 2: 411-429 (2014).
- Fishman SL, Sonmez H, Basman C, et al. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol Med. 24(1): L59 (2018).
- Koska J, Saremi A, Howell S, et al. Advanced glycation end products, oxidation products, and incident cardiovascular events in patients with type 2 diabetes. Diabetes Care. 41(3): 570-576.
- Kiuchi K. Increased serum concentrations of advanced glycation end products: A marker of coronary artery disease activity in type 2 diabetic patients. Heart. 85(1): 87-91 (2001).
- Shen Y, Pu LJ, Lu L, et al. Serum advanced glycation end-products and receptors as prognostic biomarkers in diabetics undergoing coronary artery stent implantation. Can J Cardiol. 28(6): 737-743 (2012).
- Kosmopoulos M, Drekolias D, Zavras PD, et al. Impact of Advanced Glycation End products (AGEs) signaling in coronary artery disease. Biochim Biophys Acta Mol Basis Dis. 1865(3): 611-619 (2019).
- Liu Q, Hua B, Su W, et al. AGEs impair Kv channel-mediated vasodilation of coronary arteries by activating the NF-κB signaling pathway in ZDF rats. Biomed Pharmacother. 120: 109527 (2019).
- Adamopoulos C, Farmaki E, Spilioti E, et al. Advanced glycation end-products induce endoplasmic reticulum stress in human aortic endothelial cells. Clin Chem Lab Med. 52(1):151-160 (2014).
- Prasad A, Bekker P, Tsimikas S, et al. Advanced glycation end products and diabetic cardiovascular disease. Cardiology in Review. 20(4): 177-183 (2012).
- Su W, Li W, Chen H, et al. Advanced glycation end products impair voltage-gated k+ channels-mediated coronary vasodilation in diabetic rats. PLOS ONE. 10(11): e0142865 (2015).
- Elvira B, Warsi J, Munoz C, et al. SPAK and OSR1 Sensitivity of Voltage-Gated K+ Channel Kv1.5. J Membr Biol. 248(1): 59-66 (2014).
- Yi F, Wang H, Chai Q, et al. Regulation of large conductance ca2+-activated k+(bk) channel β 1 subunit expression by muscle ring finger protein 1 in diabetic vessels. J Biol Chem. 289(15): 10853-10864 (2014).
- Li Y, Wang XL, Sun X, et al. Regulation of vascular large-conductance calcium-activated potassium channels by Nrf2 signaling. Diab Vasc Dis Res. 14(4): 353-362 (2017).
- Ashcroft FM, Rorsman P. KATP channels and islet hormone secretion: New insights and controversies. Nat Rev Endocrinol. 9(11): 660-669 (2013).
- Xu M, Hu H, Deng D, et al. Prediabetes is associated with genetic variations in the gene encoding the Kir6.2 subunit of the pancreatic ATP-sensitive potassium channel (KCNJ11 ): A case-control study in a Han Chinese youth population. J Diabetes. 10(2): 121-129 (2017).
- Dong P, Liu M, Liu C, et al. Exenatide inhibits the K Ca 3.1 channels of aortic vascular smooth muscle in diabetic rats. Acta Cardiol Sin. 33(6): 648-655 (2017).
- Xu R, Li C, Wu Y, et al. Role of kca3.1 channels in macrophage polarization and its relevance in atherosclerotic plaque instability highlights. Arterioscler Thromb Vasc Biol. 37(2): 226-236 (2016).
- Kazama I, Tamada T, Tachi M. (2015). Usefulness of targeting lymphocyte Kv1.3-channels in the treatment of respiratory diseases. Inflamm Res. 64(10): 753-765 (2015).
- Kan XH, Gao HQ, Ma ZY, et al. Kv1.3 potassium channel mediates macrophage migration in atherosclerosis by regulating ERK activity. Arch Biochem Biophys. 591: 150-156 (2016).
- Ling MY, Ma ZY, Wang YY, et al. Up-regulated ATP-sensitive potassium channels play a role in increased inflammation and plaque vulnerability in macrophages. Atherosclerosis. 226(2): 348-355 (2013).
- Maiese K. New insights for oxidative stress and diabetes mellitus. Oxid Med Cell Longev. 2015: 1-17 (2015).
- Jha JC, Ho F, Dan C, et al. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci (Lond). 132(16): 1811-1836 (2019).
- Ungurianu A, Margina D, Gradinaru D, et al. Lipoprotein redox status evaluation as a marker of cardiovascular disease risk in patients with inflammatory disease. Mol Med Rep. 15(1): 256-262 (2016).
- Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 127(18): 1888-1902 (2013).
- Gray SP, Di Marco E, Kennedy K, et al. Reactive oxygen species can provide atheroprotection via nox4-dependent inhibition of inflammation and vascular remodelingsignificance. Arterioscler Thromb Vasc Biol. 36(2): 295-307 (2015).
- Hu P, Wu X, Khandelwal AR, et al. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim Biophys Acta Mol Basis Dis. 1863(6): 1382-1391 (2017).
- Sifuentes- Franco S, Padilla-Tejeda, DE, Carrillo-Ibarra S, et al. Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int J Endocrinol. 2018: 1-13 (2018).
- Magenta A, Greco S, Gaetano C, et al. Oxidative stress and micrornas in vascular diseases. Int J Mol Sci. 14(9): 17319-17346 (2013).
[CrossRef][Google Scholar][PubMed]
- Spadaccio C, Nenna A, Nappi F, et al. Pharmacologic approaches against Advanced Glycation End Products (AGEs) in diabetic cardiovascular disease. Res Cardiovasc Med. 4(2): 5 (2015).
- Yamagishi S, Fukami K, Matsui T, et al. Crosstalk between Advanced Glycation End products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc Diabetologia. 14(1): 2 (2015).
- Bergin M, Norman I, Foley M, et al. Practice implications and recommendations for managing codeine misuse and dependence. Acta Pharm. 65(4): 351-364 (2015).
- Gray SP, Jha JC, Kennedy K, et al. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno-and atheroprotection even in established micro-and macrovascular disease. Diabetologia. 60 (5): 927-937 (2017).
- Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. (2016).
- Sirtuin 1a diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. (2018).
- Uribarri J, Cai W, Ramdas M, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: Potential role of AGER1 and SIRT1. Diabetes Care. 34(7): 1610-616 (2011).