Short Article - Interventional Cardiology (2012) Volume 4, Issue 6
Mechanisms of late stent-related myocardial infarction: insights from optical coherence tomography
- Corresponding Author:
- Periklis A Davlouros
Cardiology Department, Patras University Hospital
Rion 26504, Patras, Greece
Tel: +30 2610999281
E-mail: pdav@otenet.gr
Abstract
Keywords
bare metal stent, drug-eluting stent, myocardial infarction, optical coherence tomography, thrombosis
Following implantation of coronary stents, late (beyond 1 year) major cardiovascular events (MACE), including myocardial infarction (MI), can originate from the culprit stented lesion, from significant lesions left untreated, or from disease progression in other territories of the coronary tree [1–3]. In patients presenting with an acute coronary syndrome (ACS), the PROSPECT study, has shown that the rate of such MACE may be up to 20% at 3 years of follow-up [3]. Similarly, the COURAGE study has reported a 19% cumulative incidence of death and MI at 4.6 years following stenting of stable patients [4]. It seems that disease progression and ‘stentrelated’ events share an equal proportion as the underlying mechanism of MI following coronary stenting [2,3]. Stent-related MI includes stent thrombosis (ST), with or without underlying restenosis.
Intracoronary imaging, including intravascular ultrasound, angioscopy and optical coherence tomography (OCT), has been used in an effort to study the mechanisms of stent-related MI [5–8]. In particular, OCT represents a relatively new imaging technique, employing the analysis of near-infrared light back-reflection to create images [9]. The greatest advantage of OCT is its high axial resolution, which is in the range of 10 μm, compared with that of intravascular ultrasound, which is 100–150 μm. Its safety using a nonocclusive contrast flushing technique has been established [10], whereas the frequency- domain interferometry analysis system constitutes an innovation allowing faster image acquisition (up to 20 mm/s, maximum recording 55 mm) and greater scan depths, making scanning of larger caliper vessels possible [11,12].
OCT findings in late ‘stent-related MI’ and/or stent thrombosis: the role of uncovered & malapposed struts
Although the use of drug-eluting stents (DES) reduced restenosis, it is associated with up to 0.6% annual incidence of late ST, at least with first-generation DES [13]. Stent thrombosis – especially DES ST – is a multifactorial phenomenon with coronary lesion-, stent- and patient-related characteristics being among its most important predictors [14,15]. Pathological post-mortem and in vivo studies have shown that stent characteristics associated with ST include stent underexpansion, incomplete stent strut apposition (malapposition) and incomplete stent strut endothelialization (delayed healing) [5,16,17]. Detailed in vivo studying of the above characteristics has become feasible with application of high-fidelity intracoronary imaging techniques like OCT. The efficacy and reproducibility of the latter for the assessment of neointimal stent coverage, strut apposition and thrombus identification has been established in numerous studies to date [18–33].
▪ Uncovered struts in DES of patients with late events
Both case reports [34] and the only two existing studies systematically examining patients presenting with ‘stent-related’ MI or late ST with OCT, have shown an increased frequency and length of uncovered and malapposed struts associated with those late events [7,8]. Previous pathological studies of first-generation DES (sirolimus- eluting stents [SES] and paclitaxel-eluting stents [PES]) have shown delayed healing [16], and especially the presence of >30% uncovered struts per cross-section, to be highly predictive of DES thrombosis [17]. We have recently reported a series of 17 patients with DES (n = 7) and baremetal stents (BMS; n = 10) ‘related late MI’ cases studied with OCT, in which all DES cases demonstrated uncovered struts [8]. More specifically, there were 15% malapposed struts per stent, and as high as 43% total cross-sections, with >30% uncovered struts per stent. Similarly, Guagliumi et al. studied 18 patients with definite late DES thrombosis with OCT and found that 78% exhibited uncovered struts, and 72% had >30% uncovered struts/section [7]. These authors also studied matched control subjects with DES and found that the frequency of cross-sections with >30% uncovered struts was only 4.1% [7]. The median time from DES implantation to late stent thrombosis in this study was 615 days (range 172–1836 days), and 83% of patients had very late ST (beyond 1 year). In our study, DES related MI happened at a median of 240 days, with a range of 90–1860 days, with three out of seven cases occurring beyond 1 year [8]. Thus, both studies confirm that uncovered DES struts might persist for several years and might be a frequent underlying finding in patients with ‘DES-related MI’ and/or late DES thrombosis.
▪ Malapposed struts in DES of patients with late events
An intravascular ultrasound study in patients with very late ST following DES implantation reported an increased incidence of stent malapposition compared with those without ST (Figure 1) [35]. We found a 15% incidence of malapposed struts per stent in our seven cases of ‘DES-related MI’ [8]. Guagliumi et al. found malapposed stent struts in 78% of patients with late ST, which was significantly greater than in patients without late ST [7]. Pathologic and in vivo histologic studies of ST have attributed late, presumably acquired, malapposition of SES and PES to localized hypersensitivity, inflammation and necrosis induced by the eluted drug and/or the polymer of first-generation DES [5,36]. Similarly, Guagliumi et al. found, eosinophilic-rich inflammatory infiltrates in tissue from stents demonstrating thrombosis [7]. Another possible explanation for late acquired malapposition could be thrombus resolution following performance of the index procedure due to ACS, as strut malapposition with OCT has been reported more frequently at followup in stents implanted for ACS compared with non-ACS cases [28,37]. However, the issue of acquired malapposition has been disputed by a prospective OCT study of a SES, showing that only a small proportion of struts that were well apposed immediately after intervention developed subsequent malapposition at a 10-month follow-up [30]. Therefore, we cannot exclude that malapposed DES struts found in patients with late events post-PCI do not represent persistent initial incomplete apposition. Additionally late, presumably acquired, malapposition and extensive vessel remodeling, as stated by Guagliumi et al., might only be a marker of underlying vascular toxicity and inflammation, which might be the direct cause of ST [7].
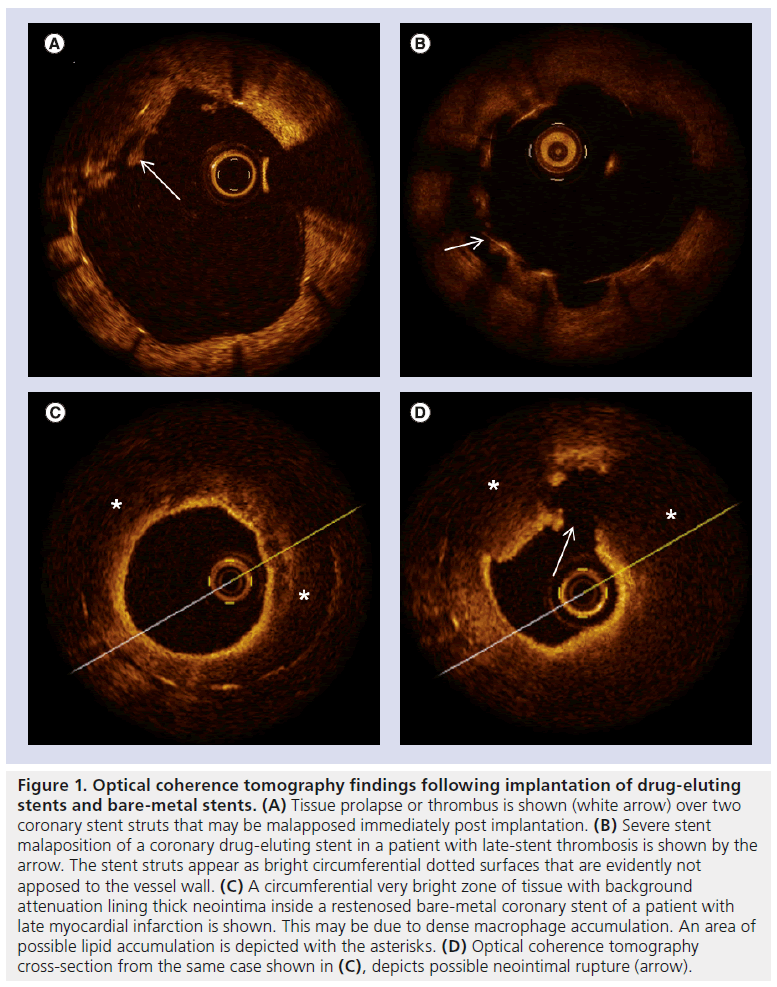
Figure 1: Optical coherence tomography findings following implantation of drug-eluting stents and bare-metal stents. (A) Tissue prolapse or thrombus is shown (white arrow) over two coronary stent struts that may be malapposed immediately post implantation. (B) Severe stent malaposition of a coronary drug-eluting stent in a patient with late-stent thrombosis is shown by the arrow. The stent struts appear as bright circumferential dotted surfaces that are evidently not apposed to the vessel wall. (C) A circumferential very bright zone of tissue with background attenuation lining thick neointima inside a restenosed bare-metal coronary stent of a patient with late myocardial infarction is shown. This may be due to dense macrophage accumulation. An area of possible lipid accumulation is depicted with the asterisks. (D) Optical coherence tomography cross-section from the same case shown in (C), depicts possible neointimal rupture (arrow).
▪ Cross-sectional & prospective OCT studies of surrogates of late DES-related events
Numerous studies have tried to examine, prospectively with OCT, the issue of delayed healing and malapposition of DES [10,18–24,26–33]. In these studies, the frequency of uncovered struts reported at 6–12-month follow-up in asymptomatic patients treated with first-generation DES varied from 0 to 8.4% [29,31,32]. The frequency of cross-sections with >30% uncovered struts was only 2% at 2 years in asymptomatic patients after SES implantation in one of those studies [31]. Similarly, this proportion was found to be 6.25%, at a 6-month routine OCT followup of a PES implanted in patients with ACS, by our group [32]. Although the rate of uncovered struts decreases progressively by 12 months as shown in a SES study, complete coverage is still rare [38]. Delayed healing and incomplete endothelialization of first-generation SES and PES have been partly attributed to chronic inflammation [5,16,17]. More specifically, the durable polymer coat of those stents has been proposed to have a proinflammatory action, retarding vessel healing and endothelial strut coverage [16,23]. In this sense, a biodegradable polymer carrier may be an advantage compared with old generation stents, in terms of vessel reaction and strut endothelialization [32,39]. Finally, the active drug eluted by DES may have a role in delayed healing, as uneven healing has been reported with PES with evidence of late ST [17]. Indeed, PES demonstrate more in-stent late loss and uncovered struts, as well as worse apposition to the vessel wall in comparison with everolimuseluting stents [40]. On the other hand, stents with similar biodegradable polymer platforms demonstrate more uncovered struts at 6 months postimplantation when eluting newer more potent drugs like biolimus A9, compared with paclitaxel [33]. The main problem with such studies (apart from the cross-sectional design of the majority) is that the rate of MACE following stenting was quite low, making it difficult to draw safe conclusions regarding the exact role of such surrogates. Even more importantly, the rate of ST with newer generation stents seems to be very low [41].
▪ Uncovered & malapposed struts in BMS of patients with late events
Late ‘BMS-related MI’ and/or thrombosis has also been described; however, its mechanisms may differ from those of DES, with restenosis being the most frequent finding in such cases [8]. In our experience, up to 50% of BMS in patients presenting with late events postimplantation may demonstrate uncovered struts, although the incidence of uncovered (3.6%) and malapposed (1.6%) struts per stent appears to be very small compared with DES cases. This is indirectly putting in dispute the possible contribution of uncovered and malapposed struts in late events following BMS implantation.
OCT findings in late ‘stent-related MI’ and/or stent thrombosis: the role of restenosis & neointimal-atherosclerosis
▪ Neointimal features in patients with BMS-related late events
In our study, patients with ‘BMS related MI’ (n = 10) presented significantly later, at a median of 95 months (range: 3–180), compared with patients with DES (Figure 1) [8]. Interestingly, 70% of these cases presented as ST segment elevation myocardial infarction with visible angiographic thrombus, and 80% demonstrated binary restenosis. On the other hand, in our experience, thrombus laden is not a consistent OCT finding in restenotic lesions. The existence of visible angiographic thrombus in the majority of BMS-related MI cases suggests that ST – at least for BMS – may be associated with restenosis. The latter is due to hyperproliferative neointimal growth inside the stent, and constitutes an important problem following implantation of, with reported rates of 15–40% at 6 months [42]. Clinical studies in turn have shown that BMS restenosis, contrary to previous thoughts that it may have a benign clinical presentation [43], may also present with ACS [44–47]. This is in accordance with our findings.
Most importantly, OCT in patients with BMS-related late events discloses features of atherosclerosis-like lipid cores, calcium deposits and neovascularization [8,48–51]. Images consistent with neointimal rupture may be detected with OCT in up to 70% of cases [8]. These findings imply that the potential mechanism of ‘BMS-related MI’ late postimplantation may be ‘atherosclerotic neointimal transformation’, with subsequent rupture similar to native atherosclerosis. The existence of BMS neointimal atherosclerosis has been previously reported in pathologic studies [52,53]. This presumed ‘atherosclerotic transformation’ of the neointima seems to happen late post BMS implantation [53]. The abovementioned small OCT studies and case series have come to confirm in vivo such neointimal atherosclerotic features late post-BMS implantation, associated with significant narrowing, tissue rupture and thrombus, which sometimes present as ACS [8,48–51]. However, a direct comparison of OCT images with pathologic specimens from the same cases does not exist. Some investigators have proposed that this atherosclerotic process may progress from the vessel wall through the space between struts into the lumen of the BMS [49,50]. Serial OCT studies are needed to clarify the presumed process of ‘neointimal atherosclerotic transformation’ following stenting.
▪ Neointimal features in patients with BMS-related late events
DES restenosis, although rare, may also present clinically with ACS [2,54], and recent evidence suggests that there may be a late catch-up phenomenon in some patients following DES implantation [55]. In our study, none of the ‘DES-related MI’ cases demonstrated restenosis or neointimal atherosclerotic features with OCT [8]. However, the number of such cases was small and the time of the events ranged considerably. A prospective angioscopic study in patients without events, however, demonstrated atherosclerotic findings within DES, speculating that this may represent another possible substrate for DES thrombosis [6]. Additionally, in a pathologic study of thrombosed stents, atherosclerotic changes were found in both DES and BMS neointima, and occurred significantly more frequently (35%) and earlier (after 4 months) in DES lesions, compared with BMS lesions (10%, and beyond 24 months) [53]. The authors speculated that inhibition of neointimal formation by the eluted drug of DES might promote atherosclerosis. The above constitute indirect evidence that late events following DES implantation may be associated with other mechanisms, namely atherosclerotic, apart from delayed healing and malapposition. Gonzalo et al. reported various OCT patterns of restenotic tissue (84% DES cases); however, the median follow-up time was only 12 months [24]. Recently, Kang et al. reported a 90% frequency of lipid-containing neointima on OCT examination late following DES implantation, with 50–60% of cases demonstrating thin cap intima, intimal rupture and thrombi [56]. Such findings were observed in both stable and unstable patients, however, were more frequent in the latter. Most importantly, the authors reported that neointimal fibrous cap thickness decreased over time, with a follow-up imaging >20 months post implantation being the best cut-off value to predict the presence of thin cap containing intima, and 70% of patients with late DES restenosis (beyond 20 months postimplantation) revealing thin cap intima on OCT. It may be that a long time interval is needed for the full spectrum of ‘neoatherosclerosis’ to be manifested. Nevertheless, this still seems to happen earlier with DES compared with BMS [53,56]. Finally, in a recent study, Guagliumi et al. also provided direct evidence that DES ‘neoatherosclerosis’ may be involved in clinical events. However, in that study only two patients with DES had no other OCT surrogates of ST apart from a disrupted plaque with thrombus within, or immediately adjacent, to the DES [7]. A recent case report of a very late DES thrombosis of a fully covered DES, without evidence of malapposition on OCT, and despite dual antiplatelet therapy, underlines the multifactorial nature of this phenomenon, and the need for further OCT studies [57].
Future perspective
It is quite evident that more studies regarding late BMS and DES ‘failure’ are eagerly needed. OCT has helped us understand the potential mechanisms of stent related late events. This experience, in turn, might be used for therapeutic purposes. For example, it may be that OCT could be used for PCI optimization immediately following stenting if incomplete apposition is detected. In this case, further dilatation at high pressure with noncompliant balloons might be beneficial. In cases of malapposition discovered late post implantation (which might be persisting initial, or acquired) in the context of a new clinical event, the same practice might also be of benefit. The potential efficiency or the possible benefit of such strategies should be established with properly designed prospective randomized studies.
Neointimal ‘atherosclerotic transformation’ is another potential explanation for MACE occuring late following both DES and BMS implantation. Although there are only a few studies regarding the latter phenomenon, it would be very interesting to investigate with serial OCT studies, the potential effects of established atherosclerotic modifying therapies like statins on this kind of neointimal ‘atherosclerotic transformation’. Additionally, it may help us to understand the potential mechanisms of late catch-up following DES implantation, and the reasons why some patients with in-stent neoatherosclerosis will culminate in ACS, and others will not, or which are the potential DES-treated lesions that will develop DES neoatherosclerosis.
Finally, although the accuracy and reproducibility of OCT in assessing neointimal stent coverage and strut apposition is fairly good [18–33], there is less validation of OCT findings regarding neointimal atherosclerotic findings. Certainly, correlation of such OCT features with pathologic findings are needed.
Conclusion
OCT constitutes an extremely useful imaging technique for studying late events, namely MI and/or ST, following implantation of DES and BMS. In the case of ‘BMS-related events’ studied with OCT, it seems that restenosis and/or ‘neointimal atherosclerotic transformation’ are the most frequent features. On the other hand, OCT case reports and studies of such late events point to delayed healing and malapposition as the prevailing but not the sole mechanism of ‘DES related events’. There is recent evidence that ‘neointimal atherosclerotic transformation’ may also happen in DES, and this may be the substrate of late catch-up phenomenon and another potential explanation for late DES failure. It remains to be proven whether the mechanisms and/or the chronology of ‘stent-related MI’ may differ according to the type of stent.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ There are only a few in vivo imaging studies in patients with late events following drug-eluting stent (DES) or bare-metal stent (BMS) implantation. These studies point to an important role of uncovered and malapposed struts in these events, namely stent thrombosis.
▪ It seems that uncovered and malapposed struts are more frequent in DES-related events compared with BMS related events, without totally excluding a potential role in the latter case.
▪ Uncovered DES struts are considered the result of the antiproliferative properties of the eluted drug, as well as of potential inflammation induced by the polymer carrier. Malapposed DES struts may be the result of initial incomplete stent apposition, or the result of late acquired malapposition due to vessel wall inflammation, although the latter might be less important, especially with new-generation DES.
▪ Pathologic and optical coherence tomography studies of neointimal features in both DES and BMS have recently introduced the concept of ‘neo-atherosclerosis’.
▪ Investigators speculate that this constitutes a kind of ‘atherosclerotic transformation’ of the neointimal tissue, progressing from the adjacent vessel wall through the stent struts.
▪ Plaque rupture and/or erosion with subsequent thrombosis of such ‘atherosclerotic neointima’ might underline some cases of late stent thrombosis. This would explain the mechanism of late events in restenotic lesions.
▪ Atherosclerotic changes may be found earlier in DES compared with BMS lesions of patients with late events, and this might be due to inhibition of neointimal formation by the eluted drug of DES, promoting atherosclerosis. However, this was suggested by only one pathologic study, and there is no large optical coherence tomography study confirming in vivo this potential chronologic difference between DES and BMS.
References
- Kimura T, Abe K, Shizuta S et al. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation 105(25), 2986–2991 (2002).
- Alexopoulos D, Xanthopoulou I, Davlouros P et al. Mechanisms of nonfatal acute myocardial infarction late after stent implantation: the relative impact of disease progression, stent restenosis, and stent thrombosis. Am. Heart J. 159(3), 439–445 (2010).
- Stone GW, Maehara A, Lansky AJ et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364(3), 226–235 (2011).
- Boden WE, O’Rourke RA, Teo KK et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 356(15), 1503–1516 (2007).
- Cook S, Ladich E, Nakazawa G et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120(5), 391–399 (2009).
- Higo T, Ueda Y, Oyabu J et al. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc. Imaging 2(5), 616–624 (2009).
- Guagliumi G, Sirbu V, Musumeci G et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc. Interv. 5(1), 12–20 (2012).
- Davlouros PA, Karantalis V, Xanthopoulou I et al. Mechanisms of non-fatal stent-related myocardial infarction late following coronary stenting with drug-eluting stents and bare metal stents. Insights from optical coherence tomography. Circ. J. 75(12), 2789–2797 (2011).
- Barlis P, Di Mario C, Van Beusekom H, Gonzalo N, Regar E. Novelties in cardiac imaging – optical coherence tomography (OCT). EuroIntervention 4(Suppl. C), C22–C26 (2008).
- Barlis P, Gonzalo N, Di Mario C et al. A multicentre evaluation of the safety of intracoronary optical coherence tomography. EuroIntervention 5(1), 90–95 (2009).
- Barlis P, Schmitt JM. Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention 4(4), 529–533 (2009).
- Davlouros P, Damelou A, Karantalis V et al. Evaluation of culprit saphenous vein graft lesions with optical coherence tomography in patients with acute coronary syndromes. JACC Cardiovasc. Interv. 4(6), 683–693 (2011).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Kereiakes DJ, Choo JK, Young JJ, Broderick TM. Thrombosis and drug-eluting stents: a critical appraisal. Rev. Cardiovasc. Med. 5(1), 9–15 (2004).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435–2441 (2007).
- Ishigami K, Uemura S, Morikawa Y et al. Long-term follow-up of neointimal coverage of sirolimus-eluting stents – evaluation with optical coherence tomography. Circ. J. 73(12), 2300–2307 (2009).
- Tian F, Chen YD, Sun ZJ et al. Evaluation of the late stent malposition after drug-eluting stent implantation by optical coherence tomography. Zhonghua Xin Xue Guan Bing Za Zhi 37(7), 585–589 (2009).
- Kim JS, Fan C, Choi D et al. Different patterns of neointimal coverage between acute coronary syndrome and stable angina after various types of drug-eluting stents implantation; 9month follow-up optical coherence tomography study. Int. J. Cardiol. 146(3), 341–346 (2011).
- Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. Int. J. Cardiol. 134(2), 180–188 (2009).
- Motreff P, Souteyrand G, Levesque S et al. Comparative analysis of neointimal coverage with paclitaxel and zotarolimus drug-eluting stents, using optical coherence tomography 6 months after implantation. Arch. Cardiovasc. Dis. 102(8–9), 617–624 (2009).
- Moore P, Barlis P, Spiro J et al. A randomized optical coherence tomography study of coronary stent strut coverage and luminal protrusion with rapamycin-eluting stents. JACC Cardiovasc. Interv. 2(5), 437–444 (2009).
- Gonzalo N, Serruys PW, Okamura T et al. Optical coherence tomography patterns of stent restenosis. Am. Heart J. 158(2), 284–293 (2009).
- Barlis P, Dimopoulos K, Tanigawa J et al. Quantitative analysis of intracoronary optical coherence tomography measurements of stent strut apposition and tissue coverage. Int. J. Cardiol. 141(2), 151–156 (2010).
- Yao ZH, Matsubara T, Inada T, Suzuki Y, Suzuki T. Neointimal coverage of sirolimuseluting stents 6 months and 12 months after implantation: evaluation by optical coherence tomography. Chinese Med. J. 121(6), 503–507 (2008).
- Chen BX, Ma FY, Luo W et al. Neointimal coverage of bare-metal and sirolimus-eluting stents evaluated with optical coherence tomography. Heart (Br. Cardiac. Soc.) 94(5), 566–570 (2008).
- Takano M, Yamamoto M, Xie Y et al. Serial long-term evaluation of neointimal stent coverage and thrombus after sirolimus-eluting stent implantation by use of coronary angioscopy. Heart (Br. Cardiac. Soc.) 93(12), 1533–1536 (2007).
- Matsumoto D, Shite J, Shinke T et al. Neointimal coverage of sirolimus-eluting stents at 6month follow-up: evaluated by optical coherence tomography. Eur. Heart J. 28(8), 961–967 (2007).
- Ozaki Y, Okumura M, Ismail TF et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur. Heart J. 31(12), 1470–1476 (2010).
- Takano M, Yamamoto M, Inami S et al. Long-term follow-up evaluation after sirolimus-eluting stent implantation by optical coherence tomography: do uncovered struts persist? J. Am. Coll. Cardiol. 51(9), 968–969 (2008).
- Davlouros P, Nikokiris G, Karantalis V et al. Neointimal coverage and stent strut apposition six months after implantation of a paclitaxel eluting stent in acute coronary syndromes: An optical coherence tomography study. Int. J. Cardiol. 151(2), 155–159 (2011).
- Davlouros PA, Mavronasiou E, Xanthopoulou I et al. An optical coherence tomography study of two new generation stents with biodegradable polymer carrier, eluting paclitaxel vs. biolimus-A9. Int. J. Cardiol. 157(3), 341–346 (2012).
- Schinkel AF, Barlis P, Van Beusekom HM, Serruys PW, Regar E. Images in intervention. Optical coherence tomography findings in very late (4 years) paclitaxel-eluting stent thrombosis. JACC Cardiovasc. Interv. 1(4), 449–451 (2008).
- Cook S, Wenaweser P, Togni M et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 115(18), 2426–2434 (2007).
- Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004).
- Davlouros PA, Nikokiris G, Karantalis V et al. Neointimal coverage and stent strut apposition six months after implantation of a paclitaxel eluting stent in acute coronary syndromes: an optical coherence tomography study. Int. J. Cardiol. 151(2), 155–159 (2011).
- Katoh H, Shite J, Shinke T et al. Delayed neointimalization on sirolimus-eluting stents: 6month and 12month follow up by optical coherence tomography. Circ. J. 73(6), 1033–1037 (2009).
- Barlis P, Regar E, Serruys PW et al. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur. Heart J. 31(2), 165–176 (2010).
- Takano M, Murakami D, Yamamoto M et al. Six-month follow-up evaluation for everolimus-eluting stents by intracoronary optical coherence tomography: comparison with paclitaxel-eluting stents. Int. J. Cardiol. doi: 10.1016/j.ijcard.2011.10.102 (2011) (Epub ahead of print).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 372(9644), 1163–1173 (2008).
- Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann. Intern. Med. 138(10), 777–786 (2003).
- Greenberg D, Bakhai A, Cohen DJ. Can we afford to eliminate restenosis? Can we afford not to? J. Am. Coll. Cardiol. 43(4), 513–518 (2004).
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 151(6), 1260–1264 (2006).
- Bainey KR, Norris CM, Graham MM, Ghali WA, Knudtson ML, Welsh RC. Clinical in-stent restenosis with bare metal stents: is it truly a benign phenomenon? Int. J. Cardiol. 128(3), 378–382 (2008).
- Assali AR, Moustapha A, Sdringola S et al. Acute coronary syndrome may occur with in-stent restenosis and is associated with adverse outcomes (the PRESTO trial). Am. J. Cardiol. 98(6), 729–733 (2006).
- Doyle B, Rihal CS, O’Sullivan CJ et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation 116(21), 2391–2398 (2007).
- Davlouros PA, Karantalis V, Mavronassiou E, Alexopoulos D. Neointimal tissue rupture as a mechanism of myocardial infarction very late following implantation of bare metal stents. Insights from optical coherence tomography. Int. J. Cardiol. 148(3), 348–349 (2011).
- Takano M, Yamamoto M, Inami S et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J. Am. Coll. Cardiol. 55(1), 26–32 (2009).
- Hou J, Qi H, Zhang M et al. Development of lipid-rich plaque inside bare metal stent: possible mechanism of late stent thrombosis? An optical coherence tomography study. Heart (Br. Cardiac. Soc.) 96(15), 1187–1190 (2010).
- Kashiwagi M, Kitabata H, Tanaka A et al. Very late clinical cardiac event after BMS implantation: in vivo optical coherence tomography examination. JACC Cardiovasc. Interv. 3(5), 525–527 (2010).
- Hasegawa K, Tamai H, Kyo E et al. Histopathological findings of new in-stent lesions developed beyond five years. Catheter Cardiovasc. Interv. 68(4), 554–558 (2006).
- Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc. Imaging 2(5), 625–628 (2009).
- Rathore S, Kinoshita Y, Terashima M et al. A comparison of clinical presentations, angiographic patterns and outcomes of in-stent restenosis between bare metal stents and drug eluting stents. EuroIntervention 5(7), 841–846 (2010).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Kang SJ, Mintz GS, Akasaka T et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 123(25), 2954–2963 (2011).
- Nishiguchi T, Kitabata H, Tanaka A et al. Very late stent thrombosis after drug-eluting stent in segment with neointimal tissue coverage. JACC Cardiovasc. Imaging 3(4), 445–446 (2010).