Research Article - Neuropsychiatry (2017) Volume 7, Issue 2
Metabolomics analysis implies noninvolvement of the kynurenine pathway neurotoxins in the interferon-gamma- induced neurotoxicity of adult human astrocytes
- Corresponding Author:
- Sadayuki Hashioka
Department of Psychiatry, Faculty of Medicine, Shimane University, 89-1 Enya-cho, Izumo, Shimane, 693-8501 Japan
Tel: 81.853.20.2262
E-mail: hashioka@f2.dion.ne.jp
Abstract
Emerging evidence indicates that the kynurenine pathway (KP) contributes to neurodegenerative diseases associated with glial activation. Interferon (IFN)-γ is a potent activator of indole-2,3-dioxygenase, the first and rate-limiting enzyme of the KP. Our previous studies have shown that adult human astrocytes become neurotoxic when activated by IFN-γ. We now used high performance liquid chromatographymass spectrometry in both the positive- and negative- ionization mode of electrospray interface, to examine whether the IFN-γ-activated adult human astrocytes secrete neurotoxic KP metabolites, such as quinolinic acid (QUIN), 3-hydroxykynurenine (3-HK) and 3-hydroxyanthranilic acid (3-HAA). Kynurenine was detected in cell culture supernatants of IFN-γ-stimulated astrocytes, but not in supernatants of unstimulated astrocytes. On the other hand, QUIN, 3-HK and 3-HAA were not detected in samples from either IFN-γ- stimulated or unstimulated cells. These results indicate that the KP may not be involved in the IFN-γ-induced neurotoxicity of adult human astrocytes. Therefore, neurotoxins other than KP metabolites could be responsible for the IFN-γ-induced astrocyte neurotoxicity.
Keywords
Human astrocytes, Interferon-γ, Kynurenine pathway, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, Quinolinic acid, High performance liquid chromatography-mass spectrometry
Abbreviation
KP: Kynurenine pathway; 3-HK: 3-hydroxykynurenine; 3-HAA: 3-hydroxyanthranilic acid; QUIN: Quinolinic acid; IFN: Interferon; IDO: Indole-2,3-dioxygenase; HPLC-MS: High performance liquid chromatography-mass spectrometry; ESI: Electrospray interface.
Introduction
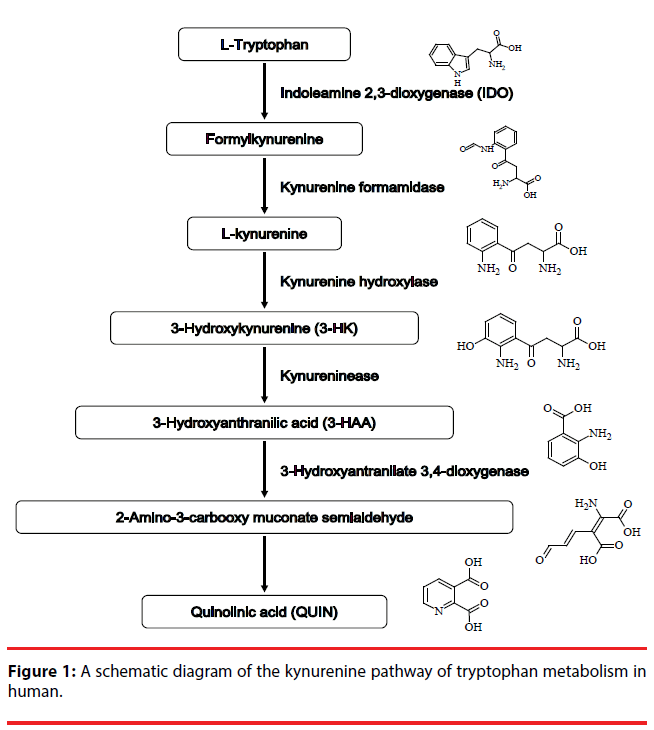
The kynurenine pathway KP (Figure 1) metabolizes more than 95% of ingested tryptophan, an essential amino acid, and generates metabolites collectively called kynurenines [1]. Several metabolites of the KP, including kynurenine, 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA) and quinolinic acid (QUIN), are considered to be neurotoxic and have essential roles in activation of N-methyl-D-aspartate (NMDA) receptor and in free radical production [2]. Since NMDA receptor-mediated excitotoxicity and excessive free radical production play important roles in the mechanism of neuronal damage in neurodegenerative disorders, the KP may be a key player in their pathogeneses [3]. Indeed, increasing evidence shows elevated levels of QUIN, an endogenous NMDA agonist, in the brain or cerebrospinal fluid of patients with neurodegenerative disorders, such as Alzheimer’s disease (AD) [4], Huntington’s disease [5] and amyotrophic lateral sclerosis [6]. Both 3-HK and 3-HAA are endogenous generators of free radicals [2,7]. These metabolites have been demonstrated to reduce the viability of cultured cerebellar granule neurons [8]. Moreover, increased brain levels of 3-HK were demonstrated in Parkinson’s disease patients [9] and elevated serum levels of 3-HK were reported in AD patients [10].
We have previously demonstrated that adult human astrocytes become neurotoxic when activated by interferon (IFN)-γ [11,12]. It is well known that astrocytes are activated in neuroinflammatory processes associated with a broad spectrum of neurodegenerative diseases [13,14]. Interestingly, IFN-γ is one of the inducers of indole-2,3-dioxygenase (IDO), which is the first rate-limiting enzyme in the KP. In addition, IFN-γ-stimulated fetal human astrocytes have been shown to express IDO [15]. These findings prompted us to examine whether the IFN-γ- induced neurotoxicity of adult human astrocytes involves neurotoxic metabolites of the KP. To facilitate the detection of such metabolites in the cell culture supernatants from IFN-γ-treated astrocytes of adult human, we employed high performance liquid chromatography (HPLC)- mass spectrometry (MS) in both the positiveand negative-ionization mode of electrospray interface (ESI).
Materials and Methods
▪ Chemicals
Kynurenine, 3-HAA and QUIN were purchased from Tokyo Chemical Industry (Tokyo, Japan), while 3-HK was purchased from Sigma-Aldrich (St. Louis, MO, USA). A mixture of the four chemical standards was dissolved in 50% (w/v) methanol and the final concentration of each chemical was 50μg/mL. Four standard mixtures were used for HPLC-MS analysis in both the positive- and negative-ionization mode of ESI. All the solvents used in this study were purchased from Wako (Osaka, Japan). The organic solvents (acetonitrile, ultrapure water, and formic acid) for HPLC-MS were of liquid chromatography- MS grade.
▪ Preparation of cell culture supernatant samples
Adult human astrocytes were obtained from three epileptic patients undergoing temporal lobe surgery. The specimens were from normal tissue overlying the epileptic foci. The use of human brain materials was approved by the Clinical Research Ethics Board for Human Subjects of the University of British Columbia, Canada. The epileptic patients were a 46 yearold male, a 57 year-old female and a 26 yearold male. Astrocytes were isolated as described previously [11,12]. Primary astrocytes isolated from each patient were considered as a separate sample. Astrocytes were grown to confluence in Dulbecco’s modified Eagle medium-nutrient mixture F12 Ham (DMEM-F12) supplemented with 10% fetal bovine serum (FBS), penicillin (200 U/ml) and streptomycin (200 μg/ml) (all from ThermoFisher Scientific, Waltham, MA, USA) on 10cm dishes in a cell incubator (37°C, humidified 5% CO2). Astrocytes were rinsed thrice with warmed FBS-free/phenol red-free medium (ThermoFisher Scientific) and were then cultured in 7 mL of the FBS- and phenol red-free medium containing B27 supplement (ThermoFisher Scientific). Subsequently, the cells were treated with 50 U/ml of IFN-γ (PeproTech, Rocky Hill, NJ, USA) or left unstimulated in humidified 5% CO2, 95% air atmosphere at 37°C for 48 h. The cell-free supernatants were collected and frozen at -80°C until analyzed by HPLC-MS as described below. Under our experimental conditions, immunocytochemistry using antibody against the astrocytic marker glial fibrillar acidic protein (GFAP) confirmed that more than 99% cells were GFAP positive [12].
▪Metabolite extraction from the frozen cell-free supernatants
The frozen cell-free supernatants were thawed on an ice bath. Within 30 min of thawing the supernatants, iced absolute methanol (0.3 mL) was added to 0.1 mL of the sample. Subsequently, the sample tube was vortexed and mixed to remove the protein fraction. After centrifugation of the mixture at 15,000 × g for 10 min at 4°C, the supernatant samples were filtrated with polytetrafluoroethylene hydrophilic membranes (Merck Millipore, Japan).
▪ HPLC-MS analytical conditions
HPLC-MS was performed with an Agilent 1200 system (Agilent Technologies, Palo Alto, CA) coupled to a Finnigan LTQ Orbitrap XL (ThermoFisher Scientific), which was equipped with an electrospray source operating in the positive- and negative-ionization mode, and with a lock-spray interface for accurate mass measurement. Five different chemicals (lidcaine, prochloraz, reserpine, bombesin, and aureobasidin A) were employed as the lockmass compounds for the positive-ionization mode of ESI. A different set of five chemicals (2,4 dichlorophenoxyacetic acid, ampicillin, 3-[(3-cholamidopropyl)dimethylammonio] propanesulfonate, tetra-N-acetylchitotetraose, and aureobasidin A) was employed as the lockmass compounds for the negative-ionization mode of ESI. An aliquot of the extracted sample (5 μL) was injected into a TSKgel column ODS- 100V (5 μm, 3 x 50 mm; Tosoh, Tokyo, Japan) with mobile phases which consisted of 0.1% (v/v) aqueous formic acid (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B). The gradient program was as follows: 3-97% solvent B for the first 15 min, 97% solvent B for the next 5min, and 3% solvent B from 20 to 25 min, with a flow rate of 0.4 mL/min. The column oven temperature was set at 40°C. HPLC-MS analysis was performed in both the positive-ionization mode and negative-ionization mode of ESI.
▪ Data analysis of HPLC-MS
All data obtained from the HPLC-MS analysis were acquired with the Xcalibur™ software (ThermoFisher Scientific). To determine the lowest concentrations detectable in our system for the four KP metabolites, namely, kynurenine, 3-HK, 3-HAA and QUIN in cell culture supernatants, we focused the HPLCMS analysis on the adduct ion forms ([M+H]+ or [M+H]-) which occur in each of the four targeted KP metabolites using the Xcalibur™ software. We calculated the peak area values for the four KP metabolites based on extracted ion chromatogram of m/z [M+H] ± 3 ppm.
▪ Determination of the lowest detectable concentrations for the KP metabolites using the HPLC-MS analysis
To determine the lowest detectable concentrations for the four KP metabolites (kynurenine, 3-HK, 3-HAA and QUIN), we searched for the detectable concentrations of authentic compounds by testing several different concentrations (ranging from 10 pM to 10 μM) of analytical-grade standards in our HPLC-MS analytical system. We used cell culture medium to prepare solutions of kynurenine, 3-HK, 3-HAA and QUIN at the final concentrations of 10 μM, 1 μM, 100 nM, 10 nM, 1 nM, 100 pM and 10 pM. Each of the above standard dilutions of the four KP metabolites (kynurenine, 3-HK, 3-HAA and QUIN) was independently injected three times into the HPLC-MS equipment in 5-μL aliquots. After HPLC-MS analysis, the peak area of m/z [M+H] ± 3 ppm was divided by the area of authentic compounds, to calculate the peak area values for these compounds. In the ESI positive-ionization mode of our HPLCMS analytical system, the peak area values for kynurenine, 3-HK, 3-HAA and QUIN were determined to be m/z=209.0921 ± 3 ppm, m/ z=225.0870 ± 3 ppm, m/z=154.0499 ± 3 ppm, and m/z=168.0291 ± 3 ppm, respectively. In the ESI negative-ionization mode of our HPLCMS analytical system, the peak area values for kynurenine, 3-HK, 3-HAA and QUIN were determined to be m/z= 207.0775± 3 ppm, m/z=223.0724 ± 3 ppm, m/z= 152.0353± 3 ppm, and m/z=166.0146± 3 ppm, respectively. Table 1 and Supplementary Table 1 show the lowest detectable concentrations for the four KP metabolites in our HPLC-MS analytical system in the positive-ionization and negativeionization modes of ESI.
| KP metabolizes (abb. name) | Chemical composition fourmula | Detected full mass (m/z) | Concentration | Elution time | Peak Area | ||
|---|---|---|---|---|---|---|---|
| Sample (n1) | Sample (n2) | Sample (n3) | |||||
| kynurenine | C10H12N2O3 | 209.0923 [M+H]+ |
10 µM | 2.63 min | 19558367 | 20210107 | 19162270 |
| 1 µM | 2017830 | 1970963 | 2018336 | ||||
| 100 nM | 199352 | 196436 | 192607 | ||||
| 10 nM | N.D. | N.D. | N.D. | ||||
| 1 nM | N.D. | N.D. | N.D. | ||||
| 100 pM | N.D. | N.D. | N.D. | ||||
| 10 pM | N.D. | N.D. | N.D. | ||||
| control | N.D. | N.D. | N.D. | ||||
| 3-hydroxykynurenine (3-HK) |
C10H12N2O4 | 225.0872 [M+H]+ |
10 µM | 1.53 min | 6597988 | 7080201 | 6555178 |
| 1 µM | 532920 | 561289 | 492719 | ||||
| 100 nM | N.D. | N.D. | N.D. | ||||
| 10 nM | N.D. | N.D. | N.D. | ||||
| 1 nM | N.D. | N.D. | N.D. | ||||
| 100 pM | N.D. | N.D. | N.D. | ||||
| 10 pM | N.D. | N.D. | N.D. | ||||
| control | N.D. | N.D. | N.D. | ||||
| 3-hydroxyanthranilic acid (3-HAA) |
C7H7NO3 | 154.0501 [M+H]+ |
10 µM | 4.17 min | 12345992 | 12211420 | 12658353 |
| 1 µM | 1157383 | 1171459 | 1319920 | ||||
| 100 nM | N.D. | 195202 | 216426 | ||||
| 10 nM | N.D. | N.D. | N.D. | ||||
| 1 nM | N.D. | N.D. | N.D. | ||||
| 100 pM | N.D. | N.D. | N.D. | ||||
| 10 pM | N.D. | N.D. | N.D. | ||||
| control | N.D. | N.D. | N.D. | ||||
| quinolinic acid (QUIN) |
C7H5NO4 | 168.0293 [M+H]+ |
10 µM | 1.32 min | 156334 | 169688 | 105259 |
| 1 µM | N.D. | N.D. | N.D. | ||||
| 100 nM | N.D. | N.D. | N.D. | ||||
| 10 nM | N.D. | N.D. | N.D. | ||||
| 1 nM | N.D. | N.D. | N.D. | ||||
| 100 pM | N.D. | N.D. | N.D. | ||||
| 10 pM | N.D. | N.D. | N.D. | ||||
| blank control | N.D. | N.D. | N.D. | ||||
| N.D. describes as not detected for the tageted metabolite Control describes as using only not detected for the tageted metabolite. |
|||||||
All HPLC-MS data were acquired by using the positive-ionization mode of ESI.
Table 1: Determination of the lowest detectable concentrations for the four KP metabolites using the HPLC-MS analysis with the positive ionization mode of ESI.
Results
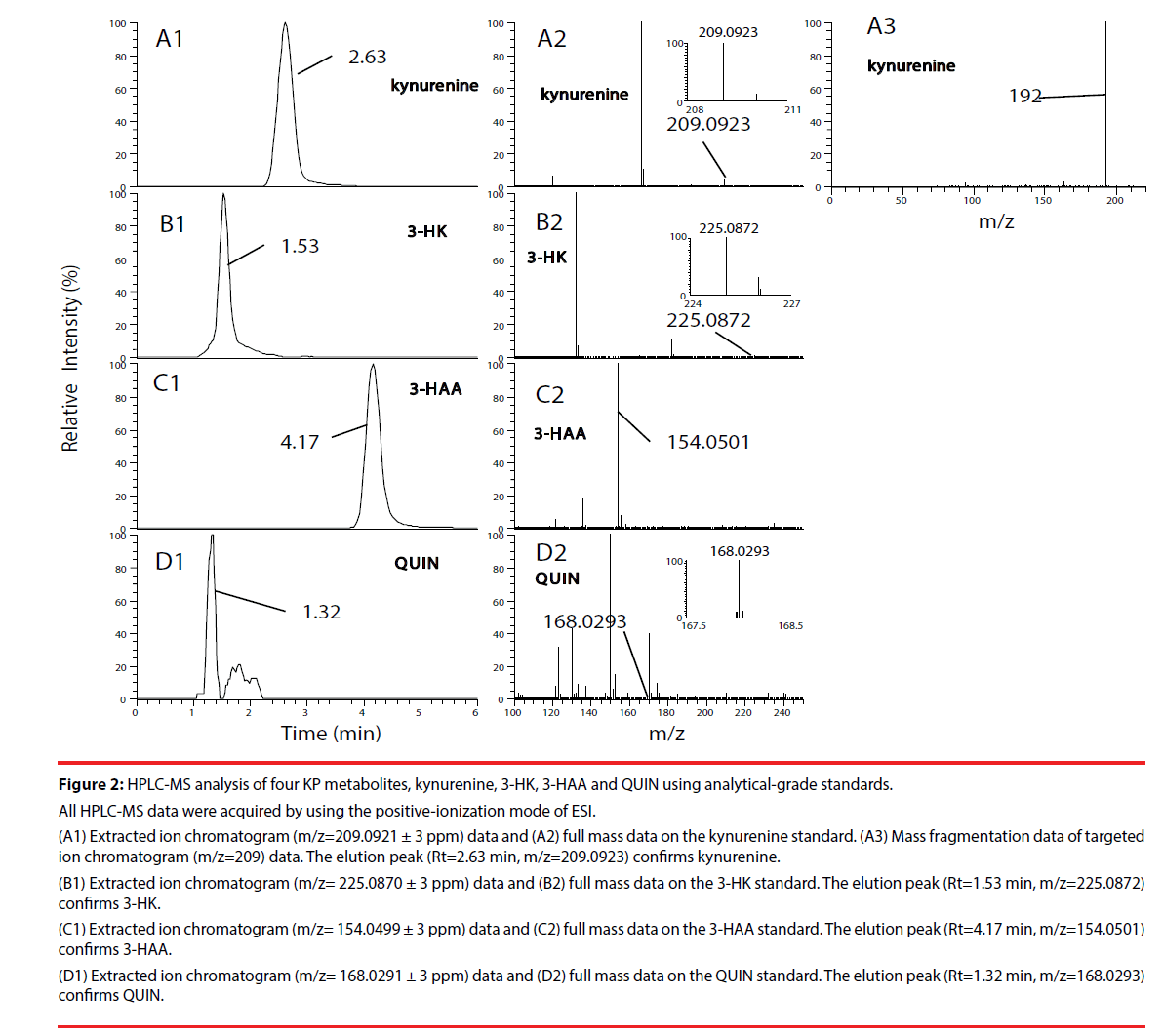
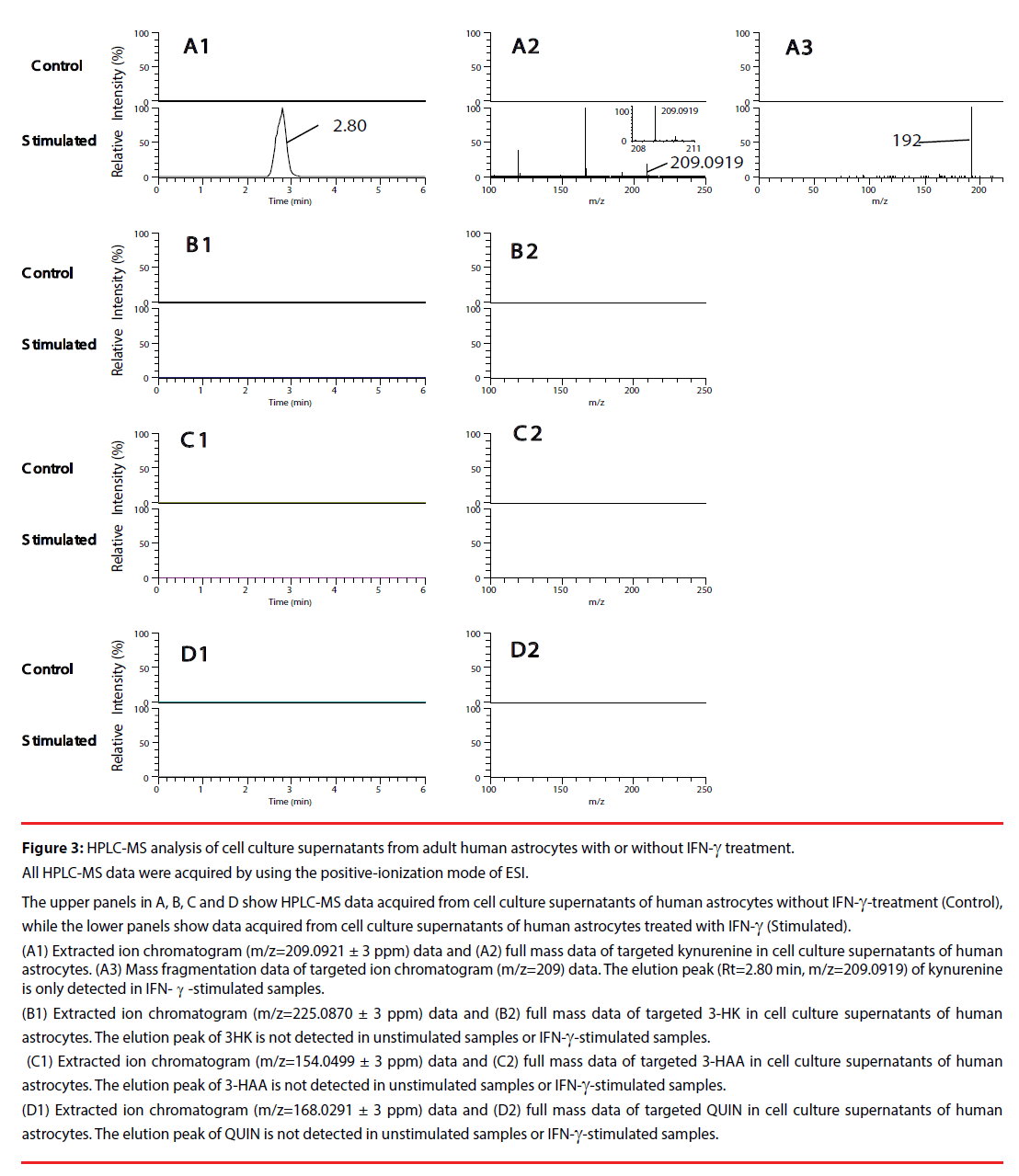
We first, by employing authentic compounds, confirmed the detection of the four KP metabolites, kynurenine, 3-HK, 3-HAA and QUIN, in our HPLC-MS analytical system with the positive-ionization mode (Figure 2) and negative-ionization mode (Supplementary Figure 1) of ESI. Next, cell culture supernatants of adult human astrocytes were analyzed by using our HPLC-MS system in both the positive-ionization mode (Figure 3) and negative-ionization mode (Supplementary Figure 2) of ESI. Kynurenine was detected in cell culture supernatants of adult human astrocytes stimulated with IFN-γ, but not in supernatants of unstimulated astrocytes. QUIN, 3-HK and 3-HAA were not detected in either IFN-γ- stimulated samples or in unstimulated control samples (Figure 3 and Supplementary Figure 2).
Figure 2: HPLC-MS analysis of four KP metabolites, kynurenine, 3-HK, 3-HAA and QUIN using analytical-grade standards.
All HPLC-MS data were acquired by using the positive-ionization mode of ESI.
(A1) Extracted ion chromatogram (m/z=209.0921 ± 3 ppm) data and (A2) full mass data on the kynurenine standard. (A3) Mass fragmentation data of targeted
ion chromatogram (m/z=209) data. The elution peak (Rt=2.63 min, m/z=209.0923) confirms kynurenine.
(B1) Extracted ion chromatogram (m/z= 225.0870 ± 3 ppm) data and (B2) full mass data on the 3-HK standard. The elution peak (Rt=1.53 min, m/z=225.0872)
confirms 3-HK.
(C1) Extracted ion chromatogram (m/z= 154.0499 ± 3 ppm) data and (C2) full mass data on the 3-HAA standard. The elution peak (Rt=4.17 min, m/z=154.0501)
confirms 3-HAA.
(D1) Extracted ion chromatogram (m/z= 168.0291 ± 3 ppm) data and (D2) full mass data on the QUIN standard. The elution peak (Rt=1.32 min, m/z=168.0293)
confirms QUIN.
Figure 3: HPLC-MS analysis of cell culture supernatants from adult human astrocytes with or without IFN-γ treatment.
All HPLC-MS data were acquired by using the positive-ionization mode of ESI.
The upper panels in A, B, C and D show HPLC-MS data acquired from cell culture supernatants of human astrocytes without IFN-γ-treatment (Control),
while the lower panels show data acquired from cell culture supernatants of human astrocytes treated with IFN-γ (Stimulated).
(A1) Extracted ion chromatogram (m/z=209.0921 ± 3 ppm) data and (A2) full mass data of targeted kynurenine in cell culture supernatants of human
astrocytes. (A3) Mass fragmentation data of targeted ion chromatogram (m/z=209) data. The elution peak (Rt=2.80 min, m/z=209.0919) of kynurenine
is only detected in IFN-γ-stimulated samples.
(B1) Extracted ion chromatogram (m/z=225.0870 ± 3 ppm) data and (B2) full mass data of targeted 3-HK in cell culture supernatants of human
astrocytes. The elution peak of 3HK is not detected in unstimulated samples or IFN-γ-stimulated samples.
(C1) Extracted ion chromatogram (m/z=154.0499 ± 3 ppm) data and (C2) full mass data of targeted 3-HAA in cell culture supernatants of human
astrocytes. The elution peak of 3-HAA is not detected in unstimulated samples or IFN-γ-stimulated samples.
(D1) Extracted ion chromatogram (m/z=168.0291 ± 3 ppm) data and (D2) full mass data of targeted QUIN in cell culture supernatants of human
astrocytes. The elution peak of QUIN is not detected in unstimulated samples or IFN-γ-stimulated samples.
The HPLC-MS analysis system used in the present study differs from those used in previous investigations which detected QUIN, 3-HK and 3-HAA in cell culture supernatants of IFN- γ-treated human monocytes [16] and QUIN in rat plasma [17]. Therefore, we prepared the KP metabolite solutions dissolved in cell culture medium at several different concentrations (ranging from 10 pM to 10 μM), in order to determine the lowest detectable concentrations for the four KP metabolites in our HPLC-MS system. The lowest detectable concentrations for kynurenine, 3-HK, 3-HAA and QUIN were determined to be 100 nM, 1 μM, 100 nM, and 10 μM, respectively, under the positiveionization mode of ESI (Table 1). Under the negative-ionization mode of ESI, the lowest detectable concentration for all four metabolites was determined to be 1 μM (Supplementary Table 1).
Discussion
Recent evidence indicates that the KP contributes to the pathogenesis in neurodegenerative diseases associated with glial activation. It has also been reported that IFN-γ activates IDO, the initial ratelimiting enzyme of the KP (reviewed in Tan, et al. [18] ). Our previous studies have shown that IFN-γ-activated adult human astrocytes secrete neurotoxin(s) [11,12]. Now we aimed to determine whether the KP is involved in the IFN-γ-induced neurotoxicity of adult human astrocytes. Three of the KP metabolites, QUIN, 3-HK and 3-HAA have been detected in cell culture supernatants of human monocytes treated with IFN-γ by using an HPLC-ESI-positive-MS method [16]. Another study demonstrated that the negative ionization approach provided the highest sensitivity for the detection of QUIN in rat plasma [17,18]. We therefore performed the HPLC-MS in both the positive- and negativeionization mode of ESI. Nevertheless, we were unable to detect the neurotoxic kynurenines QUIN, 3-HK or 3-HAA in supernatants from IFN-γ-stimulated adult human astrocytes by using either the positive or negative ESI mode. These results indicate that the KP may not be involved in the IFN-γ-induced neurotoxicity of adult human astrocytes, even though we cannot completely rule out the possibility that very low concentrations of the KP metabolites at undetectable levels in our HPLC-MS system contribute to the neurotoxic activity of astrocytes. Our results also suggest that increased levels of the neurotoxic kynurenines, including QUIN, shown in neurodegenerative disorders may stem from activated microglia, not astrocytes, since cultured human microglia have been demonstrated to produce QUIN [19]. Although the exact roles of the KP in astrocytes remain unclear, a study using pharmacological inhibition of the KP suggests that the KP directly facilitates the maintenance of intracellular levels of nicotinamide adenine dinucleotide and sirtuin deacetylase-1 activity in human astrocytes [20].
Very few studies have examined astrocytic production of the neurotoxic KP metabolites using human cells. Our results showing undetectable levels of QUIN, 3-HK and 3-HAA in supernatants from IFN-γ-stimulated astrocytes of adult human are consistent with the previous limited studies. Guillemin, et al. demonstrated that fetal human astrocytes did not express the mRNA encoding kynurenine hydroxylase even when these cells were stimulated with IFN-γ [15]. Since kynurenine hydroxylase converts kynurenine into 3-HK, absence of this enzyme renders fetal human astrocytes unable to produce the downstream KP metabolites, including 3-HK, 3-HAA and QUIN (Figure 1). Indeed, it has been shown that cultured fetal human astrocytes do not synthesize detectable levels of QUIN or 3-HK [15]. Although Guillemin and colleagues did not examine whether 3-HAA is secreted by IFN-γ-stimulated fetal human astrocytes, we confirmed no detectable levels of 3-HAA in supernatants from IFN-γ-stimulated adult human astrocytes. Heyes, et al. also reported that human astrocytoma U373-MG cells activated by IFN-γ showed no detectable production of QUIN [21]. Based on these findings, it can be concluded that, regardless of their age or proliferative status, human astrocytes do not produce high levels of 3-HK, 3-HAA or QUIN even when stimulated with IFN-γ. In contrast to their human counterparts, gerbil astrocytes have been shown to produce increased levels of QUIN when stimulated with lipopolysaccharide [22]. Therefore, astrocytic capacity to produce neurotoxic kynurenines appears to vary between species and between stimulants.
It still needs to be established conclusively which neurotoxins secreted in supernatants are responsible for the IFN-γ-induced neurotoxicity of adult human astrocytes. This study was performed to discover whether toxic metabolites of the KP are responsible for the human astrocyte neurotoxicity. However, our results do not support the hypothesis that the KP mediates the IFN- γ-induced neurotoxicity of adult human astrocytes. Therefore, neurotoxins other than KP metabolites should be responsible for the IFN-γ-induced astrocyte neurotoxicity. It is likely that no single molecule is responsible for the astrocytic neurotoxicity, since microarray analysis revealed 1192 genes that were differentially expressed in murine astrocytes in response to IFN-γ by a factor of 1.5 or greater. Moreover, approximately one-fourth of these genes were of unknown identity [23]. Further exploration of the compounds responsible for the astrocyte neurotoxicity is clearly warranted since an understanding of these mechanisms could lead to the discovery of new therapeutic targets for neurodegenerative diseases.
Author Contributions
SH, HS and AK participated in the design of the study. SH, DN and AK carried out all experiments, collected the data and performed the statistical analysis. SH, HS and DN interpreted the data. SH, HS and AK wrote the manuscript. TM, RW, MH, JH and AK revised the manuscript. All authors read and approved the final version of manuscript.
Acknowledgements
Sincere appreciation is extended to Drs. Edith and Patrick McGeer for their invaluable support. This research was supported by Grant-in-Aid for Scientific Research #24591721 (SH).
References
- Wolf H. Studies on Tryptophan Metabolism in Man: The Effect of Hormones and Vitamin B6on Urinary Excretion of Metabolites of the Kynurenine Pathway: Part 1. Scand. J. Clil. Lab. Invest 33(s136), 11-87 (1974).
- Vecsei L, Szalardy L, Fulop F, et al. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug. Discov 12(1), 64-82 (2013).
- Stone TW, Forrest CM, Darlington, LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J 279(8), 1386-1397 (2012).
- Guillemin GJ, Brew BJ, Noonan CE, et al. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol. Appl. Neurobiol 31(4), 395-404 (2005).
- Guidetti P, Luthi-Carter RE, Augood SJ, et al. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol. Dis 17(3), 455-461 (2004).
- Chen Y, Stankovic R, Cullen KM, et al. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox. Res 18(2), 132-142 (2010).
- Okuda S, Nishiyama N, Saito H, et al. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem 70(1), 299-307(1998).
- Smith AJ, Stone TW, Smith RA. Neurotoxicity of tryptophan metabolites. Biochem. Soc. Trans 35(Pt 5), 1287-1289 (2007).
- Ogawa T, Matson WR, Beal MF, et al. Kynurenine pathway abnormalities in Parkinson's disease. Neurology 42(9), 1702-1706 (1992).
- Schwarz MJ, Guillemin GJ, Teipel SJ, et al. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer's disease patients from controls. Eur. Arch. Psychiatry. Clin. Neurosci 263(4), 345-352 (2013).
- Hashioka S, Klegeris A, Schwab C, et al. Interferon-gamma-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol. Aging 30(12), 1924-1935 (2009).
- Hashioka S, Klegeris A, McGeer PL. Proton pump inhibitors reduce interferon-gamma-induced neurotoxicity and STAT3 phosphorylation of human astrocytes. Glia 59(5), 833-840 (2011).
- Fuller S, Steele M, Munch G. Activated astroglia during chronic inflammation in Alzheimer's disease--do they neglect their neurosupportive roles? Mutat. Res 690(1-2), 40-49 (2010).
- Hashioka S, McGeer EG, Miyaoka T, et al. Interferon-gamma-induced neurotoxicity of human astrocytes. CNS. Neurol. Disord. Drug. Targets 14(2), 251-256 (2015).
- Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem 78(4), 842-853 (2001).
- Zhu W, Stevens AP, Dettmer K, et al. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 401(10), 3249-3261 (2011).
- Moller M, Du Preez JL, Harvey BH. Development and validation of a single analytical method for the determination of tryptophan, and its kynurenine metabolites in rat plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci 898(1), 121-129 (2012).
- Tan L, Yu JT, Tan L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J. Neurol. Sci 323(1-2), 1-8 (2012).
- Heyes MP, Achim CL, Wiley CA, et al. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J 320(Pt 2), 595-597 (1996).
- Braidy N, Guillemin GJ, Grant R. Effects of Kynurenine Pathway Inhibition on NAD Metabolism and Cell Viability in Human Primary Astrocytes and Neurons. Int. J. Tryptophan. Res 4(1), 29-37 (2011).
- Heyes MP, Chen CY, Major EO, et al. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem. J 326(Pt 2), 351-356 (1997a).
- Heyes MP, Saito K, Chen CY, et al. Species heterogeneity between gerbils and rats: quinolinate production by microglia and astrocytes and accumulations in response to ischemic brain injury and systemic immune activation. J. Neurochem 69(4), 1519-1529 (1997b).
- Halonen SK, Woods T, McInnerney K, et al. Microarray analysis of IFN-gamma response genes in astrocytes. J. Neuroimmunol 175(1-2), 19-30 (2006).