Case Report - Neuropsychiatry (2018) Volume 8, Issue 2
Methylmalonic Aciduria may be an Innegligible Etiology of Cerebral Venous Sinus Thrombosis
- *Corresponding Authors:
- Ran Meng
Xuanwu Hospital, Capital Medical University
Beijing Institute for Brain Disorders, Beijing, 100053, China
Tel: +86-10-83198952
Fax: +86-10-83154745
Xunming Ji
Xuanwu Hospital, Capital Medical University
Beijing Institute for Brain Disorders, Beijing, 100053, China
Tel: +86-10- 83198952
Fax: +86-10-83154745
Abstract
Abstract
This paper analyzed a case of Methylmalonic Aciduria (MMA) in combination with Patent Foramen Ovale (PFO), presenting as cerebral venous sinus thrombosis (CVST) and cerebral venous infarctions coexisted with fresh arterial infarctions located at bilateral cerebella. From the aspects including clinical manifestations, physical examination, final diagnosis confirmation, comprehensive therapy, and clinical outcomes, this paper will be a very useful
reference to clinicians.
Keywords
Cerebral venous sinus thrombosis, Methylmalonic aciduria, Patent foramen ovale, Venous infarction, Arterial infarction, Psychiatric disorders.
Introduction
Cerebral venous sinus thrombosis (CVST) is easily misdiagnosed, thus resulting in treatment delay and subsequent poor outcomes, regarding its variable and non-specific clinical manifestations [1]. Recently, some special or complex causes of CVST are emerging, which may display more intricate clinical presentations and require customized management [2,3], although the mainstay treatment still focuses on anticoagulation [4].
Case Report
A 22-year-old female was admitted to our institution with the complaints of left arm and bilateral legs weakness for 14 days, dizziness and progressing headache for 7 days, and epileptic seizures for 3 days. She was once diagnosed as schizophrenia and had taken haloperidol tablet (4 mg, twice a day) for 4 years. No dehydration status occurred prior to this onset. Physical examination revealed hemiplegia and bilateral Babinski sign, NIHSS=7, mRS=3. Intracranial pressure (ICP) was 250 mmH2O. Peripheral blood tests showed anemia (hemoglobin 89 g/L, [normal reference range: 110-160 g/L]), and abnormally elevated homocysteine (122 μmol/L, [normal reference range: 0-20 μmol/L]) and D-dimer (1.25 mg/L, [normal reference range: 0.01-0.5 mg/L]), while with normal levels of folic acid, vitamin B12, Protein C (70%), antithrombin-III (61%), Protein S (101%) as well as negative antiphospholipid antibodies.
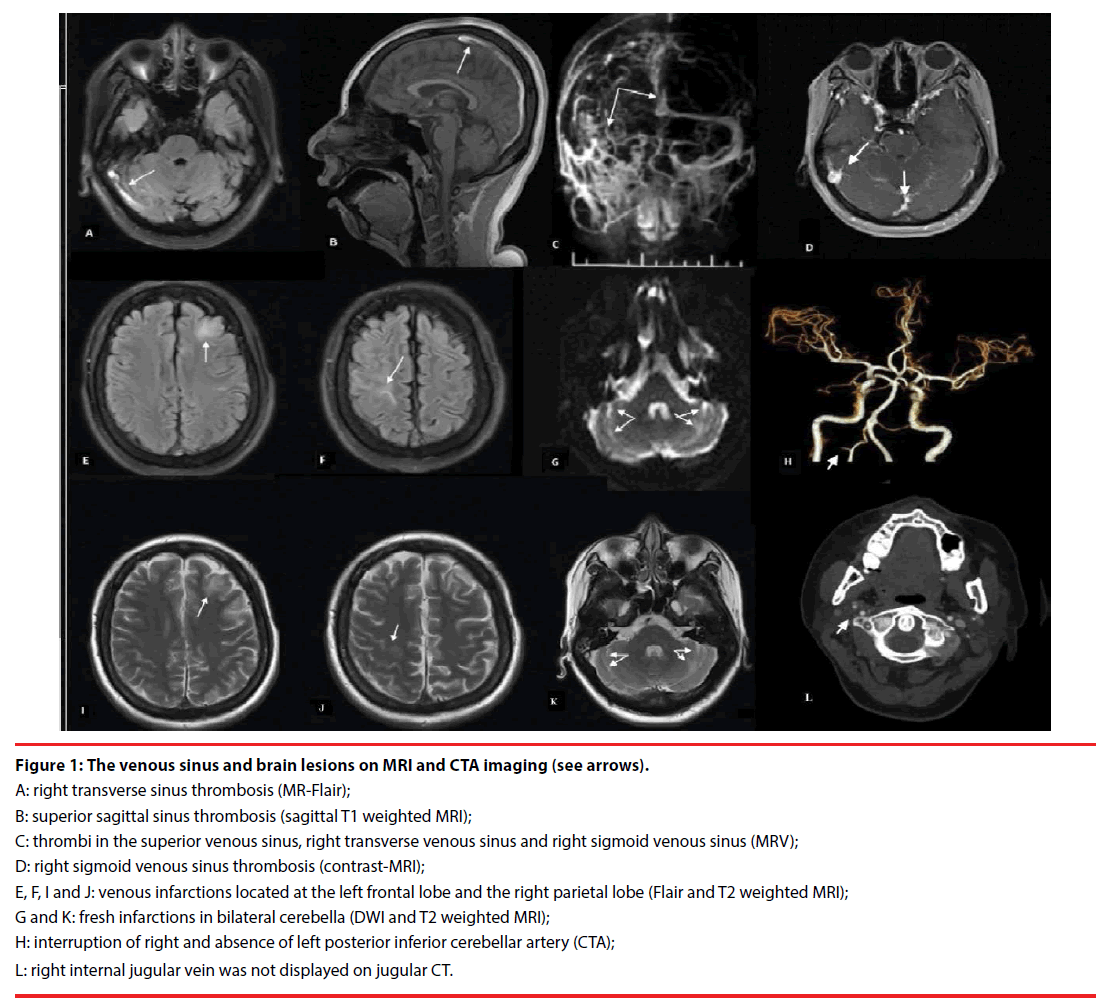
Magnetic resonance imaging (MRI) and contrast MR-venography (MRV) confirmed: the presence of thrombi in multiple cerebral venous sinuses; and distribution of newly infarctions at left frontal, right parietal lobes and bilateral cerebella. Computed tomography angiography (CTA) revealed the involvement of posterior inferior cerebellar arteries (PICA); right internal jugular vein was absent on computed tomography imaging of neck (Figure 1). Vascular ultrasound identified that the deep venous thrombi (DVT) were distributed in the right jugular vein and the deep veins of bilateral legs. Tran’s esophageal ultrasound and contrast-echocardiogram sonography confirmed the existence of patent foramen ovale (PFO, diameter, 1.2 mm). No abnormalities were found in electromyography (EMG) examination.
Figure 1: The venous sinus and brain lesions on MRI and CTA imaging (see arrows).
A: right transverse sinus thrombosis (MR-Flair);
B: superior sagittal sinus thrombosis (sagittal T1 weighted MRI);
C: thrombi in the superior venous sinus, right transverse venous sinus and right sigmoid venous sinus (MRV);
D: right sigmoid venous sinus thrombosis (contrast-MRI);
E, F, I and J: venous infarctions located at the left frontal lobe and the right parietal lobe (Flair and T2 weighted MRI);
G and K: fresh infarctions in bilateral cerebella (DWI and T2 weighted MRI);
H: interruption of right and absence of left posterior inferior cerebellar artery (CTA);
L: right internal jugular vein was not displayed on jugular CT.
The scenario of CVST was not well controlled with standard anticoagulation (low molecular heparin bridged to warfarin, INR is 2.5), and the abnormally elevated homocysteine could not be explained as CVST with PFO. Thereafter, the organic acids and blood ammonia were detected, and the findings were listed as follows: abnormally elevated urinary methylmalonic acid (MMA, 58.33 mmol, [normal reference range: 0.2-3.6 mmol]) and blood propionyl carnitine (7.95 μmol/L, [normal reference range: 0.43-4.12 μmol/L]), the ratio of propionyl carnitine/acetyl carnitine being 0.31, blood ammonia (73 μmol/L, [normal reference range: 0-100 μmol/L] and other details were displayed in our supplementary file. Furthermore, the outcomes of MMACHC gene sequencing showed mutations at sites of c. 482G>A and c. 609 G>A, which are two of the most frequent mutations reported amongst Chinese patients and accounted for approximately 48% and 7% of disease alleles, respectively [5].
This patient then received etiological treatments (Betaine, L-cainitine, and methylcobalamin) and homocysteine inhibitors accompanied with standard anticoagulation. Within 2 weeks of inpatient follow-up after the intervention: the levels of homocysteine, MMA and ICP were decreased to 34.8 μmol/L, 12.6 mmol and 150 mmH2O, respectively; NIHSS=0, mRS=0. During 9 months of outpatient follow-up: no dizziness, headache, mental disorders and epilepsy recurrence were complained; no newly formed brain lesions of either venous or arterial infarctions were identified on MRI/diffusion weighted imaging (DWI) images. Recanalizations of CVST and DVT were confirmed by MRV and vascular ultrasound.
Discussion
Cerebral venous infarction subsidiary to CVST caused by MMA in adults is uncommon. MMA, a type of organic acid metabolic disorder, often results in multi-system dysfunctions. Based on the age at initial onset, MMA can be divided into early-onset (in neonatal) and late-onset (in childhood or adult) subtypes, the latter of which displays a significantly higher incidence of neurological and psychological symptoms [6,7]. MMA can also be classified as idiopathic and acquired ones; the former is caused by abnormal metabolism of organic acid and/or vitamin B12, while the latter is likely attributed to malabsorption and/or disorders of transport [6]. In terms of the efficacy of vitamin B12 treatment, MMA consists of vitamin B12 positive and negative subtypes [8]. Obviously, taken the data above together, our case belongs to the idiopathic late-onset with hyperhomocysteinemia and vitamin B12 positive MMA subtype.
In this case, similar to the infarcted brain lesions distributed at left frontal and right parietal lobes, bilateral cerebellar infarctions were likely to be associated with MMA-related CVST. The pathogenesis of cerebral infarctions in MMA is currently understood to be induced by the disruption of mitochondrial aerobic glucose oxidation as a result of excessive accumulation of organic acids, leading to a decrease in cellular energy generation and subsequent excitotoxicity [9]. Nevertheless, it cannot rule out another possibility, that is, cerebellar infarctions may be derived from PFO related arterial emboli [10], considering the fact that blood flow of PICA was interrupted in the right and absent in the left on CTA, even though the infarctions did not seem to be located within the typical PICA territory.
It was postulated that both CVST and DVT were associated with hyperhomocysteinemia of MMA [6]. Symptomatic epilepsy was likely caused by cortical venous infarctions secondary to CVST, while psychiatric symptoms might be a feature of MMA. It should be noted that the late-onset subtype of MMA more frequently presents with psychiatric symptoms when compared with the early-onset one. Before the organic acid screening, the psychiatric disorders and CVST of this case were once regarded as two independent issues. After confirmation of the diagnosis of MMA, the psychiatric disorders were well explained. It has been well known that hyperammonemia is observed in a large percentage of patients with MMA. The ammonia-induced cellular redox changes on mitochondrial function and alterations of the glycine/glutamate cycle might contribute to MMA-induced excitability [11]. However, the blood ammonia level of this patient was within normal range.
Conclusion
This case demonstrates that screening for MMA is necessary, particularly when a young patient with CVST presenting severe hyperhomocysteinemia and psychiatric disorders. Furthermore, standard anticoagulation therapy is not adequate for MMA-related CVST, and strategies aiming at etiological levels are required on this condition.
Conflict of Interest
All authors report no disclosures or conflicts of interest.
Acknowledgments
This study was sponsored by the National Key R&D Program (2017YFC1308400) and the National Natural Science Foundation (81371289), of China.
Disclosure
All authors report no disclosures or conflicts of interest. Co-First authors Drs Da Zhou and Weijuan Wu contributed equally to this article. The authors alone are responsible for the content and writing of this paper.
References
- Hiltunen S, Putaala J, Haapaniemi E, et al. Long-term outcome after cerebral venous thrombosis: analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. J. Neurol 263(3), 477-484 (2016).
- Senadim S, Alpaydin BS, Tekin GB, et al. A rare cause of cerebral venous thrombosis: cryptococcal meningoencephalitis. Neurol. Sci 37(7), 1145-1148 (2016).
- Karti DT, Karti O, Aktert D, et al. Sildenafil-related cerebral venous sinus thrombosis and papilledema: a case report of a rare entity. Neurol. Sci 38(9), 1727-1729 (2017).
- Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(4), 1158-1192 (2011).
- Liu MY, Yang YL, Chang YC, et al. Mutation spectrum of MMACHC in Chinese patients with combined methylmalonic aciduria and homocystinuria. J. Hum. Genet 55(9), 621-626 (2010).
- Sunden SL, Renduchintala MS, Park EI, et al. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys 345(1), 171-174 (1997).
- Bodamer OA, Rosenblatt DS, Appel SH, et al. Adult-onset combined methylmalonic aciduria and homocystinuria (cblC). Neurology 56(8), 1113 (2001).
- Kolker S, Sauer SW, Hoffmann GF, et al. Pathogenesis of CNS involvement in disorders of amino and organic acid metabolism. J. Inherit. Metab. Dis 31(2), 194-204 (2008).
- Royes LF, Gabbi P, Ribeiro LR, et al. A neuronal disruption in redox homeostasis elicited by ammonia alters the glycine/glutamate (GABA) cycle and contributes to MMA-induced excitability. Amino. Acids 48(6), 1373-1389 (2016).
- Sun YP, Homma S. Patent Foramen Ovale and Stroke. Circ. J 80(8), 1665-1673 (2016).
- Wajner M, Goodman SI. Disruption of mitochondrial homeostasis in organic acidurias: insights from human and animal studies. J. Bioenerg. Biomembr 43(1), 31-38 (2011).