Commentary - Interventional Cardiology (2011) Volume 3, Issue 3
MICASA: a randomized trial using biochemical markers and cardiac magnetic resonance imaging
- Corresponding Author:
- William J van Gaal
Department of Cardiology, University of Melbourne
Northern Health, Victoria 3076, Australia
Tel: +61 384 058 389
Fax: +61 384 058 405
E-mail: william.vangaal@nh.org.au
Abstract
Keywords
catheter-based coronary interventions, coronary artery disease, CV surgery, stents
The incidence of symptomatic coronary heart disease (defined as myocardial infarction [MI], angina pectoris, coronary artery bypass grafting [CABG], percutaneous coronary intervention [PCI] and/or fatal atherosclerotic coronary heart disease) in the USA between 1989 and 2000 was as high as 41.7 cases per 1000 patient-years [1]. In the USA, over 150,000 patients undergo CABG per year, with many more undergoing PCI [2].
Current treatment guidelines for patients with left main and/or three-vessel coronary artery disease recommend CABG as the preferred treatment option. Two-vessel disease involving the proximal left anterior descending artery is considered equally suited to treatment with PCI or CABG [3]. When choosing a revascularization strategy other factors also need consideration, including operative risk and informed patient preference, with many patients opting for percutaneous treatment rather than the surgical approach.
As well as the risk of death and cerebrovascular injury, both revascularization strategies can lead to irreversible myocardial damage. Even minor elevations of troponin I (cTnI) are detectable by sensitive imaging techniques such as cardiac magnetic resonance (CMR). Irrespective of the revascularization method used, studies utilizing CMR have shown that postprocedural elevation of creatine kinase-MB (CK-MB) [4] and cTnI [5,6] following revascularization reflect irreversible myocardial necrosis. Furthermore, the extent of elevation of troponin measured at 24 h postprocedure is directly related to the absolute mass of new myocardial necrosis [5], which can be measured directly using CMR techniques. The actual incidence of periprocedural myocardial injury varies widely according to the definition used, methodology employed, population studied and the type of revascularization performed. Reports range from as low as 3% with uncomplicated PCI [7], 28% with more complex PCI [8] and up to 44% following CABG [6]. However, no randomized study has evaluated the incidence of periprocedural myocardial injury following PCI compared with CABG, in particular utilizing CMR imaging and the new joint ESC/ ACCF/AHA/WHF task force definitions of periprocedural necrosis and infarction.
Recently, the definition of periprocedural myocardial necrosis and MI following PCI and CABG was arbitrarily redefined by the joint ESC/ACCF/AHA/WHF task force [9]. Following PCI or CABG in patients with normal baseline troponin values, elevations of cardiac biomarkers above the 99th percentile upper reference limit (URL) are indicative of periprocedural myocardial necrosis. The definitions of MI differ however. Following PCI, an increase in biomarkers greater than 3 × 99th percentile URL defines PCI-related MI. However, for the definition of CABG-related MI the increase in biomarkers must be greater than 5 × 99th percentile URL, plus one of the following must be present: new pathological Q waves, new left bundle branch block, angiographically documented new graft or native vessel occlusion, or imaging evidence of new loss of viable myocardium.
There is little doubt that significant periprocedural myocardial injury is associated with worse clinical outcomes, including death. Elevated cardiac biomarkers following PCI have been shown to portend a worse prognosis [10]. To evolve therapy and minimize adverse outcomes, a greater understanding of periprocedural myocardial injury is required. This will allow development of new techniques and therapeutic strategies to predict, prevent and treat such injury.
Introduction to the trial
Patients were selected for the Myocardial Injury following Coronary Artery Surgery versus Angioplasty (MICASA) trial if they had severe disease of all three coronary arteries or double-vessel disease involving the left anterior descending artery, and/or disease of the left main coronary stem. The aims of the trial were to compare the frequency of periprocedural myocardial injury following CABG compared with PCI. Serial measurement or biomarkers were undertaken using cTnI and CK-MB, with CMR prior to and after treatment. The findings were compared according to the new definitions from the joint ESC/ACCF/AHA/WHF task force. Several angiographic scores including the SYNTAX score, Gensini score and the AHA/ACC classification, as well as measures of procedural risk (Euroscore), were evaluated for their ability to predict periprocedural myocardial injury.
▪ Design
The MICASA trial was a prospective, singlecenter, randomized (1:1) trial of myocardial injury following PCI compared with CABG [11]. This randomized controlled trial was prospectively registered [101].
Based on previous work by our group, we expected the frequency of periprocedural injury to be 44% in the CABG group [6]. We prospectively powered the study to detect a 25% difference in the primary end point (frequency of periprocedural injury) between the groups with a significance level of 0.05 and a power of 80%.
Patients who underwent coronary angiography with stable angina, unstable angina (Braunwald class 1 or 2) or documented silent ischemia on functional stress testing, at the John Radcliffe Hospital (Oxfordshire, UK), were eligible for the study. The interventional cardiologist and cardiac surgeon evaluated eligible patients with previously untreated threevessel coronary artery disease (minimum 50% stenosis in each vessel) or two-vessel coronary artery disease including an AHA type C lesion in the left anterior descending artery, and/or a functionally significant left main stem stenosis of at least 50%. Diagnostic coronary angiograms were scored according to the SYNTAX score algorithm [102]. Patients were randomized 1:1 to PCI or CABG if it was determined they could achieve equivalent anatomical revascularization based on their coronary angiogram.
Patients undergoing PCI were treated in accordance with current best practice including aspirin and clopidogrel prior to PCI, heparin intravenously and glycoprotein IIb/IIIa inhibitor (abciximab) followed by a 12-h infusion unless contraindications existed. Use of drug-eluting stents was the preferred strategy for all lesions. Angioplasty technique (use of adjunctive lesion preparation, direct stenting, postinflation and bifurcation technique) was left to operator choice and patients underwent PCI in one sitting. Aspirin was prescribed indefinitely for all patients who underwent randomization. Clopidogrel was prescribed to all PCI patients with an intended duration of at least 12 months.
Patients undergoing CABG ceased all antiplatelet agents at least 2 days prior to surgery. Myocardial protection was achieved with cold crystalloid cardioplegia delivered antegradely. Use of saphenous vein and/or arterial conduits was left to the discretion of the operator, although all patients received at least one pedicle internal mammary artery graft.
▪ Data analysis
The primary end points were the frequency of new myocardial injury following PCI and CABG, as assessed by postprocedural elevation of cTnI, and new late gadolinium enhancement (LGE) on postprocedural CMR. Secondary end points included the total amount of new myocardial necrosis (in grams) assessed by CMR, the change in left ventricular ejection fraction assessed by CMR, and periprocedural myocardial necrosis and infarction as defined by the new universal definition [9].
Troponin I and CK-MB were measured before treatment and at multiple time points in the first 24 h after revascularization. For CMR imaging, patients were studied at 1.5T Sonata (Siemens Medical Solutions). Baseline CMR assessment was performed in the fortnight prior to revascularization. Repeat CMR was performed 7 days (4–10 days) after the procedure, and a third CMR assessment was undertaken 6 months (5–7 months) after revascularization. Areas of LGE were assessed using custom software (MATLAB R2007b; The MathWorks, MA, USA), with new LGE being defined as pixels with an image intensity >2 standard deviations above the mean image intensity with the same slice.
▪ Results
A total of 80 patients were randomized into the study; however, one patient died after randomization to CABG prior to having surgery. The remaining 79 patients all underwent their assigned treatment and there were no significant differences with respect to baseline demographic variables between the treatment groups. Thus, in the surgical group, 39 patients underwent successful on-pump CABG and in the PCI group, 40 patients underwent attempted PCI. Revascularization was complete in 39 out of 40 PCI procedures (102 treated vessels), with inability to cross a chronic total occlusion in one patient. PCI was uneventful in 35 of the remaining patients, with four patients experiencing occlusive dissection or embolism. There were two patients in the PCI group and seven in the CABG group that did not undergo LGECMR analysis (one poor image quality, three impaired estimated glomerular filtration rate, five claustrophobia).
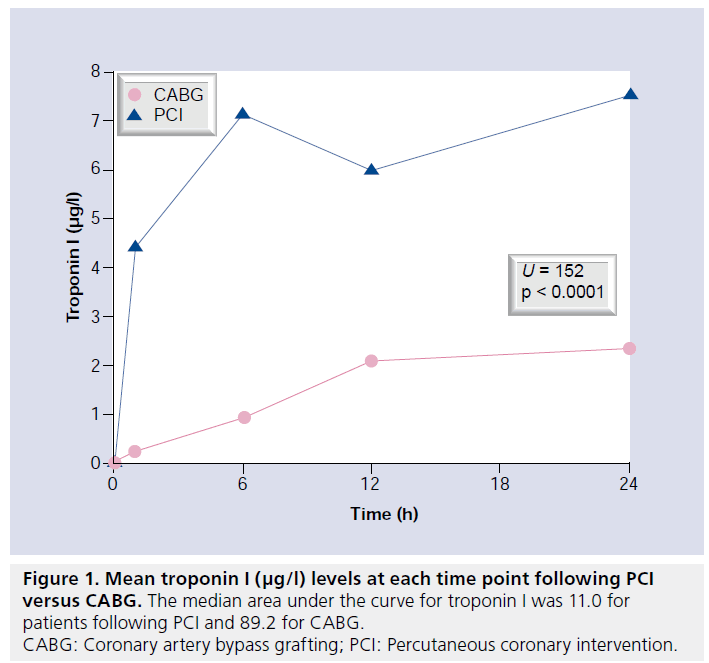
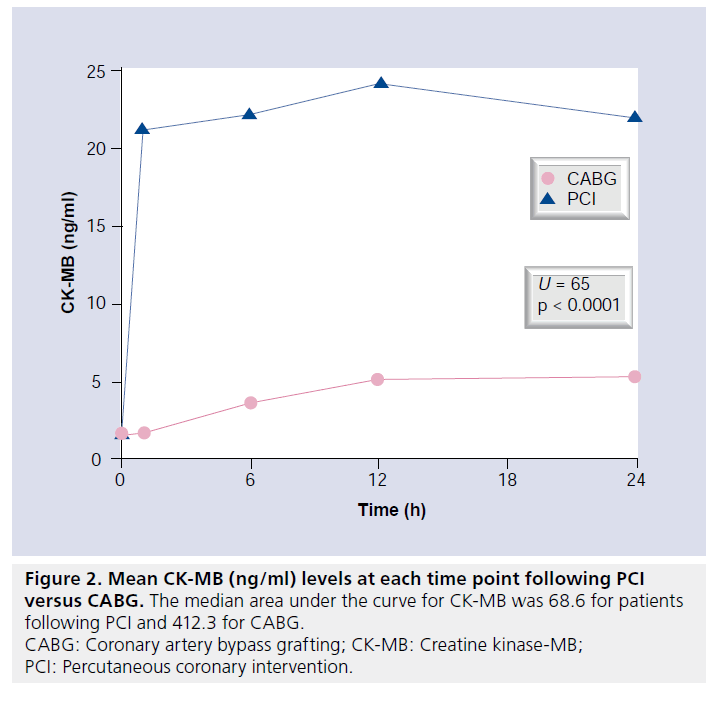
No patient developed a new Q wave MI according to the 12-lead ECG following revascularization. Elevation of cTnI was universal in the CABG group and significantly more frequent compared with the PCI group in those with biomarkers available for analysis (35 out of 35, 100% vs 30 out of 40, 75%; p = 0.0001). Mean peak cTnI and CK-MB levels in the first 24 h after revascularization were also significantly higher following CABG compared with PCI (Figures 1 & 2).
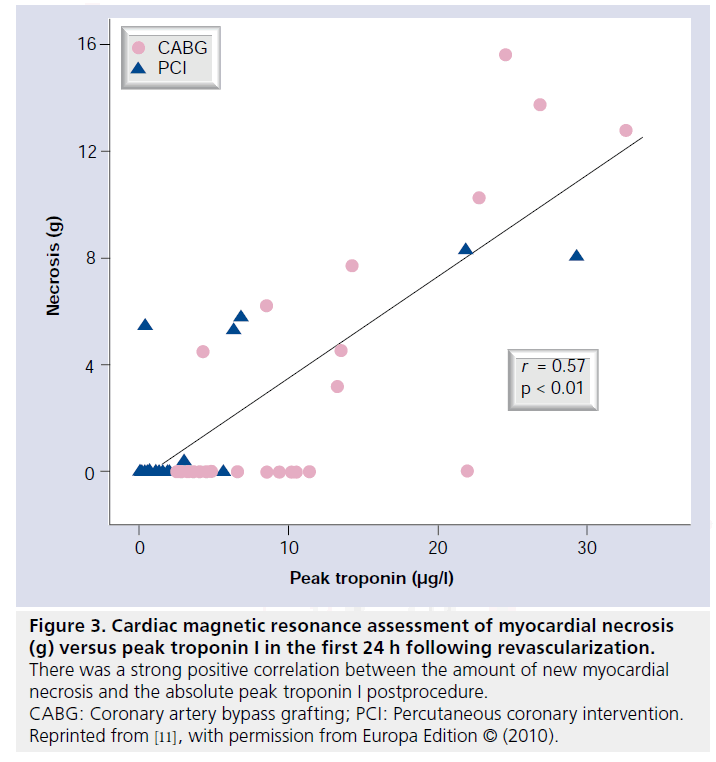
Rates of new LGE on the early postprocedure CMR were lower than expected and not statistically different between the two revascularization strategies (CABG: 9 out of 32, 28.1% vs PCI: 6 out of 38, 15.8%; p = 0.25). In patients with new LGE, the mean amount of new necrosis (grams) following CABG was 8.7 ± 4.5 versus 5.6 ± 2.8 following PCI (p = 0.12). There was a strong positive correlation between the absolute peak cTnI postprocedure and the amount of new myocardial necrosis in grams assessed by CMR (Figure 3). At 6 months, 13 patients with new LGE underwent repeat imaging with minimal change in the amount of LGE (8.2 ± 3.8 g at 7 days vs 7.9 ± 3.8 g at 6 months; p = 0.25).
Figure 3: Cardiac magnetic resonance assessment of myocardial necrosis (g) versus peak troponin I in the first 24 h following revascularization. There was a strong positive correlation between the amount of new myocardial necrosis and the absolute peak troponin I postprocedure. CABG: Coronary artery bypass grafting; PCI: Percutaneous coronary intervention. Reprinted from [11], with permission from Europa Edition © (2010).
According to the new universal definition, the incidence of periprocedural necrosis was 35 out of 35 (100%) in the CABG group and 30 out of 40 (75%) in the PCI group (p = 0.0001), which simply reflects the presence of any elevation in postprocedural cTnI. The incidence of MI was much lower in the CABG group according to the new definition (9 out of 32, 28.1% vs 30 out of 40, 75%; p = 0.0001). All of the cTnI elevations post-PCI met the criteria for necrosis as well as infarction, being more than 3 × 99th URL. The ‘apparent’ infarction rate was lower in the CABG group owing to the need for imaging evidence of infarction (not present in the PCI-related MI definition). Only six patients in the PCI group who met the new criteria for infarction had imaging evidence of infarction (LGE).
Troponin I elevation with new LGE following PCI was associated with visible angiographic complications (dissection with transient slow flow, embolus) in the majority of cases. Patients in the CABG group were more likely to have pre-existing LGE prior to revascularization and were also more likely to have periprocedural troponin elevation without evidence of new LGE.
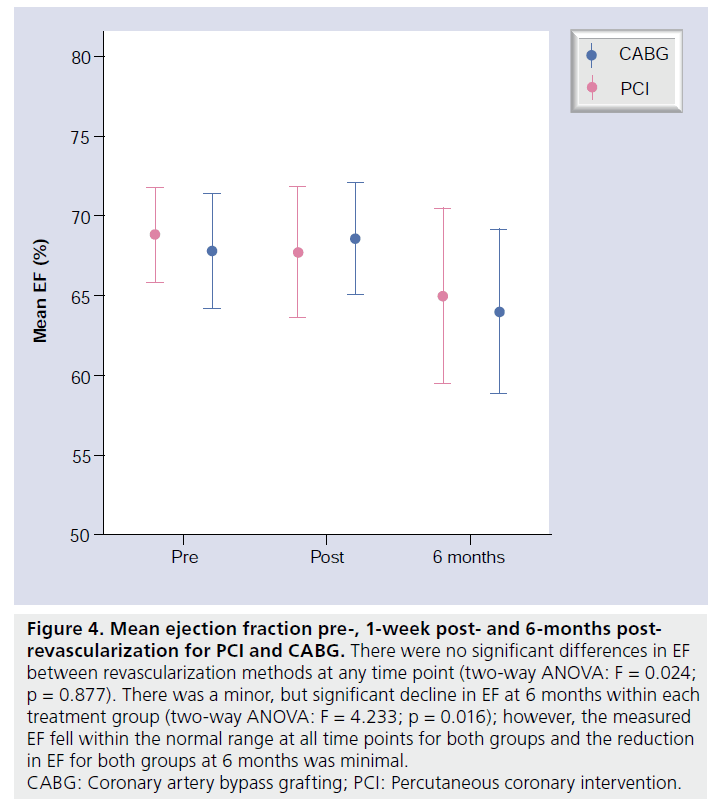
The baseline ejection fraction was similar in each group, with no significant differences following revascularization or at 6-month follow-up (Figure 4). New regional wall motion abnormalities postprocedure corresponded in all cases to vascular territories sustaining new areas of LGE.
Figure 4: Mean ejection fraction pre-, 1-week post- and 6-months postrevascularization for PCI and CABG. There were no significant differences in EF between revascularization methods at any time point (two-way ANOVA: F = 0.024; p = 0.877). There was a minor, but significant decline in EF at 6 months within each treatment group (two-way ANOVA: F = 4.233; p = 0.016); however, the measured EF fell within the normal range at all time points for both groups and the reduction in EF for both groups at 6 months was minimal. CABG: Coronary artery bypass grafting; PCI: Percutaneous coronary intervention.
None of the prespecified angiographic or clinical scores were predictive of periprocedural MI, nor was there any relationship between peak troponin and these scores. The mean patient SYNTAX scores in patients with and without new LGE were 19.9 ± 5.6 versus 23.8 ± 7.4, respectively (p = 0.07). Similarly, there was no relationship between peak troponin and the SYNTAX score (r = 0.039, p = 0.74).
Conclusion
The MICASA study demonstrates that PCI can be performed without excess LGE-defined periprocedural myocardial injury in patients with left main and/or triple-vessel disease. The amount of myocardial injury, as detected by new enzyme elevation with CABG, actually appears to be more than with PCI, although this is not translated into a significant difference as assessed by LGE-CMR.
Applying the new universal definitions of periprocedural necrosis and MI, periprocedural necrosis was significantly more frequent following CABG; however, MI was significantly more frequent following PCI. This arbitrarily manufactured difference relates to the requirement for the addition of CMR evidence of new LGE to the enzyme data to diagnose CABG-related MI. The incidence of MI in the PCI group would have fallen dramatically if the CABG criteria were applied (i.e., imaging evidence of infarction). Application of the new definition of MI profoundly affected the interpretation of our results. Whilst the AUC for troponin and CK-MB was significantly higher following CABG compared with PCI, and the incidence of CMRdefined injury was similar, application of the new definition actually resulted in a higher incidence of MI following PCI. Notably, the definition of CABG-related MI differs from PCI as it requires presence of either new Q waves, angiographically documented graft or native vessel occlusion, and/or imaging evidence of MI. Consequently, without routine CMR imaging in our study, the incidence of MI after CABG would have been 0%. The difference in definitions could result in confusion during future comparative revascularization studies.
CMR-defined injury following PCI was predicted by clinically relevant angiographic complications (embolus or dissection with slow flow).
The overall incidence of CMR-defined injury was relatively low for this complex patient subset (six out of 102 vessels). In previous studies, we have shown a 28% incidence of CMR-defined injury in complex PCI patients [8]. Factors that may explain the apparent reduction in periprocedural injury rates include the liberal use of IIb/IIIa inhibitors in the current study, fastidious technique to prevent side-branch loss by preemptive wiring of branches and predominant use of second-generation drug-eluting stent, which have thinner struts and have been shown to reduce both side-branch occlusion and the rates of non-Q wave MI during PCI [12].
Unlike previous studies, we were not able to detect LGE reliably following PCI in patients with troponin elevations under 4.0 μg/l. Factors that may explain this apparent loss of sensitivity include the multiterritory nature of the intervention, which may cause small undetectable areas of watershed ischemia, and the inclusion of some patients with pre-existing LGE. A subsequent analysis of these patients has shown that CK-MB may be the preferred biomarker for detection of periprocedural necrosis and infarction [13]. The majority of CABG patients with abnormal biomarker profiles and no new LGE had preexisting infarction, and it is possible that undetectable new injury may have occurred within these previously infarcted areas. Future studies of periprocedural myocardial injury utilizing imaging should exclude patients with any pre- existing necrosis.
Future perspective
The field of percutaneous revascularization for patients with left-main and triple-vessel disease is likely to progress further. Already many interventional cardiologists are willing to perform these procedures on high-risk surgical candidates and for those patients who make an informed decision regarding PCI. Certain subsets of patients have shown favorable/ equitable short-term (1 year) outcomes in the SYNTAX trial with PCI, notably patients with isolated left main disease not involving the bifurcation and those with low-to-intermediate SYNTAX scores (<33). Future developments in stent technology are likely to expand the group of patients suitable for PCI. Newer generation drug-eluting stents may reduce stent restenosis and thrombosis further, and are more deliverable. Novel polymer agents are now more biocompatible, and newer generation bioabsorbable polymers and stents are designed to ‘leave nothing behind’. New therapeutic agents such as prasugrel and ticagrelor have given cardiologists a much broader armamentarium in the fight against stent thrombosis, an issue of significance in patients undergoing multivessel stenting. Newer intravenous antiplatelet agents will get to market in the next 5 years and also improve the procedural outcomes for patients undergoing total percutaneous coronary revascularization.
With major adverse cardiac event rates already reduced by improvements in stent and drug technology, periprocedural injury is an easily measured surrogate marker of worse outcomes that will be employed in future trials of PCI with increasing frequency. With respect to periprocedural injury during PCI, fastidious technique aimed at prevention of side-branch loss is paramount. Distal embolization is also known to occur during PCI and can obstruct the microvasculature causing irreversible damage. Distal protection devices have proved disappointing in the native coronary circulation, whilst intracoronary vasodilators may improve flow; however, their effect on myocardial preservation is questionable. Despite best efforts, unforeseen complications such as dissection will still arise that jeopardize the myocardium and require prompt treatment. Myocardial preconditioning using intracoronary administration of adenosine has been shown to decrease myocardial damage caused by elective PCI, and the use of nitroglycerin, nicorandil, bradykinin and enalaprilat have shown promising results (e.g., reduction of ST-segment shift, less chest pain), but reduction of myocardial injury has not been demonstrated as yet. Remote preconditioning induced by three 5-min inflations of a blood pressure cuff to 200 mmHg around the upper arm, followed by 5-min intervals of reperfusion markedly improved the incidence of biomarker release and could represent an easily applicable tool to reduce injury during revascularization [14].
The definitions of periprocedural necrosis and MI for PCI and CABG should be aligned. Whilst imaging evidence of infarction is useful, only LGE-CMR has the required sensitivity to detect small areas of infarction, and is not readily available or suitable for routine use postrevascularization. Other imaging modalities and ECG evidence of new infarction are crude tools at detecting infarction and therefore not useful in incorporating into the standard definition of periprocedural MI. We would support the use of CK-MB rather than PCI as the marker of choice in defining such injury and this approach would be supported by recent meta-analytic data of CABG patients showing CK-MB to be the stronger predictor of adverse outcomes [15].
Information resource
▪ SYNTAX Score: www.syntaxscore.com/; downloadable free software allows you to calculate the SYNTAX score for patients with left main and/or triple-vessel coronary artery disease, and compare major adverse cardiac events out to 2 years.
Executive summary
Background
▪ Periprocedural injury is common following percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG).
▪ Periprocedural injury is associated with poorer clinical outcomes, including death.
▪ No randomized study has compared the frequency of periprocedural injury following PCI compared with CABG using cardiac magnetic resonance (CMR) imaging, or the new joint ESC/ACCF/AHA/WHF task force definitions of necrosis and myocardial infarction (MI).
Study overview
▪ Randomized controlled trial of PCI versus CABG.
▪ Primary end points were periprocedural injury as assessed by cardiac biomarkers, late gadolinium enhancement (LGE) on MRI, and the new joint ESC/ACCF/AHA/WHF task force definitions of necrosis and MI.
▪ Secondary end point was to evaluate angiographic and clinical predictors of periprocedural injury.
Results
▪ 80 patients enrolled (40 patients underwent PCI, 39 underwent CABG).
▪ Median AUC and mean peak troponin I and creatine kinase-MB levels were significantly higher following CABG compared with PCI in the first 24 h following treatment.
▪ The frequency of new LGE on MRI and the mean amount of new necrosis in those with new LGE were not statistically different between treatment groups.
▪ According to the new joint ESC/ACCF/AHA/WHF task force definitions, postprocedural necrosis was universal following CABG but not PCI, whilst procedure-related MI was significantly more frequent following PCI (the difference being entirely accounted for by discrepancies in the definitions of PCI and CABG-related MI).
Conclusion
▪ PCI can be performed without excess periprocedural myocardial injury in patients with left-main and/or triple-vessel disease as assessed by CMR and cardiac biomarkers.
▪ CMR-defined injury following PCI is predicted by clinically relevant angiographic complications.
▪ Application of the new definition of MI will profoundly affect the interpretation of studies comparing CABG with PCI.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Wilson CT, Fisher ES, Welch HG, SiewersAE, Lucas FL: U.S. trends In CABG hospitalvolume: the effect of adding cardiac surgeryprograms. Health Aff. 26, 162–168 (2007).
- Patel MR, Dehmer GJ, Hirshfeld JW, SmithPK, Spertus JA; ACCF/SCAI/STS/AATS/AHA/ASNC: 2009 appropriateness criteriafor coronary revascularization: a report bythe American College of CardiologyFoundation Appropriateness Criteria TaskForce, Society for CardiovascularAngiography and Interventions, Society ofThoracic Surgeons, American Association forThoracic Surgery, American HeartAssociation, and the American Society ofNuclear Cardiology Endorsed by theAmerican Society of Echocardiography, theHeart Failure Society of America, and theSociety of Cardiovascular ComputedTomography. J. Am. Coll. Cardiol. 53,530–553 (2009).
- Ricciardi MJ, Wu E, Davidson CJ et al.:Visualization of discrete microinfarction afterpercutaneous coronary interventionassociated with mild creatine kinase-MBelevation. Circulation 103, 2780–2783(2001).
- Selvanayagam JB, Porto I, Channon K et al.:Troponin elevation after percutaneouscoronary intervention directly represents theextent of irreversible myocardial injury:insights from cardiovascular magneticresonance imaging. Circulation 111,1027–1032 (2005).
- Selvanayagam JB, Petersen SE, Francis JMet al.: Effects of off-pump versus on-pumpcoronary surgery on reversible and irreversiblemyocardial injury: a randomized trial usingcardiovascular magnetic resonance imagingand biochemical markers. Circulation 109,345–350 (2004).
- Porto I, Blackman DJ, Nicolson D et al.:What is the incidence of myocardial necrosisin elective patients discharged on the sameday following percutaneous coronaryintervention? Heart 90, 1489–1490 (2004).
- Porto I, Selvanayagam JB, van Gaal WJ et al.:Plaque volume and occurrence and locationof periprocedural myocardial necrosis afterpercutaneous coronary intervention: insightsfrom delayed-enhancement magneticresonance imaging, thrombolysis inmyocardial infarction myocardial perfusiongrade analysis, and intravascular ultrasound.Circulation 114, 662–669 (2006).
- Thygesen K, Alpert JS, White HD et al.:Joint ESC/ACCF/AHA/WHF Task Force forthe Redefinition of Myocardial Infarction.Universal definition of myocardial infarction.Circulation 116, 2634–2653 (2007).
- Testa L, van Gaal WJ, Biondi Zoccai GGet al.: Myocardial infarction afterpercutaneous coronary intervention: ameta-analysis of troponin elevation applyingthe new universal definition. QJM 102,369–378 (2009).
- Sianos G, Morel MA, Kappetein AP et al.:The Syntax score: an angiographic toolgrading the complexity of coronary arterydisease. Eurointervention 1, 219–227 (2005).
- van Gaal WJ, Arnold JR, Testa L et al.:Myocardial injury following coronary arterysurgery versus angioplasty (MICASA): arandomised trial using biochemical markersand cardiac magnetic resonance imaging.Eurointervention 6, 703–710 (2011).
- Popma JJ, Mauri L, O’Shaughnessy C et al.:Frequency and clinical consequencesassociated with sidebranch occlusion duringstent implantation using zotarolimus-elutingand paclitaxel-eluting coronary stents. Circ.Cardiovasc. Intervent. 2, 133–139 (2009).
- Lim CC, van Gaal WJ, Testa L et al.: Withthe “universal definition,” measurement ofcreatine kinase-myocardial band rather thantroponin allows more accurate diagnosis ofperiprocedural necrosis and infarction aftercoronary intervention. J. Am. Coll. Cardiol.57, 653–661 (2011).
- Hoole SP, Heck PM, Sharples L et al.:Cardiac Remote Ischemic Preconditioning inCoronary Stenting (CRISP Stent) Study: aprospective, randomized control trial.Circulation 119, 820–827 (2009).
- Domanski MJ, Mahaffey K, Hasselblad Vet al.: Association of myocardial enzymeelevation and survival following coronaryartery bypass graft surgery. JAMA 305,585–591 (2011).
▪ Websites
101 Myocardial Injury following Coronary Artery bypass Surgery versus percutaneous coronary Angioplasty with stents: a randomised controlled trial using biochemical markers and cardiovascular magnetic resonance imaging www.controlled-trials.com/ ISRCTN25699844
102 Incidence & Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. National Institutes of Health. National Heart, Lung, and Blood Institute (2006)