Review Article - Interventional Cardiology (2012) Volume 4, Issue 2
Minimally invasive and transcatheter techniques in high-risk cardiac surgery patients
- Corresponding Author:
- Simon H Sündermann
Division of Cardiac & Vascular Surgery
University Hospital Zurich
Zurich, Switzerland
Tel: +41 442553298
Fax: +41 442554446
E-mail: simon.suendermann@usz.ch
Abstract
Keywords
aortic valve, high-risk patient, MIDCAB, minimally invasive surgery, mitral valve, OPCAB, TECAB, transcatheter valve procedure, tricuspid valve
Minimally invasive cardiovascular surgical techniques were popularized in the mid 1990s with the performance of the first heart valve and coronary artery bypass procedures using minimal access routes [1,2]. Compared with laparoscopic interventions, the development of minimally invasive cardiac surgical techniques poses some unique challenges. These include the anatomical position of the target organ, protected by the rigid chest wall, as well as the natural rhythmic motion of the heart [3]. As such, the first procedures were performed after techniques were developed to facilitate access and stabilize the heart.
Two aspects define minimally invasive cardiac surgery: the nature of the access and the avoidance of cardiopulmonary bypass (CPB). This definition implies that all procedures that are not performed through a conventional median sternotomy or standard thoracotomy, and/or without the use of CPB, are minimally invasive in nature. Examples include partial sternotomies, mini-thoracotomies and the use of peripheral vascular access. Off-pump coronary artery bypass (OPCAB) surgery, minimally invasive direct coronary artery bypass (MIDCAB) grafting and transcatheter valve interventions are further considered to be minimally invasive approaches.
In many instances these techniques have become the procedure of choice for elective cardiac surgical procedures and the application in selected high-risk patients have been investigated.
High-risk patients
Operative risk has traditionally been calculated using various scoring systems including the European system for cardiac operative risk evaluation (EuroSCORE) [4] and Society of Thoracic Surgeons (STS) score [5]. Individual components of these risk scores such as advanced age, redo surgery, impaired left ventricular (LV) function and renal impairment have also been used to stratify periprocedural risk. The observed aging of the general population further contributes to the development of increased comorbidities in individual patients and thus a higher operative risk [6,7]. Elderly patients also have an increased level of frailty, which is a predictor of short- and long-term mortality [8].
During recent years there has been a steady incline in the predicted operative mortality of patients, while the observed mortality has remained stable [9]. This, amongst other reasons, has been attributed to the development of new minimally invasive and image-guided techniques. Risk scoring systems have also been adapted to the changing patient profile. The EuroSCORE has recently been adjusted to include additional individual factors to predict periprocedural risk more accurately (Presented at EACTS annual meeting [2011]. For the EuroSCOREII calculator see [101]).
Minimally invasive treatment options for high-risk patients
▪ Minimally invasive coronary artery surgery
Coronary artery bypass grafting (CABG) can be performed with the use of CPB on an arrested or beating heart, or without CPB on a beating heart (OPCAB) using cardiac stabilizing devices. The first reports on OPCAB procedures were published in the early 1990s [10]. Since then, numerous trials have investigated the potential advantages of OPCAB versus conventional CABG procedures, but none could clearly demonstrate the superiority of one technique. The largest trial to date, the ROOBY-trial, showed no difference in 30-day mortality [11]. The ratio of OPCAB versus CABG procedures performed in different countries further attest to the lack of clear evidence. In the USA, approximately 20% of all CABG procedures are performed off-pump [12] and in Europe this number varies between 10 and 30%. In single centers it varies between 0 and 95% [13].
This controversy regarding a single superior procedure is even more debated about in highrisk patients requiring coronary revascularization. Al-Ruzzeh et al. retrospectively analyzed the outcome of 1398 patients with a preoperative EuroSCORE of ≥5 (defined as high risk [4]) [14]. Two hundred and eighty six patients underwent an OPCAB procedure and 1112 patients had a conventional CABG procedure performed. The end points included 30‑day mortality, major complications, atrial fibrillation and reoperation. Major complications were defined as myocardial infarction, renal dysfunction, pulmonary edema or acute respiratory distress syndrome, septicemia and cerebrovascular accidents. The 30‑day mortality was significantly higher in the conventional CABG group (7 vs 3.5%). Major complications as a combined end point also occurred significantly more often in the conventional CABG group (14.2 vs 7.3%). The authors concluded that OPCAB surgery reduces perioperative morbidity and mortality in selected high-risk patients as compared with conventional CABG surgery.
Emmert et al. analyzed the use of OPCAB surgery in patients with high-risk coronary anatomy (left main coronary artery disease) requiring revascularization. They compared 343 patients with left main coronary artery disease with 640 patients without left main coronary artery disease operated with the OPCAB technique. There was no significant difference in either group for the combined end point (30‑day mortality, postoperative renal failure, length of ICU stay [>2 days], neurological complications, the use of intraaortic balloon pump and conversion to CPB). The authors concluded that OPCAB surgery is a safe and feasible approach for patients with left main coronary artery disease [15]. Dewey and associates compared 100 patients with left main coronary artery disease, operated without the use of CPB to 793 patients with left main coronary artery disease that were operated with CPB. The 30-day mortality was 1% in the OPCAB group, compared with 4.7% in the CABG group. The OPCAB group was further associated with less postoperative inotropic support and the need for blood transfusions. The use of CPB was an independent risk factor for mortality in this series [16].
Emmert et al. also investigated the results of OPCAB versus conventional CABG surgery in 478 patients with poor ventricular function (LV ejection fraction ≤35%). The authors concluded that OPCAB surgery is a comparable alternative to conventional CABG surgery [17].
Consensus statement
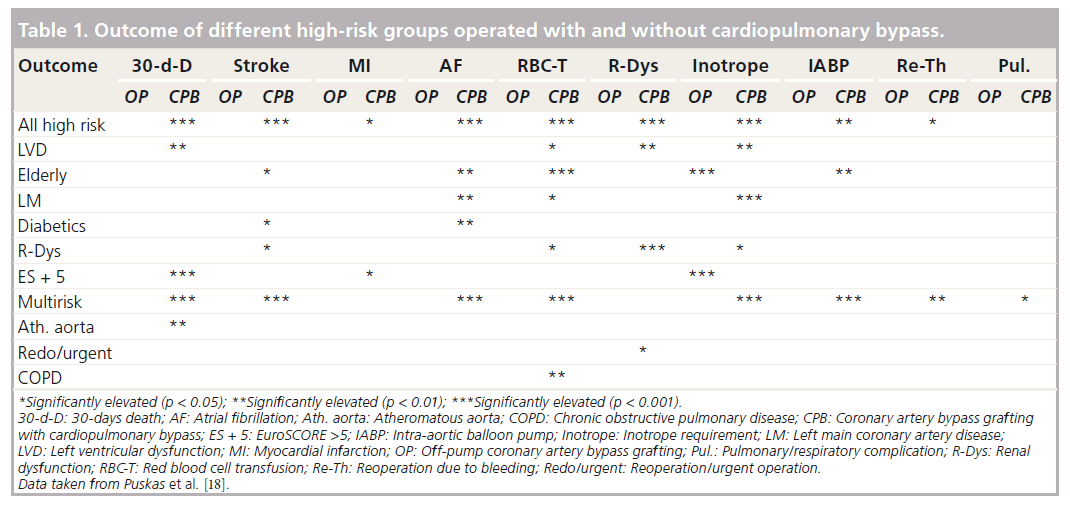
In 2005, the International Society for Minimally Invasive Cardiothoracic Surgery published a consensus report comparing OPCAB to conventional CABG surgery [18]. A separate meta-analysis for the high-risk patient population was also performed. This included 26,349 patients. The report concluded that OPCAB surgery is associated with a reduced 30-day mortality in the high-risk patient group and in some of the subgroups (poor LV function, high EuroSCORE, atheromatous aorta and a multirisk factor group), but found no differences in the 30-day mortality for other subgroups (advanced age, left main coronary artery disease, diabetes mellitus, renal dysfunction and chronic obstructive pulmonary disease). These results are summarized in Table 1.
The conclusion and recommendations of this consensus report are:
▪ OPCAB surgery should be considered as a safe alternative to conventional CABG surgery;
▪ A similar completeness of revascularization and graft patency can be achieved with OPCAB procedures;
▪ OPCAB is recommended to reduce perioperative morbidity;
▪ OPCAB may be recommended to minimize midterm cognitive dysfunction;
▪ OPCAB should be considered as an equivalent alternative as assessed by quality of life (QOL) questionnaires;
▪ OPCAB is recommended to reduce the duration of ventilation, ICU and hospital stay and resource utilization;
▪ OPCAB should be considered in high-risk patients to reduce perioperative mortality, morbidity and resource utilization.
Puskas et al. also recently analyzed the benefits of OPCAB surgery for high-risk patients. They extracted the data from 7083 OPCAB procedures and 7683 conventional CABG procedures from the STS database. Patients were divided into quartiles according to their STS score. Statistical analysis showed that OPCAB surgery is beneficial for patients with a higher STS score with a calculated threshold STS score of 2.5–3% [19].
▪ MIDCAB
The MIDCAB surgery procedure was first described by Robinson et al. in 1995 [20]. Since then, various authors published good results for selected patient populations when using this technique. The largest series by Holzhey et al. included more than 1300 patients and demonstrated a graft patency of 95% at 6 months follow-up with a 90% 7 year survival [21].
MIDCAB surgery conforms to both aspects of minimally invasive surgery. The procedure is performed without CPB via a limited incision. It can therefore be regarded as an ideal procedure for higher risk patients with contraindications to a median sternotomy or the use of CPB. In addition, it can be used during hybrid procedures together with percutaneous coronary intervention for patients with multivessel disease and high operative risk.
The role of MIDCAB procedures in high-risk patients has been investigated widely. Sorm et al. showed that use of a MIDCAB procedure is an acceptable alternative in patients over 70 years of age with single vessel coronary artery disease. The 30-day mortality in this series was 2.5% (8.7% predicted by EuroSCORE) and the 5-year survival was 80% [22].
Morishita et al. investigated the role of MIDCAB surgery in redo CABG. In a case series of 7 patients they concluded that MIDCAB via a left anterolateral thoracotomy is a safe and effective technique [23]. Sunderdiek et al. reported their MIDCAB results on 35 high-risk patients who were turned down for conventional CABG surgery (the risk factors included impaired LV-function, concomitant pulmonary disease, advanced age and redo surgery). They concluded that MIDCAB is an acceptable alternative with excellent clinical results in selected high-risk patients and reduces mortality, morbidity and length of in-hospital stay [24].
Giglio et al. described a series of 21 high-risk patients (EuroSCORE >6) who underwent a MIDCAB procedure via a J-shaped mini sternotomy. Eight of the patients were operated without the use of general anesthesia and four patients had a hybrid procedure with additional stenting to complete revascularization. There was one in-hospital death. The graft patency was 100% after 6 months as evaluated using 64-slice computer tomography [25].
There have been some reports on MIDCAB procedures performed under local anesthesia. Multiple incisions to access the various coronary vessels have also been described. A report by Watanabe described a subxyphoid approach that was used in three patients. Anesthesia was maintained with a high epidural block whilst the patients were awake during the procedure. The xyphoid cartilage was excised and the gastro-epiploic artery used as a bypass graft to the left anterior descending artery. These patients had severe respiratory comorbidities, which precluded them from having general anesthesia. All three patients survived the procedure without severe complications and were discharged after 7–10 days postoperatively [26].
▪ Totally endoscopic CABG
The term totally endoscopic coronary artery bypass (TECAB) grafting is used to describe coronary artery bypass procedures performed via port access using telemanupilators such as the da Vinci® robotic system. Although initially described for use in other cardiac surgical procedures this technique was adapted to perform CABG in the late 1990s. The first promising reports by Falk et al. described the successful harvesting of the left internal mammary artery, to be used as a bypass graft to the left anterior descending artery. This procedure was performed on an arrested heart [27]. Although this technique was further developed, there are very few reported cases of its use in high-risk patients [28]. Further refinement of this technique might lead to wider application in selected patients.
▪ Minimally invasive aortic valve surgery
Aortic valve surgery is the second most frequently performed cardiac surgical procedure worldwide and the most commonly performed heart valve operation. The vast majority of aortic valve replacements (AVRs) are performed for native valve degeneration. Surgical AVR is a routine procedure with a low incidence of mortality and morbidity [29]. In the past decade, significant developments have been made in the field of aortic valve surgery. Surgical access has become less invasive and new implantation techniques and devices using image guidance have been developed.
In 1996, Cosgrove et al. described the first minimally invasive aortic valve procedures [30]. Surgical access via a 10 cm right parasternal incision together with femoral vessel cannulation for CPB was used to perform aortic valve repair or replacement.
Alternative surgical access routes previously described include upper and lower ministernotomies as well as a transverse sternotomy [31] and right anterior thoracotomy [32].
Numerous studies have investigated the use of these techniques for high-risk patients. A systematic literature review by Schmitto, Mohr and Cohn evaluated the role of minimal surgical access approaches to the aortic valve in various subsets of high-risk patients. These included elderly patients, redo surgery and patients with poor LV function. The authors concluded that minimally invasive surgical AVR is associated with faster recovery times and a lower incidence of sepsis and wound complications, when compared with the conventional median sternotomy approach. It is further associated with decreased blood loss during redo operations therefore reducing the need for blood transfusions [29].
Apart from minimally invasive access routes, further developments have focused on the refinement of valve prosthesis that can be implanted using sutureless and transcatheter techniques. Although the role of sutureless valves implanted via minimally invasive access using CPB have not been extensively investigated, the reported reduced ischemic periods associated with this technique could prove useful in selected high-risk patients [33].
▪ Transcatheter aortic valve implantation
The rapid evolution of transcatheter aortic valve implantation (TAVI) has revolutionized the clinician’s ability to manage high-risk patients with symptomatic aortic stenosis. After success in animal models [34], the first successful implantation in a human was reported by Cribier et al. [35]. They described a percutaneous, trans-septal, antegrade approach to position a valve prosthesis within the calcified aortic valve of a patient with cardiogenic shock after a failed balloon valvulopasty.
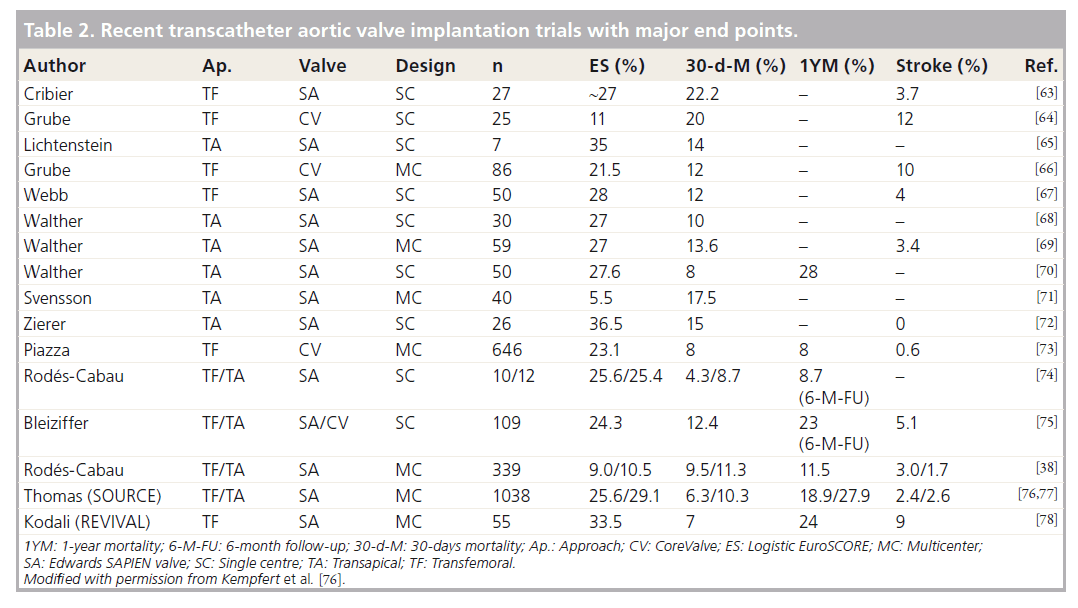
A further 2 years later, Cribier described a series of six patients of whom three had survived to 8 weeks follow-up [36]. At last follow-up there were no residual gradients and in five of the patients they reported aortic valve insufficiency ranging from mild to severe. Following this, various authors have reported on their experiences using the TAVI techniques. As such, TAVI has become one of the most researched topics in the last decade in cardiovascular surgery and intervention (Table 2).
The two approaches that are commonly used are transapical (TA) and transfemoral (TF) access. The first experiences with the TA approach were published in 2006 [37]. Transfemoral access is less invasive but not applicable to all patients, especially those with peripheral vascular disease. Rodés-Cabau et al. analyzed 345 patients with very high operative risk. Of these patients, 170 were treated with a TA TAVI and 175 with a TF TAVI. The 30 day mortality was 10.4%. The survival rates after 1 and 2 years for TF and TA access in this series were similar (TF: 75% vs TA: 78% after 1 year and TF: 65% vs TA: 64% at 2 years) [38]. These results underline the importance of a tailored approach for each patient and should ideally be discussed within a multidisciplinary heart team in order to offer the best treatment option for every patient.
The results from the PARTNER trial have further established the role of TAVI in the treatment of high-risk patients with symptomatic aortic stenosis [39,40]. The first arm of the trial has proven the superiority of TAVI versus medical management in patients considered too high risk to undergo conventional surgery. Although the TAVI group had more frequent vascular complications and higher rates of major strokes at 30 days, the all-cause mortality at 1 year were 20% less in this group.
The results of the second arm of the trial showed the noninferiority of TAVI compared with conventional AVR in high-risk patients. Although both groups had similar mortality rates at 30 days and 1 year, there were important differences in periprocedural adverse events. Whilst the rate of all neurological events and major vascular complications were significantly more in the trans-catheter group, the incidence of major bleeding and new onset atrial fibrillation were increased in the conventional surgical group. The authors of this landmark trial concluded, that trans-catheter AVR is an alternative to surgical replacement in the high-risk group of patients with aortic stenosis. Long-term followup data is, however, not yet available and further follow-ups are clearly necessary.
▪ Minimally invasive mitral/tricuspid valve surgery
The first minimally invasive mitral valve procedure was described by Navia and Cosgrove in 1996 [2]. Via a right anterior-lateral thoracotomy, two to four rib-cartilages were resected and the mitral valve exposed using a transseptal approach via the right atrium. With this approach, 25 patients were operated on without any in-hospital mortality, reoperations for bleeding, embolic complications, wound infections or failed valve repairs. In 1996, Carpentier et al. performed the first video-assisted mitral valve repair through a mini-thoracotomy using ventricular fibrillation [41]. After having collected some experience with the new technique, video-assisted endoscopes and telemanipulators were introduced by Chitwood et al. [42,43]. Soon thereafter Falk et al. used a 3D videoscope with robotic assistance, enabling solo surgery [44]. The first completely robotic mitral valve repair with the da Vinci system was performed by Carpentier et al. in 1998 [45]. The current preferred minimally invasive access route to the mitral valve is a right lateral minithoracotomy in the fourth intercostal space. This approach has also become an attractive alternative in high-risk patients.
Onnasch reported on low in-hospital mortality rates in a group of 39 patients who underwent redo-mitral valve surgery using a port-access technique with endo-aortic balloon occlusion [46]. The group included 17 previous mitral repairs, six previous mitral valve replacements, three previous AVRs, two previous atrial septal defect repairs and 11 previous CABGs.
Seeburger et al. investigated a series of 181 patients who underwent elective minimally invasive mitral valve surgery for redo during a period of almost 9 years. All patients underwent previous cardiac surgery, which included 76 isolated CABGs, 55 isolated valve operations, 16 combined CABG and valve procedures and 34 other operations. The procedures were performed via a right lateral mini-thoracotomy. In 60% of cases the redo mitral surgery consisted of a repair procedure. A total of 77% of the procedures were performed on a fibrillating heart, 6% on a beating heart with CPB and 17% on an arrested heart. The 30‑day mortality was 6.6%. The mean predicted mortality by log EuroSCORE for this series was 18%. Of this patient cohort, 3.3% required reoperation during the first 30 days (recurrent mitral regurgitation in four patients and endocarditis in one patient). The authors concluded that minimally invasive mitral valve repair is a safe and effective alternative approach in patients referred for redo cardiac surgery [47].
Casselman et al. used a video-assisted right lateral mini-thoracotomy approach without ribspreading in 80 patients who underwent redomitral valve surgery. The majority of these prospectively selected patients had a previous CABG or mitral valve procedure done. Cannulation for CPB was performed via the femoral vessels and the internal jugular vein and an endo-aortic balloon was used for aortic occlusion. Conversion to sternotomy was necessary in five patients. The 30‑day mortality was 3.8% and survival at 1 and 4 years was 94 and 86%, respectively. This group also concluded that redo-mitral valve surgery performed minimally invasively is a feasible technique with reduced operative mortality [48].
Ricci et al. described the results of 241 patients admitted for elective redo-mitral valve procedures. Some of the patients in this series had up to four previous cardiac operations. Cannulation was performed using the Heartport cannulae and aortic occlusion was achieved with endoclamping. Conversion to a sternotomy was necessary in two patients. The in-hospital mortality was 5%. The authors concluded that minimally invasive redo-mitral valve surgery is associated with reduced ICU and hospital stays and a lower incidence of wound infections [49].
Various authors have reported their experience regarding the use of minimally invasive approaches compared with conventional median sternotomy to perform mitral valve surgery in elderly patients. Grossi et al. first reported their results in 1999. The in-hospital mortality rates were similar in both groups (9 vs 7% for the minimally invasive approach) but the patients who had minimally invasive procedures performed had a significantly lower rate of wound infections, required less blood products and had a shorter length of hospital stay [50]. Lamelas et al. reported that although the procedural and CPB times were significantly longer using the minimally invasive approach, the incidence of wound infections, renal failure and total length of hospital stay were significantly reduced with minimally invasive approaches [51].
The right lateral mini thoracotomy approach can also be considered in patients with severe functional mitral regurgitation secondary to dilated cardiomyopathy, awaiting heart transplantation. Operative risk is reduced with the avoidance of a redo-sternotomy at the time of heart transplantation.
This approach can also be used in patients presenting for redo-tricuspid valve surgery. Tricuspid valve repair can also be performed on a beating heart using a minimally invasive access route. This has several advantages, including the avoidance of cross clamping of the aorta in redo patients. Botta et al. analyzed a series of patients undergoing redo surgery that also included redo tricuspid valve surgery. They demonstrated that minimally invasive approaches on a beating heart are at least as safe and feasible as performing surgery with an arrested heart [52].
▪ Image-guided, interventional mitral valve procedures
Following the reports on TAVIs, percutaneous trans-catheter mitral valve interventions became the next focus. The MitraClip is the only device in clinical use at present. Imitating the technique of the Alfieri stitch, the clip is implanted using transvenous access route and a trans-septal puncture to fixate the anterior and posterior mitral valve leaflets [53]. In 2011, Feldman et al. published the EVEREST II trial comparing percutaneous (MitraClip) and surgical mitral valve repair. A total of 279 patients were included prospectively of which 184 were assigned to percutaneous mitral valve intervention. The only reported difference in baseline characteristics was an increased New York Heart Association (NYHA) functional class in the MitraClip group. Follow up was performed at 30 days, 12 months and 24 months. The rate of reinterventions was significantly higher in the MitraClip group, whereas the incidence of major adverse events at 30‑day follow-up was reportedly higher in the surgical group. There was no difference in 30‑day and 12‑month mortality rates. Surgical mitral valve repair was associated with reduced residual mitral valve regurgitation. The authors concluded that although percutaneous mitral valve repair was less effective in reducing mitral regurgitation compared with surgical repair, there was still an improvement in LV dimensions, NYHA functional class and QOL in patients who underwent this procedure [54]. At present, the MitraClip procedure is reserved for patients deemed not fit for surgical intervention. Van den Branden et al. reported a series of ten patients with an EuroSCORE >20%, treated with the MitraClip. There was no in-hospital or 30‑day mortality. An improvement of the mitral regurgitation to ≤2 was achieved in 78% of the patients at 30‑day follow-up. No patient required reoperation and an overall improvement in QOL were reported. The authors concluded that percutaneous mitral valve repair using the edge-to-edge technique is a safe and efficient alternative therapy for high-risk patients with symptomatic mitral regurgitation [55].
Pleger et al. investigated the short-term safety and clinical efficacy in 36 high-risk patients with an STS score >15 undergoing MitraClip- Implantation. The mean STS score was 24% and mean logistic EuroSCORE was 41% in this series. The 30‑day mortality was 0% and there was a significant reduction of mitral valve regurgitation at the 30‑day follow-up. The authors also reported a significant improvement of NYHA functional class and in the 6 min walk test. They concluded that percutaneous edge-to-edge mitral valve repair could be safely performed in high-risk patients with improved clinical outcomes at early follow-up [56].
Conclusion
Minimally invasive interventions have become the gold standard for many cardiac surgical procedures in high volume centers. The term ‘minimally invasive’ generally refers to the avoidance of a median sternotomy and/or the use of CPB. The role in high-risk patients has been and is continuing to be extensively investigated.
Periprocedural risk is calculated using risk stratif ication systems including the EuroSCORE and STS score. Individual factors such as advanced age, renal failure and redo surgery are also used to define risk. The rapid progress in the development of minimally invasive procedures testifies to its emerging role in various subgroups, including high-risk patients.
▪ Minimally invasive CABG surgery
OPCAB, MIDCAB and TECAB surgeries are well-established procedures to perform myocardial revascularization. Various authors have reported on the superiority of these techniques in selected high-risk patients. The 2005 consensus statement from the International Society for Minimally Invasive Surgery recommends OPCAB surgery as procedure of choice in high-risk patients requiring coronary revascularization and for patients with individual risk factors such as advanced age or renal impairment. This recommendation is also supported by the guidelines of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC) [57]. There is currently still a lack of randomized data for MIDCAB and TECAB surgery. Furthermore, these two techniques are only used for selected patients. The learning curve associated with these procedures requires high volumes in order to maintain favorable results. This might be a reason why these procedures are not performed more frequently. The choice of procedure should be tailored to the individual patient’s risk profile in order to achieve maximum benefit.
▪ Minimally invasive valve surgery
Minimally invasive access to address mitral valve pathology has become the procedure of choice in many units throughout Europe. The reported benefits in morbidity and mortality incidence have made this an attractive alternative in high-risk patients. The first minimal access approaches to avoid a median sternotomy were performed in the mid 1990s and since then tremendous progress has been made to refine these procedures. These techniques are also associated with a learning curve and it has been demonstrated that these procedures might be beneficial in high volume centers, but not in lower volume centers [58].
Catheter-based interventions including TAVI and the MitraClip continue to evolve and as such, increase the physician’s ability to manage patients that are considered inoperable due to their risk profiles. Even in surgical high-risk patients, the noninferiority of TAVI has been proven. In Germany in 2010, 20% of all aortic valve interventions were performed using catheter-based techniques as reported in the annual report of the German Society for Thoracic and Cardiovascular Surgery (DGTHG).
Minimally invasive techniques, either defined by the use of small incisions or by the avoidance of CPB or both have proven benefits for selected high-risk patients and should be considered the therapy of choice whenever it is possible and applicable in order to reduce mortality, morbidity and length of hospital stay.
Future perspective
With ample evidence in favor of minimally invasive therapies for high-risk patients requiring cardiac surgery, it is important that new technologies and devices are further developed to improve the outcomes in this patient cohort.
New transcatheter aortic valve prostheses including the Engager™ TA system (Medtronic Inc., MN, USA) [59], the Symetis Acurate™ Valve Symetis Inc. (Ecublens, Switzerland) [60] and JenaValve™ (JenaValve Technologies, Munich, Germany) [61] prosthesis are being developed in an effort to continue improving the results of this technology. Innovative devices such as adjustable mitral valve rings and neochordea systems aim to address underlying pathology in order to achieve better anatomical repairs. The development of transcatheter based mitral valve prostheses are also being investigated [62]. These include annuloplasty bands with a anchoring system (Valtech Cardio band; Valtech Cardio Ltd, Or Yehuda, Israel) as well as different mitral valve prostheses with mechanisms to fixate the valve at the annulus. Transcatheter devices to treat functional tricuspid valve regurgitation, are also under investigation (Millipede system; Millipede, LLC, MI, USA).
Minimally invasive therapies will continue to evolve and may become the gold-standard therapies in future years. It is imperative that physicians partake in these future developments to be able to treat each patient with the best available modality.
Executive summary
Minimally invasive surgery
▪ Minimally invasive cardiac procedures are defined by:
– The avoidance of cardiopulmonary bypass;
– The access pathway.
High-risk patients
▪ The term ‘high risk’ includes:
– Elevated risk scores (EuroSCORE, STS-score);
– Advanced age;
– Redo surgery;
– Impaired left ventricular function;
– Renal impairment.
Coronary heart disease
▪ Off-pump coronary artery bypass: avoidance of cardiopulmonary bypass.
▪ Superior outcomes for high-risk patients have been demonstrated.
▪ Minimally invasive direct coronary artery bypass: off-pump coronary artery bypass surgery through a minimally invasive access pathway.
▪ Best option for selected high-risk patients with feasible coronary pathology.
▪ Totally endoscopic coronary artery bypass: might be useful for high-risk patients, but further refinement of the technique is necessary.
Aortic valve disease
▪ Minimally invasive access pathway: has been shown to be beneficial for high-risk patients.
▪ Transcatheter aortic valve implantation: good short- and mid-term results for selected high-risk patients. Technique with the potential to become the gold standard for this group. Long-term results are missing.
Mitral/tricuspid valve disease
▪ Thoracotomy: gold-standard therapy in patients with normal perioperative risk in high volume centers, favorable in patients with high operative risk.
▪ MitraClip: first CE-marked catheter based technique for selected high-risk patients with encouraging short-term results.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Subramanian VA, McCabe JC, Geller CM. Minimally invasive direct coronary artery bypass grafting: two-year clinical experience. Ann. Thorac. Surg. 64(6), 1648–1653 (1997).
- Navia JL, Cosgrove DM. Minimally invasive mitral valve operations. Ann. Thorac. Surg. 62(5), 1542–1544 (1996).
- Mack MJ. Minimally invasive cardiac surgery. Surg. Endosc. 20(S2), S488–S492 (2006).
- Nashef SA, Roques F, Michel P et al. European System for Cardiac Operative Risk Evaluation (EuroSCORE). Eur. J. Cardiothorac. Surg. 16(1), 9–13 (1999).
- Clark RE. The Society of Thoracic Surgeons National Database status report. Ann. Thorac. Surg. 57(1), 20–26 (1994).
- Alexander KP, Anstrom KJ, Muhlbaier LH et al. Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J. Am. Coll. Cardiol. 35(3), 731–738 (2000).
- Alexander KP, Newby LK, Cannon CP et al. Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation 115(19), 2549–2569 (2007).
- Sündermann S, Dademasch A, Praetorius J et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur. J. Cardiothorac. Surg. 39(1), 33–37 (2011).
- Sündermann SH, Salzberg SP. [Renaissance of surgery for coronary artery disease]. Praxis (Bern 1994) 100(1), 23–28 (2011).
- Pfister AJ, Zaki MS, Garcia JM et al. Coronary artery bypass without cardiopulmonary bypass. Ann. Thorac. Surg. 54(6), 1085–1091; discussion 1091–1092 (1992).
- Shroyer AL, Grover FL, Hattler B et al. On-pump versus off-pump coronary-artery bypass surgery. N. Engl. J. Med. 361(19), 1827–1837 (2009).
- Halkos ME, Puskas JD. Off-pump coronary surgery: where do we stand in 2010? Curr. Opin. Cardiol. 25(6), 583–588 (2010).
- Falk V, Taggart DP. Is it finally time to turn off the pump? Heart doi:10.1136/ hrt.2011.225961 (2011) (Epub ahead of print).
- Al-Ruzzeh S, Nakamura K, Athanasiou T et al. Does off-pump coronary artery bypass (OPCAB) surgery improve the outcome in high-risk patients? A comparative study of 1398 high-risk patients. Eur. J. Cardiothorac. Surg. 23(1), 50–55 (2003).
- Emmert MY, Salzberg SP, Seifert B et al. Routine off-pump coronary artery bypass grafting is safe and feasible in high-risk patients with left main disease. Ann. Thorac. Surg. 89(4), 1125–1130 (2010).
- Dewey TM, Magee MJ, Edgerton JR et al. Off-pump bypass grafting is safe in patients with left main coronary disease. Ann. Thorac. Surg. 72(3), 788–791; discussion 792 (2001).
- Emmert MY, Salzberg SP, Theusinger OM et al. Off-pump surgery for the poor ventricle? Heart Vessels. doi:10.1007/s00380-011-0146-0 (2011) (Epub ahead of print).
- Puskas J, Cheng D, Knight J. Off-pump versus conventional coronary artery bypass grafting: a meta-analysis and consensus statement from the 2004 ISMICS consensus conference. Ann. Thorac. Surg. 88(4), 1142–1147 (2005).
- Puskas JD, Thourani VH, Kilgo P et al. Off-pump coronary artery bypass disproportionately benefits high-risk patients. Ann. Thorac. Surg. 88(4), 1142–1147 (2009).
- Robinson MC, Gross DR, Zeman W, Stedje-Larsen E. Minimally invasive coronary artery bypass grafting: a new method using an anterior mediastinotomy. J. Card. Surg. 10(5), 529–536 (1995).
- Holzhey DM, Jacobs S, Mochalski M et al. Seven-year follow-up after minimally invasive direct coronary artery bypass: experience with more than 1300 patients. Ann. Thorac. Surg. 83(1), 108–114 (2007).
- Sorm Z, Harrer J, Voborník M, Cermáková E, Vojácek J. Early and long-term results of minimally invasive coronary artery bypass grafting in elderly patients. Kardiol. Pol. 69(3), 213–218 (2011).
- Morishita A, Shimakura T, Miyagishima M, Kawamoto J, Morimoto H. Minimally invasive direct redo coronary artery bypass grafting. Ann. Thorac. Cardiovasc. Surg. 8(4), 209–212 (2002).
- Sunderdiek U. Minimally invasive coronary artery bypass grafting in high-risk patients. Late follow-up with assessment of left internal mammary artery graft patency and flow by exercise transthoracic Doppler echocardiography. Cardiovasc. Surg. 11(5), 389–395 (2003).
- Giglio MD, Dell’Amore A, Aquino T et al. Minimally invasive coronary artery bypass grafting using the inferior J-shaped ministernotomy in high-risk patients. Interact. Cardiovasc. Thorac. Surg. 7(3), 402–405 (2008).
- Watanabe G, Yamaguchi S, Tomiya S, Ohtake H. Awake subxyphoid minimally invasive direct coronary artery bypass grafting yielded minimum invasive cardiac surgery for high risk patients. Interact. Cardiovasc. Thorac. Surg. 7(5), 910–912 (2008).
- Falk V, Diegeler A, Walther T et al. Total endoscopic computer enhanced coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 17(1), 38–45 (2000).
- Bonatti J, Garcia J, Rehman A et al. On-pump beating-heart with axillary artery perfusion: a solution for robotic totally endoscopic coronary artery bypass grafting? Heart Surg. Forum 12(3), E131–E133 (2009).
- Schmitto JD, Mohr FW, Cohn LH. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Curr. Opin. Cardiol. 26(2), 118–122 (2011).
- Cosgrove DM, Sabik JF. Minimally invasive approach for aortic valve operations. Ann. Thorac. Surg. 62(2), 596–597 (1996).
- Brown ML, McKellar SH, Sundt TM, Schaff HV. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 137(3), 670–679 (2009).
- Murzi M, Cerillo AG, Bevilacqua S et al. Traversing the learning curve in minimally invasive heart valve surgery: a cumulative analysis of an individual surgeon’s experience with a right minithoracotomy approach for aortic valve replacement. Eur. J. Cardiothorac. Surg. doi:10.1093/ejcts/ezr230 (2012) (Epub ahead of print).
- Flameng W, Herregods M-C, Hermans H et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J. Thorac. Cardiovasc. Surg. 142(6), 1453–1457 (2011).
- Boudjemline Y, Bonhoeffer P. Steps toward percutaneous aortic valve replacement. Circulation 105(6), 775–778 (2002).
- Cribier A, Eltchaninoff H, Bash A et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106(24), 3006–3008 (2002).
- Cribier A, Eltchaninoff H, Tron C et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J. Am. Coll. Cardiol. 43(4), 698–703 (2004).
- Ye J, Cheung A, Lichtenstein SV et al. Transapical aortic valve implantation in humans. J. Thorac. Cardiovasc. Surg. 131(5), 1194–1196 (2006).
- Rodés-Cabau J, Webb JG, Cheung A et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk. J. Am. Coll. Cardiol. 55(11), 1080–1090 (2010).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364(23), 2187–2198 (2011).
- Carpentier A, Loulmet D, Carpentier A et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C. R. Acad. Sci. III, 319(3), 219–223 (1996).
- Chitwood WR, Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann. Thorac. Surg. 63(5), 1477–1479 (1997).
- Chitwood WR, Elbeery JR, Chapman WH et al. Video-assisted minimally invasive mitral valve surgery: the ‘micro-mitral’ operation. J. Thorac. Cardiovasc. Surg. 113(2), 413–414 (1997).
- Falk V, Walther T, Autschbach R et al. Robot-assisted minimally invasive solo mitral valve operation. J. Thorac. Cardiovasc. Surg. 115(2), 470–471 (1998).
- Carpentier A, Loulmet D, Aupècle B et al. Computer assisted open heart surgery. First case operated on with success. C. R. Acad. Sci. III 321(5), 437–442 (1998).
- Onnasch JF, Schneider F, Falk V et al. Minimally invasive approach for redo-mitral valve surgery: a true benefit for the patient. J. Card. Surg. 17(1), 14–19 (2002).
- Seeburger J, Borger MA, Falk V et al. Minimally invasive mitral valve surgery after previous sternotomy: experience in 181 patients. Ann. Thorac. Surg. 87(3), 709–714 (2009).
- Casselman FP, La Meir M, Jeanmart H et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation 116(Suppl. 11), I270–I275 (2007).
- Ricci D, Pellegrini C, Aiello M et al. Port-access surgery as elective approach for mitral valve operation in re-do procedures. Eur. J. Cardiothorac. Surg. 37(4), 920–925 (2010).
- Grossi EA, Galloway AC, Ribakove GH et al. Minimally invasive port access surgery reduces operative morbidity for valve replacement in the elderly. Heart Surg. Forum 2(3), 212–215 (1999).
- Lamelas J, Sarria A, Santana O, Pineda AM, Lamas GA. Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann. Thorac. Surg. 91(1), 79–84 (2011).
- Botta L, Cannata A, Fratto P et al. The role of the minimally invasive beating heart technique in reoperative valve surgery. J. Card. Surg. 27(1), 24–28 (2011).
- Mack MJ. New techniques for percutaneous repair of the mitral valve. Heart Fail. Rev. 11(3), 259–268 (2006).
- Feldman T, Foster E, Glower DD et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 364(15), 1395–1406 (2011).
- Van den Branden BJL, Post MC, Swaans MJ et al. Percutaneous mitral valve repair using the edge-to-edge technique in a high-risk population. Neth. Heart J. 18(9), 437–443 (2010).
- Pleger ST, Mereles D, Schulz-Schönhagen M et al. Acute safety and 30-day outcome after percutaneous edge-to-edge repair of mitral regurgitation in very high-risk patients. Am. J. Cardiol. (2011).
- Task Force on Myocardial Revascularization of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery, European Association for Percutaneous Cardiovascular Interventions, Kolh P et al. Guidelines on myocardial revascularization. Eur. J. Cardiothorac. Surg. 38(S1), S1–S52 (2010).
- Bolling SF, Li S, O’Brien SM et al. Predictors of mitral valve repair: clinical and surgeon factors. Ann. Thorac. Surg. 90(6), 1904–1911; discussion 1912 (2010).
- Falk V, Walther T, Schwammenthal E et al. Transapical aortic valve implantation with a self-expanding anatomically oriented valve. Eur. Heart J. 32(7), 878–887 (2011).
- Kempfert J, Rastan A, Beyersdorf F. Trans-apical aortic valve implantation using a new self-expandable bioprosthesis: initial outcomes. Eur. J. Cardiothorac. Surg. 40(5), 1114–1119 (2011).
- Kempfert J, Rastan AJ, Mohr FW, Walther T. A new self-expanding transcatheter aortic valve for transapical implantation – first in man implantation of the JenaValve™. Eur. J. Cardiothorac. Surg. (2011).
- Chiam PT, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. JACC Cardiovasc. Interv. 4(1), 1–13 (2011).
- Cribier A, Eltchaninoff H, Tron C et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J. Am. Coll. Cardiol. 47(6), 1214–1223 (2006).
- Grube E, Laborde JC, Gerckens U et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 114(15), 1616–1624 (2006).
- Lichtenstein SV, Cheung A, Ye J et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 114(6), 591–596 (2006).
- Grube E, Schuler G, Buellesfeld L et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30‑day clinical outcome. J. Am. Coll. Cardiol. 50(1), 69–76 (2007).
- Webb JG, Pasupati S, Humphries K et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116(7), 755–763 (2007).
- Walther T, Falk V, Borger MA et al. Minimally invasive transapical beating heart aortic valve implantation – proof of concept. Eur. J. Cardiothorac. Surg. 31(1), 9–15 (2007).
- Walther T, Simon P, Dewey T et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 116(Suppl. 11), I240–I245 (2007).
- Walther T, Falk V, Kempfert J et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur. J. Cardiothorac. Surg. 33(6), 983–988 (2008).
- Svensson LG, Dewey T, Kapadia S et al. United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann. Thorac. Surg. 86(1), 46–54; discussion 54–55 (2008).
- Zierer A, Wimmer-Greinecker G, Martens S, Moritz A, Doss M. The transapical approach for aortic valve implantation. J. Thorac. Cardiovasc. Surg. 136(4), 948–953 (2008).
- Piazza N, Grube E, Gerckens U et al. Procedural and 30‑day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1‑year following CE mark approval. EuroIntervention 4(2), 242–249 (2008).
- Rodés-Cabau J, Dumont E, De Larochellière R et al. Feasibility and initial results of percutaneous aortic valve implantation including selection of the transfemoral or transapical approach in patients with severe aortic stenosis. Am. J. Cardiol. 102(9), 1240–1246 (2008).
- Bleiziffer S, Ruge H, Mazzitelli D et al. Results of percutaneous and transapical transcatheter aortic valve implantation performed by a surgical team. Eur. J. Cardiothorac. Surg. 35(4), 615–621 (2009).
- Thomas M, Schymik G, Walther T et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122(1), 62–69 (2010).
- Thomas M, Schymik G, Walther T et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 124(4), 425–433 (2011).
- Kodali SK, O’Neill WW, Moses JW et al. Early and late (one year) outcomes following transcatheter aortic valve implantation in patients with severe aortic stenosis (from the United States REVIVAL trial). Am. J. Cardiol. 107(7), 1058–1064 (2011).
- Kempfert J, Van Linden A, Holzhey D et al. The evolution of transapical aortic valve implantation and new perspectives. Minim. Invasive Ther. Allied Technol. 20(2), 107–116 (2011).
▪▪ Landmark paper comparing conventional coronary artery bypass surgery versus off-pump coronary artery bypass (OPCAB) procedures.
▪ Largest series investigating the role of OPCAB surgery in patients with high operative risk.
▪ Issues a statement about OPCAB versus conventional bypass surgery including a large high-risk patient population.
▪ Large series investigating the results of minimally invasive coronary artery bypass surgery.
▪ Highly sophisticated review about minimally invasive access for aortic valve replacement in high-risk patients.
▪▪ Landmark paper comparing transcatheter versus conventional aortic valve replacement in high-risk patients.
▪ Largest investigation in the field of interventional mitral valve repair with the MitraClip.
▪ First report about the transapical access pathway for transcatheter aortic valve intervention in patients not feasible for transfemoral transcatheter aortic valve intervention.
▪ Website
101. EuroSCOREII calculator (2011). www.euroscore.org/calc.html