Review Article - Interventional Cardiology (2013) Volume 5, Issue 3
Minimizing complications following stent implantation: outcomes and follow-up
- Corresponding Author:
- Robert J Applegate
Wake Forest School of Medicine
Section of Cardiology, Winston-Salem
NC 27157-1045, USA
Tel: +1 336 716 2718
Fax: +1 336 716 5324
E-mail: bapplega@wakehealth.edu
Abstract
Keywords
bare-metal stent, drug-eluting stent, in-stent restenosis, intraprocedural anticoagulation, stent complication, stent thrombosis
Myocardial revascularization began in 1968 with the development of surgical bypass of the coronary arteries by Rene Favaloro [1]. The use of percutaneous coronary intervention (PCI) was first introduced by Grüntzig et al. in 1977 by percutaneous transluminal coronary angioplasty (PTCA) [2]. However, in the first 50 patients who underwent PTCA, the complication rate was high, with a primary success rate of only 64%, and emergency coronary artery bypass grafting (CABG) was needed in 14% of patients [2]. As operators became more experienced with PTCA, the success rate improved, although complications remained. PTCA was frequently complicated by vessel closure during the procedure, as well as angiographic restenosis with an occurrence rate of 30–40% [3]. The need for improved catheter-based interventions for coronary artery disease led to an evaluation of other potential techniques, including directional atherectomy, rotational atherectomy and excimer laser angioplasty [4]. In 1987, Schatz et al. introduced the first successful coronary stent, the Palmaz-Schatz bare-metal stent (BMS), to be implanted in humans [5,6]. Two randomized clinical trials were published in 1994 comparing coronary stent implantation with PTCA; the BENESTENT study and STRESS study established that intracoronary stents significantly reduced the incidence of angiographic restenosis and repeat revascularization compared with PTCA [7,8]. The benefits of stent implantation occur by allowing the stent to act as a scaffold to tack up intimal and medial dissections and provide radial support to oppose elastic recoil [9]. Since that time, percutaneous coronary stent placement has become the preferred method of percutaneous revascularization, with approximately 600,000 procedures performed each year in the USA [10]. Although coronary stenting has been proven to have improved outcomes compared with PTCA, periprocedural complications remain. Improvements in interventional technique, stent technology and antiplatelet therapy have reduced the incidence of these complications; however, further progress still needs to be made.
Complications
Complications after PCI occur, although fortunately the risk of major complications during most interventions remains low. The degree of complications range from minor events (i.e., minor bleeding or mild periprocedural biomarker elevation) to major events (i.e., major bleeding, vascular-access complications requiring surgical repair, renal failure or stent thrombosis (ST) resulting in myocardial infarction or death). Most complications after PCI occur in the periprocedural period related to the procedure itself, with fewer events occurring in the longer-term follow-up period. A review of recent National Cardiovascular Data Registry data reports a 4.53% risk of any periprocedural adverse event in patients undergoing PCI for indications other than ST-elevation myocardial infarction (STEMI) [11]. The overall in-hospital mortality rate varied by individual factors ranging from 0.65%, in elective PCI, to 4.81%, in patients with STEMI, with an overall rate of 1.27% [12]. Long-term complications after PCI are largely related to recurrent ischemia from progression of atherosclerotic coronary artery disease or restenosis at the site of revascularization. The following sections will review the acute and chronic complications of PCI and strategies to prevent such events (Box 1).
Box 1: Complications after coronary artery stenting.
▪ Acute complications
Acute stent-related coronary perforation
Coronary artery perforation (CAP) is a potentially life-threatening complication, occurring in 0.1–0.84% of patients undergoing stent placement [13,14]. CAP occurs more commonly during complex coronary interventions where coronary atheroablation is utilized, although it does occur during stent implantation, with an incidence of 0.05–0.15%, when angioplasty and/or stent placement are utilized [13,15]. The etiology of CAP during stent placement can occur from guidewire-related perforations, specifically stiffer, hydrophilic wires, oversized stent implantation with a balloon–artery ratio of >1.1:1, or high-pressure balloon dilatation [13,16-18]. The Ellis classification scheme is the most widely used system, with CAP classified into three types: type I, extraluminal crater without extravasation; type II, pericardial or myocardial blushing; and type III, perforation ≥1 mm diameter with contrast streaming or cavity spilling [19]. Complications from CAP include periprocedural myocardial infarction, cardiac tamponade and death with incidence increasing for severity of perforation [13-15].
Management of patients with CAP ranges from close inpatient observation to emergency CABG. All patients with identified CAP should have heparin reversed with protamine infusion, and patients receiving bivalirudin or glycoprotein (GP) IIb/IIIa inhibitors discontinued. Prolonged balloon inflation with a standard angioplasty balloon can be quickly utilized and prevent continued extravasation into the pericardium. If a patient is hemodynamically unstable, emergent pericardiocentesis should be performed. When there is continued contrast extravasation despite prolonged balloon inflation, a polytetrafluoroethylene-covered stent can be deployed for perforations in large, mid or proximal perforations. Polytetrafluoroethylenecovered stents are limited owing to the bulkiness of the stent, poor deliverability and lack of antiproliferative drug elution. Pericardial-coated stents have been developed for treatment of CAP, with superior deliverability and greater biocompatibility. Distal or small-vessel perforations that do not respond to prolonged balloon inflation can undergo metal–coil or gel–foam embolization to seal the perforation. Nonsurgical management is often successful with 2.9–5.2% of patients with all classifications of CAP requiring emergency CABG, although with type III perforation, CABG rates have been reported to range from 16 to 60% [15,16,20,21].
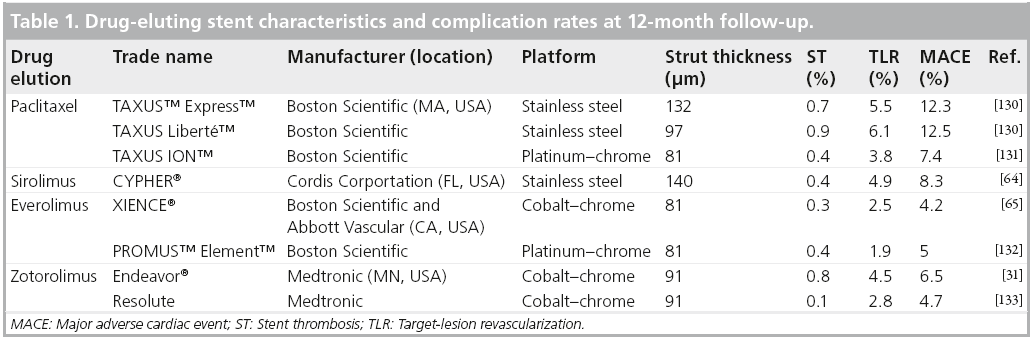
Stent thrombosis
ST is a rare, but dreaded, complication of PCI and often presents as death or nonfatal acute myocardial infarction [22]. ST is defined by the time interval from PCI to occurrence by using acute (<24 h), early (0–30 days), late (31 days to 1 year) and very late (>1 year), with the majority of events occurring early in both drug-eluting stents (DES) and BMS [23]. The results of a 2006 meta-analysis of 19 randomized controlled trials found 1-year ST incidences of 0.87% for BMS and 0.99% for firstgeneration DES (p = nonsignificant) [24]. Beyond 1 year (very late), ST is more commonly associated with DES placement and is rarely seen after BMS. Stone et al. demonstrated equivalent 4-year ST rates between BMS and first-generation DES paclitaxel-eluting stents (PES) and sirolimus-eluting stents (SES); however, there was a statistically significant difference in ST after 1 year, with only two events occurring in patients with BMS [25]. In addition, the Swedish Coronary Angiography and Angioplasty Registry reported a 1.18-times higher 3-year mortality rate in patients receiving first-generation DES, which was driven by events occurring 6 months after implantation [26]. These results brought into question the safety of DES, particularly in off-label use. Subsequent studies with longer-term follow-up showed no difference in cardiovascular death, myocardial infarction or ST between BMS and first-generation DES [27,28]. A large meta-analysis, including both randomized controlled trials and observational data, found that first-generation DES had equivalent rates of mortality and myocardial infarction compared with BMS, and in the observational studies, a significant reduction in these outcomes was seen [29]. The development of second-generation DES has led to reduced rates of ST (Table 1). A 2012 meta-analysis of 11 trials and 16,000 patients showed the use of everolimus-eluting stents (EES) compared with first-generation DES, resulted in a 0.6% absolute risk reduction in ST, with lower risk occurring at all time intervals [30]. Similarly, zotarolimus-eluting stent (ZES) placement demonstrated a significantly lower incidence of ST compared with first-generation DES placement [31]. When comparing EES with second-generation DES, EES was found to have a lower rate of ST compared with ZES (0.3 vs 1.2%; p = 0.01), as well as other DES [30,32,33].
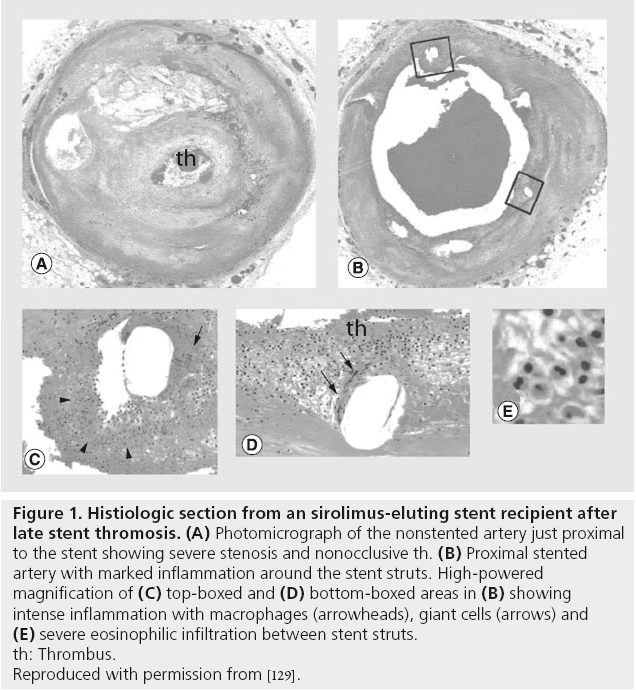
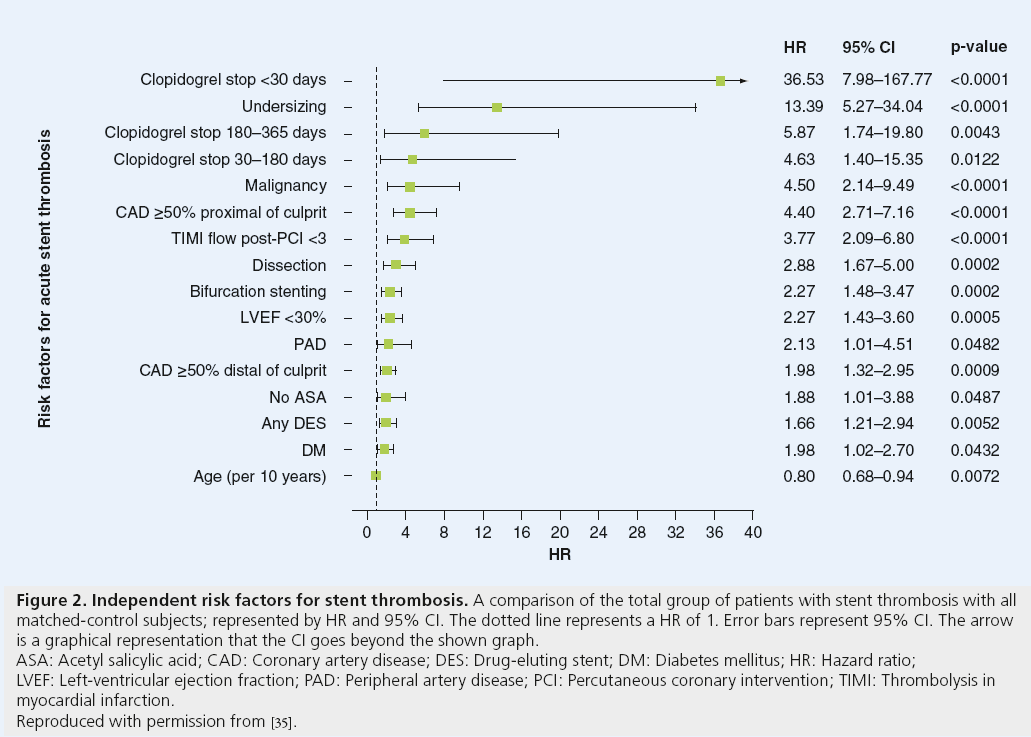
Intracoronary balloon angioplasty and stenting results in vascular injury, with resultant exposure of thrombogenic factors of the subintima and subsequent endothelial inflammation, platelet activation and long-term neointimal proliferation. Early, late and very-late ST result from different mechanisms. This inflammatory response at time of PCI, as well as the inherent thrombogenicity of the foreign stent itself, is responsible for the development of ST in the early and late time period. The pathophysiology of very-late ST seen with DES has histopathologic features with cellular thrombus infiltrate and elevated intracoronary inflammatory markers distinct from early DES ST, BMS ST and native coronary thrombosis (Figure 1) [34]. Very-late DES ST has been attributed to abnormal vascular responses to DES, including incomplete endothelialization, hypersensitivity reaction to the stent platform or antiproliferative medication coating and aneurysm formation. Predictors for developing ST have been identified after BMS and DES placement (Figures 2 and 3). The main predictor of early and late ST is the premature discontinuation of thienopyridine therapy (odds ratio [OR]: 36.5) [35]. Other clinical factors, such as acute or chronic kidney disease, depressed left-ventricular systolic function, diabetes mellitus, malignancy and cocaine use have been independently associated with increased rates of ST [35-39]. Procedural risk factors in ST include number and length of stents placed, stent undersizing, vessel dissection, stent fracture, stenting of vein grafts and complexity of stenosis [35,40-43].
Figure 1: Histiologic section from an sirolimus-eluting stent recipient after
late stent thromosis. (A) Photomicrograph of the nonstented artery just proximal
to the stent showing severe stenosis and nonocclusive th. (B) Proximal stented
artery with marked inflammation around the stent struts. High-powered
magnification of (C) top-boxed and (D) bottom-boxed areas in (B) showing
intense inflammation with macrophages (arrowheads), giant cells (arrows) and (E) severe eosinophilic infiltration between stent struts.
th: Thrombus.
Reproduced with permission from [129].
Figure 2: Independent risk factors for stent thrombosis. A comparison of the total group of patients with stent thrombosis with all
matched-control subjects; represented by HR and 95% CI. The dotted line represents a HR of 1. Error bars represent 95% CI. The arrow
is a graphical representation that the CI goes beyond the shown graph.
ASA: Acetyl salicylic acid; CAD: Coronary artery disease; DES: Drug-eluting stent; DM: Diabetes mellitus; HR: Hazard ratio;
LVEF: Left-ventricular ejection fraction; PAD: Peripheral artery disease; PCI: Percutaneous coronary intervention; TIMI: Thrombolysis in
myocardial infarction.
Reproduced with permission from [35].
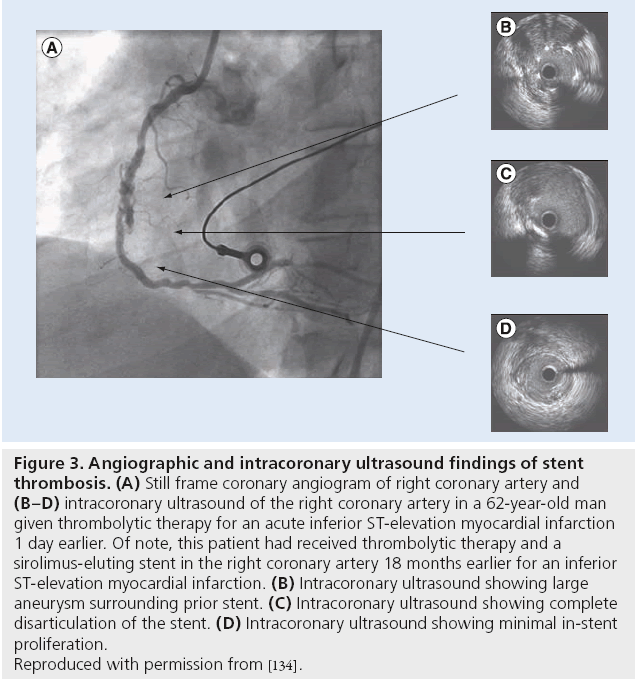
Figure 3: Angiographic and intracoronary ultrasound findings of stent
thrombosis. (A) Still frame coronary angiogram of right coronary artery and (B–D) intracoronary ultrasound of the right coronary artery in a 62-year-old man
given thrombolytic therapy for an acute inferior ST-elevation myocardial infarction
1 day earlier. Of note, this patient had received thrombolytic therapy and a
sirolimus-eluting stent in the right coronary artery 18 months earlier for an inferior
ST-elevation myocardial infarction. (B) Intracoronary ultrasound showing large
aneurysm surrounding prior stent. (C) Intracoronary ultrasound showing complete
disarticulation of the stent. (D) Intracoronary ultrasound showing minimal in-stent
proliferation.
Reproduced with permission from [134].
Periprocedural biomarker elevation
Periprocedural cardiac biomarker elevation is a common complication, with varying significance depending on clinical context occurring in up to 44% of PCIs [44,45]. Major complications of myocardial infarction associated with PCIs include abrupt vessel closure, dissection, jailing of side-branch vessel and distal embolization with no reflow. Routine monitoring of periprocedural cardiac markers has been a common practice; however, the development of highly sensitive cardiac biomarkers and evolving definitions of periprocedural myocardial infarction has made interpretation difficult.
The adverse outcomes associated with periprocedural biomarker elevation are well known. Several earlier studies, including a meta-analysis of 23,230 patients, have shown a statistically significant increased mortality rate in patients with elevated creatine kinase-MB greater than three-times the upper limit of normal [45-50]. Subsequent studies have not been as conclusive. The ACUITY trial evaluated 7773 patients with non-STEMI who underwent PCI. The results suggested that the development of a spontaneous myocardial infarction unrelated to the procedure was associated with subsequent mortality, and periprocedural biomarker elevation did not have an independent association [51]. Several additional studies showed consistent results with ACUITY, revealing that poor outcomes were related to patient comorbidities, preprocedure biomarker elevation and procedure complexity, not postprocedure biomarker levels [51-54]. Conversely, a meta-analysis of 20 studies evaluating troponin elevation after PCI found a significant association with increased mortality (OR: 1.35; 95% CI: 1.13–1.60) [55].
The disparities in the data may be attributed to the varying definitions of periprocedural myocardial infarction, heterogeneous patient populations and differences in duration of follow-up. The discrepancy in the data has led to controversy surrounding the significance of biomarker elevation post-PCI, especially in mild biomarker elevation in the absence of clinical symptoms of myocardial ischemia, and the development of highly sensitive troponin assays has increased this incidence [56,57]. In an attempt to provide a clear consensus, the ‘universal definition’ of PCI-related myocardial infarction (type 4a) was recently published. A type 4a myocardial infarction in patients with normal baseline troponin level is defined as a rise in troponin greater than five-times 99th percentile upper limit of normal occurring within 48 h of the intervention, with one of the following: evidence of prolonged ischemia (>20 min) as demonstrated by prolonged chest pain; ischemic ST changes or new pathological Q waves; evidence of a flow-limiting complication by angiography; or evidence of new loss of myocardial viability or new regional wall-motion abnormality as seen by imaging [58]. If preprocedure troponin is elevated, but stable or falling, a >20% increase in addition to one of the above clinical criteria is required for a diagnosis type 4a myocardial infarction [58]. In the current merican College of Cardiology Foundation/American Heart Association/ Society for Cardiac Angiography and Interventions guideline for PCI, it is recommended that patients who have symptoms of myocardial infarction during or after PCI, or asymptomatic patients with evidence of angiographic complications, have either creatine kinase-MB and/or troponin measured (class I, level of evidence C) [59]. It may be reasonable for all patients after PCI to have routine measurement of cardiac biomarkers (class IIb, level of evidence: C) [59]. While there are conflicting results on the independent association of cardiac biomarker elevation post-PCI with mortality, more standardized data are needed to determine the significance of periprocedural enzyme elevation.
▪ Chronic complications In-stent restenosis
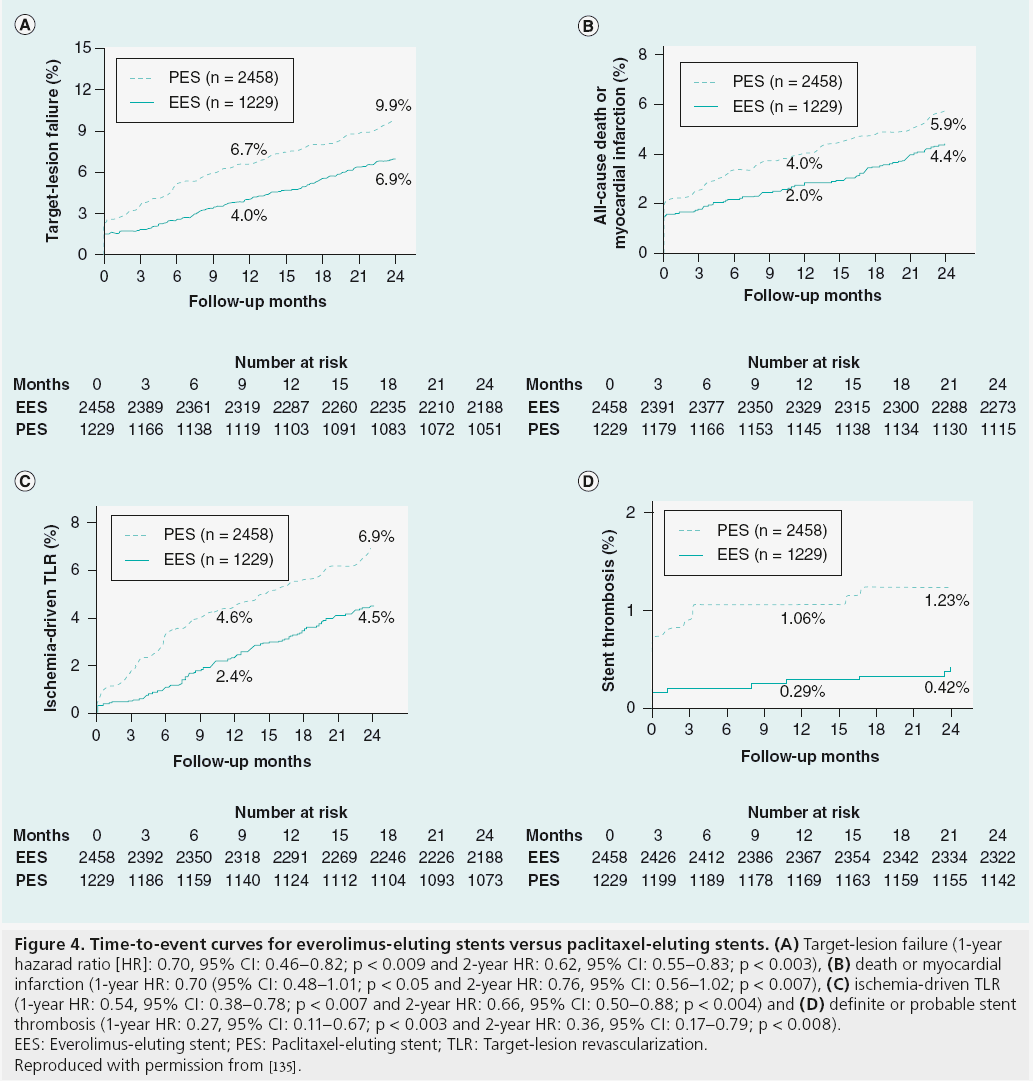
In-stent restenosis (ISR) is defined angiographically as >50% diameter stenosis on repeat angiography or clinically as anginal symptoms requiring target-lesion revascularization. The clinical presentation of ISR, unlike ST, typically involves stable angina, although up to 25% of patients can present with an acute coronary syndrome (ACS) [60,61]. The onset of symptoms occurs months after stent implantation and is further delayed if DES is deployed [62]. The development of intracoronary stenting has greatly reduced the rate of target-lesion restenosis compared with PTCA. The introduction of DES resulted in a significant decrease in the incidence of ISR compared with BMS. The incidence of ISR at 6 months was significantly less after BMS compared with PTCA (22 vs 32%; p = 0.02) [7]. ISR rates with firstgeneration DES were further reduced compared with BMS, as shown in randomized controlled trials, and were shown to have ISR rates of <6% at 9-month follow-up [63,64]. Second-generation DES provided improvement in stent platform and medications, resulting in further reduction in ISR rates. Randomized trial data have shown superiority of EES compared with PES for rates of target-lesion revascularization (TLR; 2.5 vs 4.6%; p = 0.001; Figure 4) [65].
Figure 4: Time-to-event curves for everolimus-eluting stents versus paclitaxel-eluting stents. (A) Target-lesion failure (1-year hazarad ratio [HR]: 0.70, 95% CI: 0.46–0.82; p < 0.009 and 2-year HR: 0.62, 95% CI: 0.55–0.83; p < 0.003), (B) death or myocardial
infarction (1-year HR: 0.70 (95% CI: 0.48–1.01; p < 0.05 and 2-year HR: 0.76, 95% CI: 0.56–1.02; p < 0.007), (C) ischemia-driven TLR
(1-year HR: 0.54, 95% CI: 0.38–0.78; p < 0.007 and 2-year HR: 0.66, 95% CI: 0.50–0.88; p < 0.004) and (D) definite or probable stent
thrombosis (1-year HR: 0.27, 95% CI: 0.11–0.67; p < 0.003 and 2-year HR: 0.36, 95% CI: 0.17–0.79; p < 0.008).
EES: Everolimus-eluting stent; PES: Paclitaxel-eluting stent; TLR: Target-lesion revascularization.
Reproduced with permission from [135].
The mechanism for ISR is similar for both BMS and DES. As described above, PCI results in vascular trauma with subsequent inflammatory and healing response. Excessive response to vascular injury by neointimal hyperplasia and vascular remodeling over weeks to months after intervention are primarily responsible for ISR [66]. The mechanisms for the abnormal response to vascular injury can be attributed to biological, mechanical and technical factors. Biological factors common to both DES and BMS is a hypersensitivity response to the metal alloy of the stent itself. BMS and first-generation DES stent-platform materials were mostly stainless steel; it has been suggested that nickel and molybdenum released from the stainless-steel stent served as an allergic trigger for ISR [67]. A second-generation DES stent platform is cobalt chromium, which appears to have less hyperproliferative effects. Unique to DES, the antiproliferative drug itself or stent polymer coating can result in hypersensitivity reactions. Also, resistance to the chemotherapeutic agent of the DES can result in excessive neointimal hyperplasia without the desired antirestenotic effects. Delayed restenosis after DES implantation occurring after 1 year has been reported, resulting in smaller absolute difference in luminal diameter and TLR compared with BMS, resulting in a ‘catch up’ phenomenon. While both first- and second-generation DES are associated with delayed restenosis, first-generation DES have a higher late TLR rate than second generation DES, with continued risk up to 5 years postimplantation [68,69].
Mechanical factors of ISR include stent underexpansion and stent fracture. Kang et al. found that 42% of patients with ISR after DES had evidence of stent underexpansion [70]. Stent underexpansion at the time of PCI can be difficult to detect with angiography alone, and the use of intravascular ultrasound can be useful in the diagnosis. Stent strut fracture results in complete or partial stent separation, and thus decreases mechanical support and local drug delivery. The extent of stent fracture can range from single-strut fracture (grade I) to complete transection (grade V) [71]. Vessel tortuosity, longer stents, right coronary artery location, stent overlap and use of SES have all been associated with an increased risk of stent fracture [72,73]. Technical factors contributing to ISR include barotrauma outside of the stented segment, discontinuous serial stent coverage and suboptimal stent placement [64,74,75]. Moses et al. showed, in a subgroup analyses of patients randomized to receive SES, that the portions of the vessel that were exposed to balloon injury and were not covered by the SES were the primary sites for ISR [64]. Stent gap, like stent fracture, results in interruption of drug deposition in the vessel wall and an increased risk of ISR [74]. Underestimating the length of the diseased vessel, or misplacement of the stent during PCI resulting in the diseased artery not being covered by the stent, has been associated with increased risk of target-vessel revascularization and myocardial infarction at 1-year follow-up [75]. Predictors of developing ISR include patient, lesion and procedural characteristics, and are similar between BMS and DES [76-78]. Of the patient characteristics, diabetes mellitus is the strongest clinical predictor of ISR; however, the rate of ISR is lower with DES compared with BMS in diabetic patients, there remains to be an increased incidence compared with nondiabetics [78,79]. Lesion length, diameter, vessel size and complexity have all been associated with increased rates of ISR [76-78,80]. Procedural predictors, such as stent length and strut platform thickness have been shown to increase the incidence of ISR. The ISAR-STEREO-2 trial showed a significant reduction in angiographic restenosis at 6 months and target-vessel revascularization at 1 year in patients receiving BMS with thin (50 μM) versus thick (140 μM) struts [81]. With newer DES platforms with thinner stent struts, longitudinal stent deformation as a result of decreased longitudinal integrity can occur with resultant predisposition to ISR, as well as ST [82]. Patients undergoing stenting of multiple lesions and utilization of longer stent length have an increased risk for the development of ISR [76-78,80].
Minimizing complications
▪ Stent choice
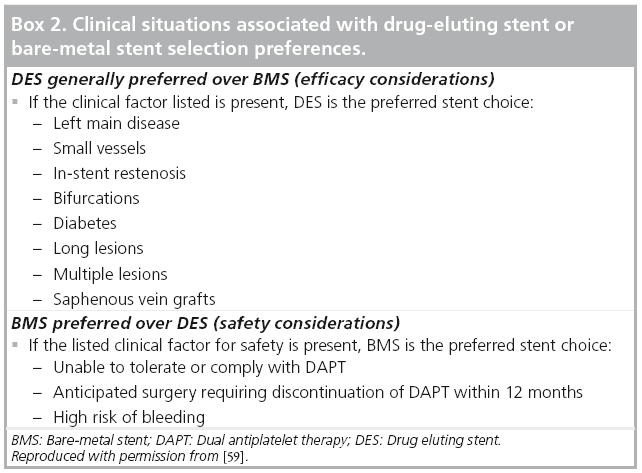
There are currently five types of stents available in the USA: BMS with either stainless steel or cobalt chromium alloy stent platforms, SES, PES, EES and ZES. As reviewed above, randomized controlled data have shown DES, specifically second-generation DES, to be superior to BMS, with a reduced rate of in-stent restenosis, ST and need for repeat TLR [7,8,25,26,30,31,33,63-65]. The antiproliferative drug elution with DES results in delayed endothelialization of the stent and thus requires longer duration of dual antiplatelet therapy (DAPT; 12 months) and leads to an increased risk of bleeding in some patients. DES should be the preferred stent choice, especially in patients that are at an increased risk of ISR or have a large area of myocardium at risk if stent complications occur (Box 2). BMS is the preferred stent choice in patients that are unable to comply with DAPT, have planned upcoming surgical procedures in the next 12 months or are considered to be a high risk for bleeding complications that may result in early discontinuation of DAPT.
▪ Intraprocedural pharmacotherapy
The use of antithrombotic therapy during PCI to reduce the incidence of thrombus formation is mandatory. More aggressive anticoagulation regimens have been implemented since the onset of PCI, resulting in a reduction in early ST, myocardial infarction and death, although at a cost of increased bleeding and vascular complications. The development of radial access for coronary intervention provides decreased vascular and bleeding complications compared with femoral access, allowing for improved outcomes despite aggressive anticoagulation [83]. Antithrombotic therapy is centered on the inhibition of platelet activation and the coagulation cascade. Multiple antithrombotic regimens have been employed with varying results.
Antiplatelet pharmacologic therapy targets cyclooxygenase inhibition, ADP P2Y12 receptor blockade and, in certain patients, GP IIb/IIIa inhibition. Aspirin has been shown to reduce the frequency of ischemic complications after PCI [84]. The minimum dose of aspirin to prevent complications is unclear; however, it is recommended that all patients undergoing PCI receive 325 mg of aspirin prior to the intervention [59]. The current available ADP P2Y12 receptor blockers include clopidogrel, prasugrel, ticagrelor and ticlopidine, with clopidogrel being the most widely used and well studied of the group.
Ticlopidine was the earliest developed ADP P2Y12 inhibiator, and was shown to reduce major adverse cardiovascular events, as well as subacute ST compared with warfarin plus aspirin [85,86]. Due to multiple adverse side effects associated with the usage of ticlopidine, and the development of newer antiplatelet agents, ticlopidine is infrequently used in the USA. Clopidogrel has proven to be beneficial in secondary prevention of myocardial infarction, stroke and vascular death in patients with ACS [87]. Clopidogrel is a prodrug requiring activation in the liver, with onset of action between 4 and 6 h. Several studies have evaluated the optimal loading dose for clopidogrel with PCI to have adequate anticoagulation at the time of procedure. A loading dose of 600 mg of clopidogrel provides greater platelet inhibition compared with 300 mg dose; however, there is no additional benefit for doses greater than 600 mg [88]. A meta-analysis of over 25,000 patients in seven studies undergoing PCI showed that a 600 mg loading dose of clopidogrel resulted in improved cardiovascular outcomes without an increase in bleeding complications, compared with a 300 mg loading dose [89]. Since clopidogrel is a prodrug requiring hepatic activation through the CYP2C19 enzyme, patients with decreased CYP2C19 function poorly metabolize the drug, resulting in higher cardiovascular events after PCI compared with normal [90]. Clopidogrel resistance testing is commercially available, although it has only had modest ability to predict clinical outcomes [90,91]. The efficacy of prasugrel compared with clopidogrel was evaluated in the TRITON-TIMI 38 trial, a randomized trial of 13,608 patients with ACS undergoing PCI [92]. Prasugrel usage resulted in a significant reduction in cardiovascular death, nonfatal myocardial infarction or nonfatal stroke compared with clopidogrel (9.9 vs 12.1%, hazard ratio [HR]: 0.81; 95% CI: 0.73–0.90), as well as a reduction in ST (1.1 vs 2.4%); however, it was associated with an increased rate of major bleeding (2.4 vs 1.8%, HR: 1.32; 95% CI: 1.03–1.68) [92]. Ticagrelor, a reversible ADP P2Y12 inhibitor that does not require conversion to an active metabolite, was compared with clopidogrel in patients with ACS in the PLATO trial [93]. Ticagrelor resulted in a reduction in the primary end point of cardiovascular death, myocardial infarction or stroke (9.8 vs 11.7%, HR: 0.84; 95% CI: 0.77–0.92), though again a higher rate of major bleeding not related to CABG was seen [93].
GP IIb/IIIa inhibitors, abciximab and the small molecule inhibitors eptifibatide and tirofiban, have been shown to improve outcomes in clinical trials of patients undergoing PCI. Earlier trials, conducted before the routine use of DAPT with ADP P2Y12 inhibition during PCI, demonstrated a reduction in ischemic events, mainly driven by a decrease in periprocedural cardiac biomarker elevation, with the use of GP IIb/IIIa agents. However, they were associated with an increased bleeding risk [94,95]. Trial data in the modern era of PCI with routine DAPT have been less clear. The ISAR-REACT trial of 2159 patients undergoing elective PCI, after receiving 600 mg clopidogrel loading, randomized patients to abciximab plus heparin versus heparin alone [96]. Results showed no difference in outcomes of death, myocardial infarction or urgent target-vessel revascularization (p = 0.82), and thus decreased enthusiasm for the routine use of GP IIb/IIIa inhibitors in patients undergoing PCI. However, the ISARREACT 2 trial, evaluating the use of abciximab in patients with high-risk ACS undergoing PCI receiving clopidogrel 600 mg loading, revealed a significant reduction in the primary end point of death, myocardial infarction or target-vessel revascularization at 30 days (8.9 vs 11.9%, relative risk: 0.75; 95% CI: 0.58–0.97), specifically in patients with elevated troponin, without a significantly increased bleeding risk [97]. Similar results were seen in high-risk patients undergoing PCI receiving eptifibatide in the ESPRIT trial, suggesting that GP IIb/IIIa inhibition should be reserved for patients at the highest risk [98].
Parenteral anticoagulation therapy during intervention is centered on the use of heparin, enoxaparin or bivalirudin. Unfractionated heparin (UFH) has historically been the most commonly used anticoagulant for PCI and has become the standard by which other anticoagulants are measured by. The degree of anticoagulation with UFH is determined by the activated clotting time, with analysis showing that higher degrees of anticoagulation (activated clotting time of >325 s) increase risk of bleeding without additional reduction in thrombotic events [99]. When GP IIb/IIIa inhibitors are used, a lower dose of heparin with target activated clotting time of 200–250 s has been shown to decrease major bleeding, without an increase in ischemic events [100]. UFH has several limitations, including a narrow therapeutic window and risk of heparin-induced thrombocytopenia, resulting in the subsequent development of newer antithrombotic agents.
Low-molecular weight heparin has been evaluated for use during PCI, with enoxaparin being the most widely studied therapeutic agent. The SYNERGY trial randomized 10,027 patients with ACS undergoing PCI to subcutaneous enoxaparin versus UFH [101]. There was no significant difference in all-cause mortality, nonfatal myocardial infarction or procedural complications at 30-day follow-up. There was an increase in bleeding complications in patients receiving enoxaparin, although significance varied owing to different bleeding definitions. The use of intravenous (iv.) enoxaparin has also been evaluated in randomized clinical trials. The STEEPLE trial evaluated over 3500 patients undergoing PCI receiving either 0.5 mg/kg iv. enoxaparin, 0.75 mg/kg iv. enoxaparin or UFH. Patients receiving 0.5 mg/kg iv. enoxaparin had a reduced rate of bleeding (5.9 vs 8.5%, 95% CI: -4.7 to -0.6; p = 0.01); however, the trial was not large enough to evaluate the prevention of ischemic complications [99]. A subsequent meta-analysis comparing iv. low-molecular weight heparin with UFH found that iv. low-molecular weight heparin resulted in a significant reduction in major bleeding (OR: 0.57; 95% CI: 0.40–0.82) with no significant difference in death, myocardial infarction or target-vessel revascularization [102].
Fondaparinux, a synthetically derived pentasaccharide, is an indirect factor Xa inhibitor that has been evaluated for the use in patients undergoing PCI. The OASIS-5 trial randomized over 20,000 patients with ACS to fondaparinux or enoxaparin [103]. Patients who underwent coronary intervention had similar ischemic outcomes (5.8 vs 5.7%, HR: -1.01; 95% CI: 0.9– 1.13), and patients receiving fondaparinux had decreased major bleeding complications (2.4 vs 5.1%, HR: 0.46; p < 0.00001). However, patients undergoing PCI had a higher rate of catheterrelated thrombosis (1.3 vs 0.5%; p = 0.001). Similar increased incidence of catheter-related thrombosis was again noted in the OASIS 6 trial [103]. Current guidelines state fondaparinux should not be used as the sole antithrombotic therapy in patients undergoing PCI [59].
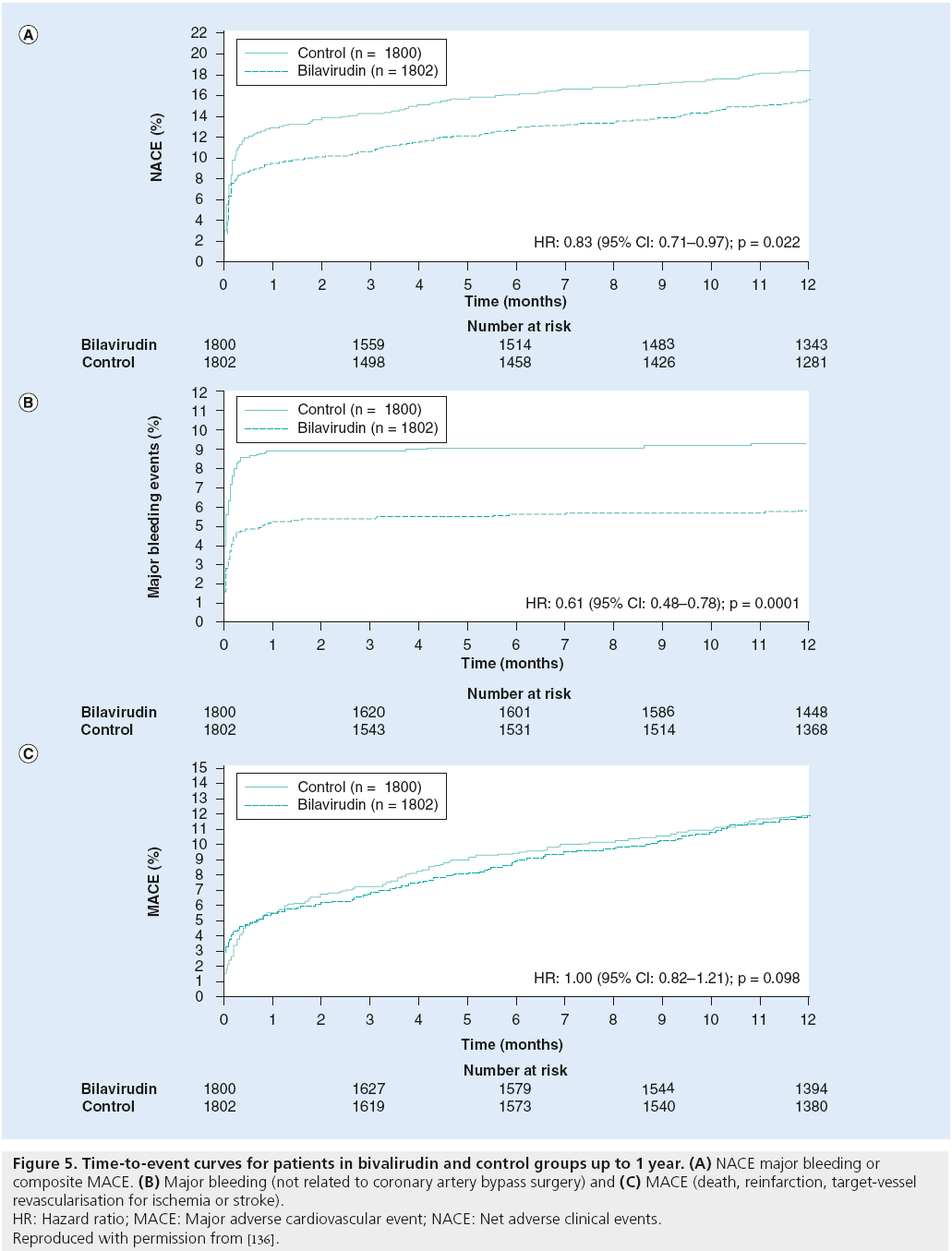
More recently, the direct thrombin inhibitors bivalirudin and argatroban have been evaluated for use in patients undergoing PCI. The REPLACE-2 trial randomized over 6000 stable or low-risk ACS patients undergoing PCI to bivalirudin, with provisional GP IIb/IIIa inhibition versus UFH plus GP IIb/IIIa inhibition. Bivalirudin was found to be noninferior to UFH plus GP IIb/IIIa inhibition with regard to 30-day mortality, myocardial infarction, urgent target-vessel revascularization or major bleeding, and was associated with a significant reduction in bleeding complications (2.4 vs 4.1%; p < 0.001) [104]. Subsequently, the ACUITY trial evaluated the use of bivalirudin in patients with ACS [105]. Patients were randomized to one of three antithrombotic regimens: UFH or enoxaparin plus GP IIb/IIIa inhibitor; bivalirudin plus GP IIb/IIIa inhibitor; or bivalirudin alone. Bivalirudin alone was noninferior to the other two regimens, with regard to the composite ischemic end point, but resulted in significantly reduced major bleeding (3.0 vs 5.7%; Figure 5) [105]. The role of bivalirudin in STEMI was evaluated in the HORIZONS-AMI study, which randomized 3602 patients with STEMI undergoing PCI to receive UFH plus GP IIb/IIIa inhibition or bivalirudin [106]. Bivalirudin was associated with a lower rate of bleeding (4.9 vs 8.3%; p < 0.001), as well as significantly lower 30-day rates of death from a cardiovascular cause (1.8 vs 2.9%, relative risk: 0.62, 95% CI: 0.40–0.95; p = 0.03) and allcause mortality (2.1 vs 3.1%, relative risk: 0.66, 95% CI: 0.44–1.00; p = 0.047) [106]. There was no significant difference in rates of reinfarction, target-vessel revascularization and stroke. Interestingly, the bivalirudin arm was associated with an increase in acute ST (<24 h); however, as above, it still resulted in a decreased 30-day rate of cardiovascular death.
Figure 5: Time-to-event curves for patients in bivalirudin and control groups up to 1 year. (A) NACE major bleeding or composite MACE. (B) Major bleeding (not related to coronary artery bypass surgery) and (C) MACE (death, reinfarction, target-vessel
revascularisation for ischemia or stroke).
HR: Hazard ratio; MACE: Major adverse cardiovascular event; NACE: Net adverse clinical events.
Reproduced with permission from [136].
▪ DAPT post-PCI
The optimal duration of DAPT with aspirin and ADP P2Y12 inhibition after PCI is uncertain, with current guidelines largely based on observational data and expert opinion. The challenge of determining the optimal length of DAPT is balancing the benefit of thrombosis prevention with the risk of increased bleeding and excess cost. Current guidelines recommend DAPT for at least 12 months after PCI, with DES or BMS in the case of an ACS [59]. In patients receiving BMS for non-ACS indications, 1 month of DAPT is recommended and ideally 12 months if there are no contraindications to continuing usage [59]. Recent data have suggested that DAPT for longer durations has not improved outcomes. Park et al. randomized 2701 patients treated with DES either to discontinue clopidogrel after 12 months or to continue it past 12 months [107]. The results revealed a nonsignificant difference in reducing the rate of myocardial infarction or cardiovascular death (HR: 1.65; 95% CI: 0.80–3.36), although few patients reached the primary end point [107]. The PRODIGY trial was a randomized clinical trial evaluating the effect of DAPT for 6 months versus 24 months in patients with ACS or stable angina who underwent PCI with BMS or DES [108]. Results showed that overall risk of all-cause mortality, nonfatal myocardial infarction or stroke at 2 years follow-up were similar (HR: 0.98; 95% CI: 0.74–1.29). Patients were also randomized to one of three different DES (PES, EES or ZES) or BMS, without change in outcomes in any of the stent types. Patients who received DES did not see a difference in ST between the 6 and 24 months of DAPT (0.42 vs 0.56%; p > 0.99). There was a significant reduction in major bleeding events in the patients receiving 6-month DAPT compared with those with 24 months of therapy (HR: 0.38; 95% CI: 0.15–0.97) [108]. Additional similar randomized trials have shown similar results, with no significant differences in adverse cardiovascular events with shorter durations of DAPT, although they were limited by sample size and duration of follow-up [109,110]. A meta-analysis of four randomized trials evaluated the clinical outcomes of the duration of DAPT after PCI [111]. The median DAPT duration was 16.8 months for the extended DAPT group versus 6.2 months for the control group. In the 8158 patients analyzed, there was no reduction in the primary end point of all-cause death (OR: 1.15; 95% CI: 0.85–1.54) or in secondary end points of myocardial infarction (OR: 0.95; 95% CI: 0.66–1.36) or ST (OR: 0.88; 95% CI: 0.43–1.81) [111].
Given the limitations of the current data, additional trials are required to determine the optimal duration of DAPT after PCI. Until further studies are completed, patients undergoing PCI with DES or BMS for ACS should continue DAPT for at least 12 months and possibly longer depending on individual risk of ST and risk of major bleeding complications. Patients undergoing BMS for non- ACS indications should have 1 month minimum of DAPT, and preferably 12 months if there are no contraindications. In patients with an indication for oral anticoagulation and DAPT, there is an increased risk of bleeding complications. Patients requiring ‘triple therapy’ should be maintained on low-dose aspirin and have goal international normalized ratio in the lower therapeutic range (2.0–2.5) to reduce bleeding risk. Recent randomized data suggest that the omission of aspirin in patients on triple therapy results in decreased bleeding complications, without an increase in thrombotic events; however, further studies are needed [112].
Clinical follow-up
▪ Stress testing
Noninvasive stress testing, with or without an imaging modality of nuclear imaging, echocardiography or MRI after PCI is frequently utilized, although the data for improved outcomes with the practice are lacking. An analysis of a national health insurance claims database with 21,046 patients status post-PCI found that 61% underwent stress testing within 2 years of intervention, and only 5% of these patients required revascularization [113]. The routine use of stress testing in asymptomatic patients after PCI has not been shown to improve outcomes [114,115]. The 2011 Appropriate Use Criteria for Echocardiography by the American College of Cardiology Foundation Appropriate Use Criteria Task Force/American Society of Echocardiography state that stress echocardiography less than 2 years following PCI in asymptomatic individuals is inappropriate; however, the appropriateness after 2 or more years is uncertain [116]. The American College of Cardiology Foundation/American Heart Association/ Society for Cardiac Angiography and Interventions guidelines for PCI suggest that routine periodic stress testing of asymptomatic patients after PCI without clinical indications should not be performed (class III recommendation, level of evidence C), but it is reasonable to perform treadmill exercise testing in this patient population prior to enrolling in a formal cardiac rehabilitation program [59]. Based on the current data, there is no indication for routine stress testing in asymptomatic patients after PCI. If clinical symptoms develop, a stress-imaging modality should be used to increase sensitivity for detecting restenosis.
▪ Secondary prevention
Secondary prevention measures for patients with coronary artery disease are essential to management after PCI in order to reduce adverse outcomes and the need for revascularization, and to improve quality of life. Aggressive lipid management should be utilized with lifestyle modification and statin therapy. The target goal of LDL cholesterol is a 30% reduction in baseline LDL, and a level of <100 mg/dl with a more aggressive treatment goal of <70 mg/dl in high-risk individuals [117-119]. Once the LDL goal is achieved, patients with triglyceride levels >200 mg/dl should have statins increased to lower non-HDL cholesterol to <130 mg/dl [120]. Blood pressure control with lifestyle modification and pharmacotherapy should be implemented with a goal of <140/80 mmHg. b-blockers and angiotensin-converting enzyme inhibitors are the preferred therapy if no contraindications exist [121]. Patients with diabetes should undergo optimal glucose management in coordination with the primary care provider and/or endocrinologist. All patients should be counseled on complete smoking cessation. Participation in a comprehensive cardiac rehabilitation program should be advised to all eligible patients after PCI at the time of discharge or at first followup appointment [122]. Cardiac rehabilitation has been shown to significantly reduce cardiovascular mortality and recurrent myocardial infarction, as well as improve exercise capacity and risk-factor management in patients after PCI [123].
Conclusion and future perspective
With the aging population in the USA, the prevalence of coronary artery disease will only continue to rise, as will the number of individuals undergoing PCI. While technique and technology have advanced greatly over the past decades, there remains room for improvement to reduce complications and improve outcomes. Stent technology continues to progress, with the most promising new development being bioresorbable stents. In the short term, the benefit of PCI is to provide vessel stability and prevent restenosis after intervention. Once the time period in which restenosis occurs has passed, the remaining stent platform remains a nidus for complications. The prospect of implanting bioresorbable stents was established in the ABSORB trial [124]. A stent composed of bioabsorbable poly-l-lactic acid, with a polymer coating of everolimus containing poly-d,l-lactide, was utilized in 30 patients with de novo coronary lesions resulting in only one non- STEMI and no cases of cardiac death or ST at 4-year follow-up [125]. A second iteration of this stent was created to improve the radial strength and reduce early and late recoil, and a randomized clinical trial (ABSORB II) comparing this bioresorbable stent with a standard EES is currently in progress [126]. Other new stent technology includes dual-therapy stenting, which combines the antiproliferative therapy of conventional DES with proendothelialization effects. The prohealing effects are achieved by eluting anti-human CD34 antibodies that enhance endothelialization by attracting endothelial progenitor cells. Clinical trials of dual-therapy stents are underway, and early results reveal rapid strut endothelialization [127,128]. Bioabsorbable polymer DES and polymerfree DES are under development, on the basis that removing the polymer will result in decreased ST while still providing drug delivery to reduce ISR. These new developments have promise to reduce ISR, ST and bleeding risk, by allowing for the early withdrawal of DAPT without increased complications.
Executive summary
Acute stent-related coronary artery perforation
▪ Rare life-threatening complication of coronary artery stenting occurring from guidewire-related perforations, oversized stent implantation or high-pressure balloon dilatation.
Stent thrombosis
▪ Complication of coronary stenting occurring acute (<24 h), early (0–30 days), late (31 days to 1 year) and very late (>1 year), with the majority of events in the early time period.
▪ Drug-eluting stents (DES) with greater risk of very-late stent thrombosis, compared with bare-metal stents (BMS).
In-stent restenosis
▪ Defined angiographically as >50% diameter stenosis on repeat angiography, or clinically as anginal symptoms requiring target-lesion revascularization.
▪ Reduced rates of in-stent restenosis with DES, specifically second-generation DES, compared with BMS.
Intraprocedural pharmacotherapy
▪ Antithrombotic therapy during coronary stenting is centered on the inhibition of platelet activation and the coagulation cascade.
▪ Trial data in the modern era of percutaneous coronary intervention with routine dual antiplatelet therapy (DAPT) have shown little difference in outcomes and an increased risk of bleeding in patients receiving routine glycoprotein IIb/IIIa inhibition, and should be reserved for high-risk patients with elevated troponin.
▪ The use of the direct thrombin inhibitor, bivalirudin, has resulted in improved outcomes in certain populations, with reduced bleeding complications compared with previous anticoagulation regimens with coronary stenting.
DAPT after stenting
▪ Patients undergoing coronary stenting with DES for any indication, or BMS for acute coronary syndrome, should continue DAPT for at least 12 months and possibly longer depending on individual risk of stent thrombosis and risk of major bleeding complications.
▪ For patients receiving BMS for nonacute coronary syndrome indications, 1 month of DAPT is recommended, and ideally 12 months, if there are no contraindications to continuing usage.
Secondary prevention
▪ Secondary prevention measures of lipid management, blood pressure control, glucose control and referral to cardiac rehabilitation are essential to management after coronary stenting with resultant improved outcomes.
Financial and competing interests disclosure
RJ Applegate has a research grant from Abbott Vascular; honoraria from Abbott Vascular; and is a consultant and on the advisory board for Abbott Vascular. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Favaloro RG. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: operative technique. Ann. Thorac. Surg. 5(4), 334–339 (1968).
- Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 301(2), 61–68 (1979).
- Holmes DR Jr, Vlietstra RE, Smith HC et al. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am. J. Cardiol. 53(12), C77–C81 (1984).
- Arjomand H, Turi ZG, McCormick D, Goldberg S. Percutaneous coronary intervention: historical perspectives, current status, and future directions. Am. Heart J. 146(5), 787–796 (2003).
- Schatz RA, Palmaz JC, Tio FO, Garcia F, Garcia O, Reuter SR. Balloon-expandable intracoronary stents in the adult dog. Circulation 76(2), 450–457 (1987).
- Sigwart U, Kaufmann U, Mirkovitch V, Joffre F, Kappemberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316(12), 701–706 (1987).
- Serruys PW, DeJaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 331(8), 489–495 (1994).
- Fischman DL, Leon MB, Baim D et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 331(8), 496–501 (1994).
- Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N. Engl. J. Med. 354(5), 483–495 (2006).
- Chan PS, Patel MR, Klein LW et al. Appropriateness of percutaneous coronary intervention. JAMA 306(1), 53–61 (2011).
- Dehmer GJ, Weaver D, Roe MT et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J. Am. Coll. Cardiol. 60(20), 2017–2031 (2012).
- Peterson ED, Dai D, Delong ER et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 55(18), 1923–1932 (2010).
- Shimony A, Joseph L, Mottillo S, Eisenberg MJ. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can. J. Cardiol. 27(6), 843–850 (2011).
- Stankovic G, Orlic D, Corvaja N et al. Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations. Am. J. Cardiol. 93(2), 213–216 (2004).
- Al-Lamee R, Ielasi A, Latib A et al. Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. JACC Cardiovasc. Interv. 4(1), 87–95 (2011).
- Javaid A, Buch AN, Satler LF et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 98(7), 911–914 (2006).
- Shimony A, Zahger D, Van Straten M et al. Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 104(12), 1674–1677 (2009).
- Kini AS, Rafael OC, Sarkar K et al. Changing outcomes and treatment strategies for wire induced coronary perforations in the era of bivalirudin use. Catheter Cardiovasc. Interv. 74(5), 700–707 (2009).
- Ellis SG, Ajluni S, Arnold AZ et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation 90(6), 2725–2730 (1994).
- Witzke CF, Martin-Herrero F, Clarke SC, Pomerantzev E, Palacios IF. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J. Invasiv. Cardiol. 16(6), 257–301 (2004).
- Kiernan TJ, Yan BP, Ruggiero N et al. Coronary artery perforations in the contemporary interventional era. J. Interv. Cardiol. 22(4), 350–353 (2009).
- Ong AT, Hoye A, Aoki J et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J. Am. Coll. Cardiol. 45(6), 947–953 (2005).
- Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17), 2344–2351 (2007).
- Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart 92(5), 641–649 (2006).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Lagerqvist B, James SK, Stenestrand U et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 356(10), 1009–1019 (2007).
- Caixeta A, Leon MB, Lansky AJ et al. 5-year clinical outcomes after sirolimus-eluting stent implantation: insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J. Am. Coll. Cardiol. 54(10), 894–902 (2009).
- Ellis SG, Stone GW, Cox DA et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: treatment of de novo coronary disease using a single paclitaxel-eluting stent). J. Am. Coll. Cardiol. Interv. 2(12), 1248–1259 (2009).
- Kirtane AJ, Gupta A, Iyengar S et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119(25), 3198–3206 (2009).
- Palmerini T, Kirtane AJ, Serruys PW et al. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circ. Cardiovasc. Interv. 5(3), 357–364 (2012).
- Leon MB, Mauri L, Popma JJ et al. A randomized comparison of the ENDEAVOR zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions: 12-month outcomes from the ENDEAVOR IV trial. J. Am. Coll. Cardiol. 55(6), 543–554 (2010).
- Bangalore S, Kumar S, Fusaro M et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117,762 patient-years of follow-up from randomized trials. Circulation 125(23), 2873–2891 (2012).
- Silber S, Windecker S, Vranckx P, Serruys PW, on behalf of the RESOLUTE All Comers Investigators. Unrestricted randomised use of two new generation drugeluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet 377(9773), 1241–1247 (2011).
- Cook S, Ladich E, Nakazawa G et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120(5), 391–399 (2009).
- van Werkum JW, Heestermans AA, Zomer AC et al. Predictors of coronary stent thrombosis: the Dutch stent thrombosis registry. J. Am. Coll. Cardiol. 53(16), 1399–1409 (2009).
- Roy P, Bonello L, Torguson R et al. Temporal relation between clopidogrel cessation and stent thrombosis after drug-eluting stent implantation. Am. J. Cardiol. 103(6), 801–805 (2009).
- Yan BP, Duffy SJ, Clark DJ et al. Rates of stent thrombosis in bare-metal versus drugeluting stents (from a large Australian multicenter registry). Am. J. Cardiol. 101(12), 1716–1722 (2008).
- Machecourt J, Danchin N, Lablanche JM et al. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT matched-cohort registry. J. Am. Coll. Cardiol. 50(6), 501–508 (2007).
- McKee SA, Applegate RJ, Hoyle JR, Sacrinty MT, Kutcher MA, Sane DC. Cocaine use is associated with an increased risk of stent thrombosis after percutaneous coronary intervention. Am. Heart J. 154(1), 159–164 (2007).
- Aoki J, Lansky AJ, Mehran R et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the acute catheterization and urgent intervention triage strategy trial. Circulation 119(5), 687–698 (2009).
- Liu X, Doi H, Maehara A et al. A volumetric intravascular ultrasound comparison of early drug-eluting stent thrombosis versus restenosis. J. Am. Coll. Cardiol. Interv. 2(5), 428–434 (2009).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice: 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Kukreja N, Onuma Y, Garcia-Garcia HM et al. The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. Interv. 2(6), 534–541 (2009).
- Prasad A, Rihal CS, Lennon RJ, Singh M, Jaffe AS, Holmes DR Jr. Significance of periprocedural myonecrosis on outcomes after percutaneous coronary intervention: an analysis of preintervention and postintervention troponin T levels in 5,487 patients. Circ. Cardiovasc. Interv. 1(1), 10–19 (2008).
- Cavallini C, Savonitto S, Violini R et al. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary intervention: results of the CK-MB and PCI study. Eur. Heart J. 26(15), 1494–1498 (2005).
- Kong TQ, Davidson CJ, Meyers SN, Tauke JT, Parker MA, Bonow RO. Prognostic implication of creatinine kinase elevation following elective coronary artery interventions. JAMA 277(6), 461–466 (1997).
- Narins CR, Miller DP, Califf RM, Topol EJ. The relationship between periprocedural myocardial infarction and subsequent target vessel revascularization following percutaneous coronary revascularization: insights from the EPIC trial. Evaluation of IIb/IIIa platelet receptor antagonist 7E3 in preventing ischemic complications. J. Am. Coll. Cardiol. 33(3), 647–653 (1999).
- Stone GW, Mehran R, Dangas G, Lansky AJ, Kornowski R, Leon MB. Differential impact on survival of electrocardiographic Q-wave versus enzymatic myocardial infarction after percutaneous intervention: a device-specific analysis of 7,147 patients. Circulation 104(6), 642–647 (2001).
- Ioannidis JP, Karvouni E, Katritsis DG. Mortality risk conferred by small elevations of creatinine kinase-MB isoenzyme after percutaneous coronary intervention. J. Am. Coll. Cardiol. 42(8), 1406–1411 (2003).
- Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ. Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J. Am. Coll. Cardiol. 40(11), 1961–1967 (2002).
- Prasad A, Gersh BJ, Bertrand ME et al. Prognostic significance of periprocedural versus spontaneously occurring myocardial infarction after percutaneous coronary intervention in patients with acute coronary syndromes: an analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J. Am. Coll. Cardiol. 54(5), 477–486 (2009).
- Jeremias A, Baim DS, Ho KK et al. Differential mortality risk of postprocedural creatinine kinase-MB elevation following successful versus unsuccessful stent procedures. J. Am. Coll. Cardiol. 44(6), 1210–1214 (2004).
- Bakhai A, Stone GW, Mahoney E et al. Cost effectiveness of paclitaxel-eluting stents for patients undergoing percutaneous coronary revascularization: results from the TAXUSIV trial. J. Am. Coll. Cardiol. 48(2), 253–261 (2006).
- Miller WL, Garratt KN, Burritt MF, Lennon RJ, Reeder GS, Jaffe AS. Baseline troponin level: key to understanding the importance of post-PCI troponin elevations. Eur. Heart J. 27(9), 1061–1069 (2006).
- Nienhuis MB, Ottervanger JP, Bilo HJ, Dikkeschei BD, Zijlstra F. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc. Interv. 71(3), 318–324 (2008).
- Cutlip DE, Kuntz RE. Does creatinine kinase- MB elevation after percutaneous coronary intervention predict outcomes in 2005? Cardiac enzyme elevation after successful percutaneous coronary intervention is not an independent predictor of adverse outcomes. Circulation 112(6), 916–922 (2005).
- Bhatt DL, Topol EJ. Does creatinine kinase- MB elevation after percutaneous coronary intervention predict outcomes in 2005? Periprocedural cardiac enzyme elevation predicts adverse outcomes. Circulation 112(6), 906–915 (2005).
- Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 60(16), 1581–1598 (2012).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 58(24), e44–e122 (2011).
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 151(6), 1260–1264 (2006).
- Mishkel GJ, Moore AL, Markwell S, Shelton MC, Shelton ME. Long-term outcomes after management of restenosis or thrombosis of drug-eluting stents. J. Am. Coll. Cardiol. 49(2), 181–184 (2007).
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J. Invasiv. Cardiol. 20(8), 401–403 (2008).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 362(18), 1663–1674 (2010).
- Chan AW. Clinical evaluation of restenosis. In: Atherothrombosis and Coronary Artery Disease. Fuster V, Topol EJ, Nabel EG (Eds). Lippincott Williams and Wilkins, PA, USA, 1415–1441 (2004).
- Koster R, Vieluf D, Kiehn M et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 356(9245), 1895–1897 (2000).
- Kimura T, Morimoto T, Nakagawa Y et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher registry. Circulation 125(4), 584–591 (2012).
- Byrne RA, Kastrati A, Tiroch K et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drugeluting stents versus polymer-based Cypher and Endeavor drug-eluting stents. J. Am. Coll. Cardiol. 55(23), 2536–2543 (2010).
- Kang SJ, Mintz GS, Park DW et al. Mechanisms of in-stent restenosis after drugeluting stent implantation: intravascular ultrasound analysis. Circ. Cardiovasc. Interv. 4(1), 9–14 (2011).
- Nakazawa G, Finn AV, Vorpahl M et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery: a pathologic analysis. J. Am. Coll. Cardiol 54(21), 1924–1931 (2009).
- Aoki J, Nakazawa G, Tanabe K et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc. Interv. 69, 380–386 (2007).
- Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc. Interv. 69(3), 387–394 (2007).
- Kereiakes DJ, Wang H, Popma JJ et al. Periprocedural and late consequences of overlapping cypher sirolimus-eluting stents: pooled analysis of five clinical trials. J. Am. Coll. Cardiol. 48(1), 21–31 (2006).
- Costa MA, Angiolillo DJ, Tannenbaum M et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am. J. Cardiol. 101(12), 1704–1711 (2008).
- Zahn R, Hamm CW, Schneider S et al. Incidence and predictors of target vessel revascularization and clinical event rates of the sirolimus-eluting coronary stent (results from the prospective multicenter German cyper stent registry). Am. J. Cardiol. 95(11), 1302–1308 (2005).
- Zahn R, Hamm CW, Schneider S et al. Coronary stenting with the sirolimus-eluting stent in clinical practice: final results from the prospective multicenter German Cypher Stent Registry. J. Interv. Cardiol. 23(1), 18–25 (2010).
- Kastrati A, Dibra A, Mehilli J et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxeleluting stents. Circulation 113, 2293–2300 (2006).
- Mathew V, Gersh BJ, Williams BA et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in thecurrent era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 109(4), 476–480 (2004).
- Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Little WC. Effect of length and diameter of drug-eluting stents versus bare-metal stents on late outcomes. Circ. Cardiovasc. Interv. 2(1), 35–42 (2009).
- Pache J, Kastrati A, Mehilli J et al. Intracoronary stenting and angiographic results: Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO-2) Trial. J. Am. Coll. Cardiol. 41(8), 1283–1288 (2003).
- Hanratty CG, Walsh SJ. Longitudinal compression: a ‘new’ complication with modern coronary stent platforms – time to think beyond deliverability? EuroIntervention 7(7), 872–877 (2011).
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157(1), 132–140 (2009).
- Jolly SS, Pogue J, Haladyn K et al. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: Insights from the PCI-CURE study. Eur. Heart J. 30(8), 900–907 (2009).
- Leon MB, Baim DS, Popma JJ et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 339(23), 1665–1671 (1998).
- Bertrand ME, Legrand V, Boland J et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation 98(16), 1597–1603 (1998).
- Yusuf S, Zhao F, Mehta SR et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without STsegment elevation. N. Engl. J. Med. 345(7), 494–502 (2001).
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schomig E, Kastrati A, Schomig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) trial. Circulation 112(19), 2946–2950 (2005).
- Siller-Matula JM, Huber K, Christ G et al. Impact of clopidogrel loading dose on clinical outcomes in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Heart 97(2), 98–105 (2011).
- Breet NJ, van Werkum JW, Bouman HJ et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 303(8), 754–762 (2010).
- Moerenhout CM, Claeys MJ, Haine S et al. Clinical relevance of clopidogrel unresponsiveness during elective coronary stenting: experience with the point-of-care platelet function assay-100 C/ADP. Am. Heart J. 159(3), 434–438 (2010).
- Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357(20), 2001–2015 (2007).
- Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361(11), 1045–1057 (2009).
- Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N. Engl. J. Med. 330(14), 956–961 (1994).
- EPISTENT Investigators. Randomized, placebo-controlled and balloon-angioplastycontrolled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Evaluation of platelet IIb/IIIa inhibitor for stenting. Lancet 352(9122), 87–92 (1998).
- Kastrati A, Mehilli J, Schuhlen H et al. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. N. Engl. J. Med. 350(3), 232–238 (2004).
- Kastrati A, Mehilli J, Neumann F-J et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 Randomized Trial. JAMA 295(13), 1531–1538 (2006).
- Puma JA, Banko LT, Pieper KS et al. Clinical characteristics predict benefits from eptifibatide therapy during coronary stenting: insights from the Enhanced Suppression of the Platelet IIb/IIIa Receptor With Integrillin Therapy (ESPRIT) trial. J. Am. Coll. Cardiol. 47(4), 715–718 (2006).
- Montalescot G, Cohen M, Salette G et al. Impact of anticoagulation levels on outcomes in patients undergoing elective percutaneous coronary intervention: Insights from the STEEPLE trial. Eur. Heart J. 29(4), 462–471 (2008).
- Brener SJ, Moliterno DJ, Lincoff AM, Steinhubl SR, Wolski KE, Topol EJ. Relationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary intervention. Circulation 110(8), 994–998 (2004).
- Ferguson JJ, Califf RM, Antman EM et al. Enoxaparin vs. unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 292(1), 45–54 (2004).
- Dumaine R, Borentain M, Bertel O et al. Intravenous low-molecular-weight heparins compared wtih unfractionated heparin in percutaneous coronary intervention: quantitative review of randomized trials. Arch. Intern. Med. 167(22), 2423–2430 (2007).
- Yusuf S, Mehta SR, Chrolavicius S et al. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA 295(13), 1519–1530 (2006).
- Lincoff AM, Bittl JA, Harrington RA et al. Bivalirudin and provisional glycoprotein IIb/ IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 289(7), 853–863 (2003).
- Stone GW, McLaurin BT, Cox DA et al. Bivalirudin for patients with acute coronary syndromes. N. Engl. J. Med. 355(21), 2203–2216 (2006).
- Stone GW, Witzenbichler B, Guagliumi G et al. Bivalirudin during primary PCI in acute myocardial infarction. N. Engl. J. Med. 358(21), 2218–2230 (2008). 107 Park SJ, Park DW, Kim YH et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N. Engl. J. Med. 362(15), 1374–1382 (2010).
- Park SJ, Park DW, Kim YH et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N. Engl. J. Med. 362(15), 1374–1382 (2010).
- Valgimigli M, Campo G, Monti M et al. Short- versus long-term duration of dualantiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 125(16), 2015–2026 (2012).
- Gwon HC, Hahn JY, Park KW et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 125(3), 505–513 (2012).
- Kim BK, Hong MK, Shin DH et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J. Am. Coll. Cardiol. 60(15), 1340–1348 (2012).
- Cassese S, Byrne RA, Tada T, King LA, Kastrati A. Clinical impact of extended dual antiplatelet therapy after percutaneous coronary interventions in the drug-eluting stent era: a meta-analysis of randomized trials. Eur. Heart J. 33(24), 3078–3087 (2012).
- Dewilde WJ, Oirbans T, Verheugt FW et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulation therapy and undergoing percuatenous coronary intervention: an open-label, randomised, controlled trial. Lancet 381(9872), 1107–1115 (2013).
- Shah BR, Cowper PA, O’Brien SM et al. Patterns of cardiac stress testing after revascularization in community practice. J. Am. Coll. Cardiol. 56(16), 1328–1334 (2010).
- Eisenberg MJ, Wilson B, Lauzon C et al. Routine functional testing after percutaneous coronary intervention: results of the aggressive diagnosis of restenosis in high-risk patients (ADORE II) trial. Acta Cardiol. 62(2), 143–150 (2007).
- Babapulle MN, Diodati JG, Blankenship JC et al. Utility of routine exercise treadmill testing early after percutaneous coronary intervention. BMC Cardiovasc. Disord. 7, 12 (2007).
- American College of Cardiology Foundation Appropriate Use Criteria Task Force; American Society of Echocardiography; American Heart Association; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Critical Care Medicine; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; Douglas PS, Garcia MJ, Haines DE et al. ACCF/ASE/ AHA/ASNC/HFSA/HRS/SCAI/SCCM/ SCMR 2011 appropriate use criteria for echocardiography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians. J. Am. Coll. Cardiol. 57(9), 1126–1166 (2011).
- Pedersen TR, Faergeman O, Kastelein JJ et al. High-dose atorvastatin vs. usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 294(19), 2437–2445 (2005).
- LaRosa JC, Grundy SM, Waters DD et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N. Engl. J. Med. 352(14), 1425–1435 (2005).
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106(25), 3143–3421 (2002).
- Robinson JG, Wang S, Smith BJ, Jacobson TA. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J. Am. Coll. Cardiol. 53(4), 316–322 (2009).
- Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J. Am. Coll. Cardiol. 55(20), 2187–2195 (2010).
- Smith SC Jr, Benjamin EJ, Bonow RO et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 124(22), 2458–2473 (2011).
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 123(21), 2344–2352 (2011).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371(9619), 899–907 (2008).
- Dudek D, Onuma Y, Ormiston J, Thuesen L, Miguel-Hebert K, Serruys PW. Four-year clinical follow-up of the ABSORB everolimus-eluting bioresorbable vascular scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention 7(9), 1060–1061 (2012).
- Diletti R, Serruys PW, Farooq V et al. ABSORB II randomized controlled trial: a clinical evaluation to compare the safety, efficacy, and performance of the ABSORB everolimus-eluting bioresorbable vascular scaffold system against the XIENCE everolimus-eluting coronary stent system in the treatment of subjects with ichemic heart disease casued by de novo native coronary artery lesions: rationale and study design. Am. Heart J. 164(5), 654–663 (2012).
- Granada JF, Inami S, Aboodi MS et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ. Cardiovasc. Interv. 3(3), 257–266 (2010).
- Lee SW, Lam SC, Chan KK et al. Evaluation of neointimal healing and late luminal loss of endothelial progenitor cell capturing sirolimus-eluting (COMBO) stent by optical coherence tomography: the EGO-COMBO study. J. Am. Coll. Cardiol. 60(17 Suppl. B), B82–B83 (2012).
- Nebeker JR, Virmani R, Bennett CL et al. Hypersensitivity cases associated with drugeluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J. Am. Coll. Cardiol. 47(1), 175–181 (2006).
- Turco MA, Ormiston JA, Popma JJ et al. Polymer-based, paclitaxel-eluting TAXUS Liberte stent in de novo lesions: the pivotal TAXUS ATLAS trial. J. Am. Coll. Cardiol. 49(16), 1676–1683 (2007).
- Kereiakes DJ, Cannon LA, Feldman RL et al. Clinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trial. J. Am. Coll. Cardiol. 56(4), 264–271 (2010).
- Stone GW, Teirstein PS, Meredith IT et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J. Am. Coll. Cardiol. 57(16), 1700–1708 (2011).
- Yeung AC, Leon MB, Jain A et al. Clinical evaluation of the RESOLUTE zotarolimuseluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J. Am. Coll. Cardiol. 57(17), 1778–1783 (2011).
- Lambert N, Applegate R. The comparative safety of bare-metal and drug-eluting intracoronary stents. Expert Rev. Med. Devices 7(5), 611–624 (2010).
- Stone GW, Rizvi A, Sudhir K et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J. Am. Coll. Cardiol. 58(1), 19–25 (2011).
- Mehran R, Lansky AJ, Witzenbichler B et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1 year results of a randomized controlled trial. Lancet 374(9696), 1149–1159 (2009).
▪▪ Analysis of nine randomized trials with over 5200 patients receiving either paclitaxel-eluting stents, sirolimus-eluting stents or bare-metal stents.
▪▪ Meta-analysis of 11 randomized trials comparing 2-year rates of definite stent thrombosis between everolimus-eluting stents and other drug-eluting stents.
▪ Review of a large Dutch registry to identify clinical- and procedural-related factors related to stent thrombosis.
▪▪ Multicenter randomized trial comparing outcomes in over 3600 patients receiving everolimus- or paclitaxel-eluting stents.
▪ Meta-analysis of over 25,000 patients demonstrating reduced rates of major cardiovascular events in patients undergoing percutaneous coronary intervention receiving 600 mg clopidogrel compared with 300 mg clopidogrel.
▪▪ Randomized, multicenter trial demonstrating confined benefit of abciximab to patients with non-ST-segment elevation acute coronary syndrome with elevated troponin undergoing percutaneous coronary intervention in patients who received a clopidogrel loading dose.
▪▪ Randomized trial comparing anticoagulation strategies of bivalirudin alone compared with heparin plus glycoprotein IIb/IIIa inhibitors in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention.