Review Article - Interventional Cardiology (2014) Volume 6, Issue 2
Mobile health applications in cardiac care
- Corresponding Author:
- Mohanraj Karunanithi
CSIRO Computational Informatics, Australian eHealth Research Centre
(AEHRC), Brisbane, QLD, Australia
Tel: +61 732533623
Fax: +61 732533690
E-mail: mohan.karunanithi@csiro.au
Abstract
There is mounting pressure on cardiac services to find novel and accessible ways of delivering services due to increasing cardiac disease prevalence and escalating costs. Although cardiology has been one of the leading disciplines in embracing telehealth, normalization of telecardiology services has been slow to develop, with challenges in geographical connectivity, affordability, technology maturity and patient uptake often being cited as causes. The focus has mostly been on teletriage and telemonitoring in long-term care. In-hospital and face-to-face care remains a mainstay of acute cardiac management. The wide adoption of ever-more sophisticated mobile applications (apps) and devices provides mobile health with the opportunity to build on telecardiology’s aims to reduce costs while delivering safe, effective and personalized care at all stages of the patient’s journey.Keywords
arrhythmia, cardiac arrest, cardiac rehabilitation, cardiology, heart failure, mHealth, mobile phone, myocardial infarction, smartphone, telehealth
There is mounting pressure on cardiac services to find novel and accessible ways of delivering services due to increasing cardiac disease prevalence and escalating costs. Although cardiology has been one of the leading disciplines in embracing telehealth, normalization of telecardiology services has been slow to develop [1], with challenges in geographical connectivity, affordability, technology maturity and patient uptake often being cited as causes. The focus has mostly been on teletriage and telemonitoring in long-term care. In-hospital and face-to-face care remains a mainstay of acute cardiac management. The wide adoption of ever-more sophisticated mobile applications (apps) and devices provides mobile health with the opportunity to build on telecardiology’s aims to reduce costs while delivering safe, effective and personalized care at all stages of the patient’s journey.
Mobile phones are playing an increasingly important and creative role in supporting patients in their well-being and healthcare journey. Health improvement apps, including weight management, smoking cessation, fitness and stress reduction, are common and widely used. While these apps may play a role in prevention of cardiac disease, smartphones also have the potential to be a viable tool for wide community cardiac disease screening through apps such as those for arrhythmia diagnosis, and mobile monitoring devices for blood pressure (BP) measurement, which are available to the public at low cost.
Clinicians are turning to mobile devices, including mobile phones and tablet PCs, to support training and continued development, with educational apps and professional publications increasingly being available on handheld devices. Professional networking and sharing of best practice are some of the ways doctors are using mobile technologies. Clinicians can also access medical records whenever required, and studies have shown that, when clinicians read ECGs and CT scans on mobile devices, they respond more promptly, have improved data management and recordkeeping practices, and fewer medication prescribing and discharge from hospital errors [2].
Acute care is often confined to hospital and/or clinical settings because it often requires specialized clinicians and a controlled environment to ensure patients are safely managed following medical interventions. Cardiac interventions, varying from revascularization, and pacemaker (PM) and cardiac defibrillator implants, to ventricular assist device surgery, are often followed by intensive care in a hospital environment. However, hospital stays beyond intensive care frequently involve monitoring and medical management, which could feasibly be shifted to the home environment and supported through mobile health.
Uptake and completion rates for cardiac rehabilitation (CR), which is known to reduce secondary events and long-term healthcare costs, are notoriously poor. Smartphones can help address these issues by enabling remote delivery of appropriately monitored rehabilitation programs [3], while also extending care services to remote areas that traditional services are not able to reach. Similarly, mobile health can support safer and more personalized care in the long-term management of chronic disease, with real-time smart monitoring opening up new possibilities for appropriate early intervention and active risk factor management. Mobile technologies can also support patients in their self-care and medication management, and, with patients also able to access electronic health records on their mobile devices, they are enabled and empowered to better participate in their own healthcare and management of their long-term condition.
This review will first outline the general capabilities and features of mobile health that enable medical, and in particular cardiac, interventions. Secondly, it will review the clinical studies that have applied mobile health technologies in cardiac care. Through these studies, we will outline how mobile health can assist cardiac clinicians and how it has influenced patient outcomes. Finally, we will discuss the limitations and future perspectives of mobile health in cardiology.
Mobile health components & features
Mobile health is defined as medical and public health practice supported by mobile devices, such as mobile phones, tablet PCs and other handheld/portable wireless devices [4]. It is an emerging area within the spectrum of telehealth. In contrast to the internet and medical workstations used in conventional telehealth systems, mobile health uses mobile phones and untethered portable healthcare devices, which are easier to use, less expensive, flexible, compliant with patients’ lifestyle and remotely upgradeable. Due to these distinct advantages, mobile health can reach large populations and is often presented and studied as a unique subprovision of healthcare services [5].
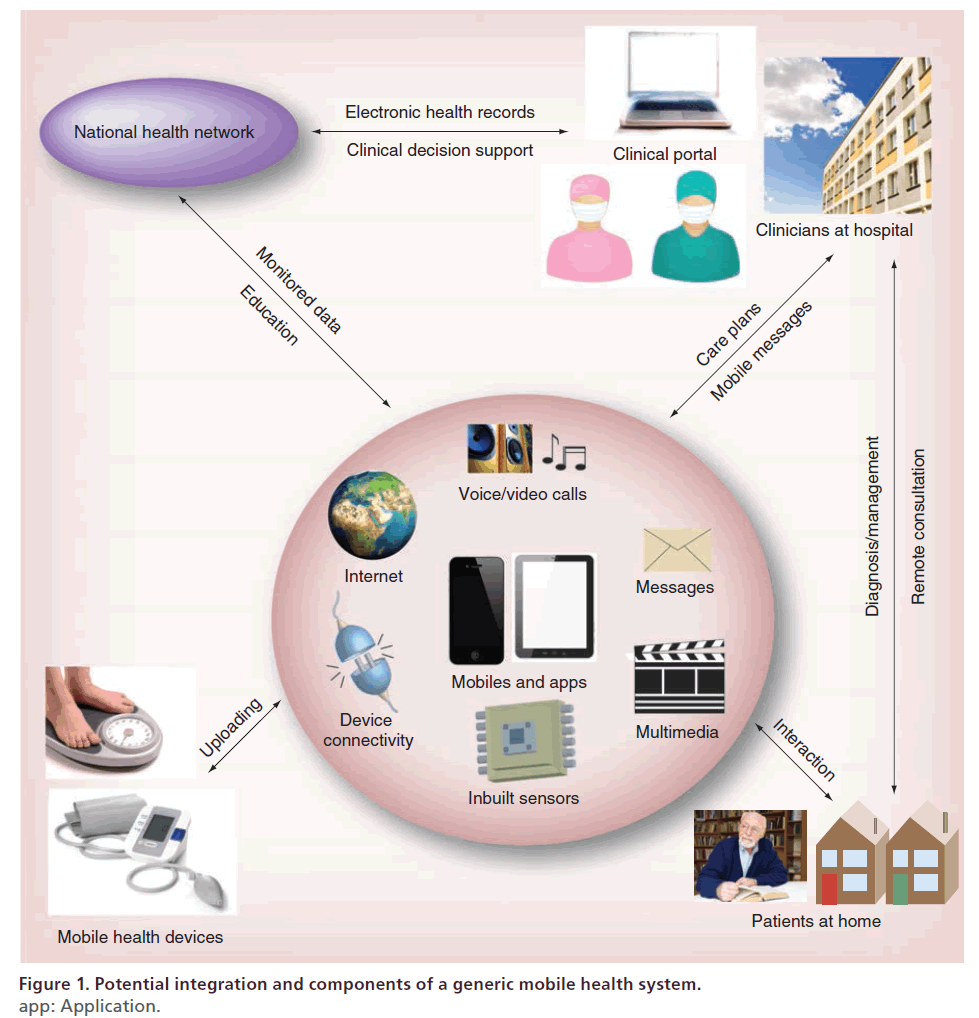
Potential integration and components of a generic mobile health system are illustrated in Figure 1. The mobile phone or tablet PC is the core device linking clinicians with patients in their own environment. Unlike early mobile phones, which were used mainly for voice communications, smartphones have integrated virtually all the core functions of a modern computer, and, hence, the available apps have revolutionized ways people now use them in their everyday lives. These functions can support patients to self-manage their diseases, and provide various communication channels for clinical interventions. The main functions of the smartphone that have enabled its clinical applications include:
Figure 1: Potential integration and components of a generic mobile health system.
app: Application.
• Voice/video calling: provides a convenient [6] and accepted [7] way for clinicians and patients to remotely communicate with each other as an alternative to face-to-face consultation;
• Short message services (SMS) and multimedia message services (MMS): the ability to transmit text messages and video clips/sound files respectively, offers a convenient and cost-effective way to deliver education materials for health behavior or risk factor modification [8];
• Multimedia functions: smartphones can access and play a large range of multimedia content from online multimedia servers, which can be updated as required and can provide intuitive, accessible health education [9,10];
• Inbuilt sensors: inbuilt touch, motion and GPS sensors, can obviate the need for additional health devices and provide clinical assessment opportunities, for example quantifying and classifying physical activities [11,12] and measuring lifestyle and social activities [13];
• Device connectivity: many telemonitoring devices such as ambulatory ECG and BP monitors, can wirelessly connect to mobile phones or tablet PCs, enabling automated data transfer that is more practical and less error prone than manual data entry;
• Internet connectivity: 3G/4G and Wi-Fi provides almost ubiquitous access to remotely monitored health data, online education materials, and communication with clinicians.
As illustrated in Figure 1, mobile devices could potentially be a personal hub that gathers and communicates patients’ health data to the health services, from where care or education can be delivered remotely by clinicians to a patient in their own environment. For example, patients nominated for home care could be equipped with mobile monitoring device(s) such as BP and ECG monitors, to perform daily (or as recommended) measurements. Wireless monitoring devices enable automatic gathering of data through a dedicated mobile medical app on the smartphone, which would relay the information to a centralized national health network. The information could also include measurements gathered from in-built sensors and manual qualitative data. The data would be structured and/or processed through clinical decision support medical apps available within an enterprise healthcare information system and made available for review by clinicians. Using this information, care could be provided through scheduled consultation via online audio or video for monitoring and diagnosis. Moreover, delivery of feedback on progress, reminders of scheduled care consultation or medications or motivational messages through SMS text or even education through video could be delivered to patients through the smartphone mobile medical app for their self-management. Furthermore, the mobile medical app would be the key source through which the patient would interact to view their progress, communicate with the care provider and guide their self-management.
Mobile health in cardiac intervention & management
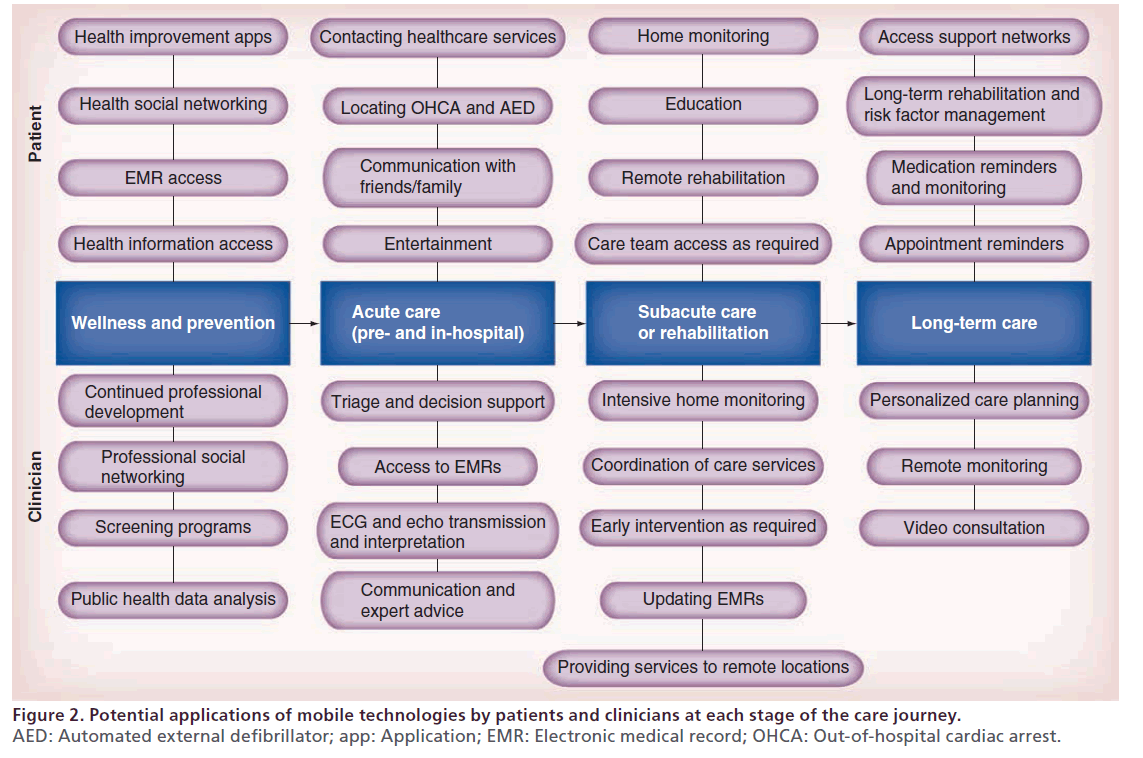
Mobile health offers significant, mainly untapped, potential to improve cardiac care from both a patient and a clinical perspective, at every stage of the patient’s care journey and in all care locations, with some potential applications highlighted in Figure 2. In this section, we describe and highlight some of the key cardiac interventions where mobile health has been applied or studied in both the acute and longer-term care settings.
These applications include recent advances and some established programs involving mobile health in cardiac arrest, arrhythmias, myocardial infarction (MI) and heart failure (HF) monitoring and management.
Mobile health in cardiac arrest
It is well known that survival to hospital discharge following an out-of-hospital cardiac arrest (OHCA) in a public location is significantly improved through early cardiopulmonary resuscitation (CPR) and defibrillation [14]. Several initiatives have evaluated how healthcare workers’ and the public’s ubiquitous access to mobile phones can strengthen each link within the chain of survival [15], with Kovic suggesting a mobile chain of survival [16], illustrated in Figure 3. Using a mobile phone to contact emergency services in lifethreatening situations has already been correlated to improved outcomes when compared with landline contact [17]. Furthermore, application of mobile technologies in the support of CPR training and implementation has been reported in several studies, which are described below.
A study involving healthcare professionals and laypeople showed improved cardiac compression rate over 2 min when supported by self-directed CPR training with feedback delivered through a smartphone app in a cardiac arrest simulation, compared with when not using the app, as well as acceptability of the technology [18]. Another study showed lay responders (LRs) receiving CPR and automated external defibrillator (AED) training had improved retention of skills through accessing a video clip via their mobile phone, which they were repeatedly encouraged to watch by SMS text message [19].
Dispatcher-assisted CPR simulation studies have shown improved confidence [20] and technique [21–23] in bystander CPR when dispatch instructions were accompanied by mobile phone-based video or animation demonstration. Improvements in technique included better rate and depth of chest compressions, correct hand positioning and reduced hands-off events during resuscitation. Even a simple audio program [24] providing CPR instruction increased bystander CPR technique in manikin simulations in 178 subjects. Similarly, advanced life support (ALS)-certified doctors showed improved ALS performance in a simulated medical emergency [25] during a randomized controlled trial (RCT) study when using the Resuscitation Council UK’s iResus© app on a smartphone.
The Mobile Life Saver (MLS) [26] is a mobile phone service that uses mobile phone positioning systems to dispatch CPR-trained LRs to nearby suspected OHCA. In a real life RCT study in Stockholm, MLS LRs arrived before the ambulance in 45% of all suspected and 56% of all true OHCAs, performing CPR in 17 and 30%, respectively. This demonstrates the potential life-saving role mobile phones can play in ensuring the earliest possible CPR.
Early defibrillation significantly improves survival in OHCA. Sakai et al. [27] developed and tested a novel mobile AED map, which allowed easier access to registered AEDs via mobile phone in emergency situations. Even though their findings did not shorten time to accessing AEDs in a simulated emergency, it significantly reduced the travel distance to access and retrieve the AED. With further technological improvements in mobile location positioning, the system is likely to improve AED usage in emergency situations.
Mobile health in arrhythmias
The prevalence of arrhythmias is increasing with an aging population. Various methods exist for the detection and ongoing monitoring of arrhythmias, which is essential in enhancing patient safety and providing optimal care. Patient choice and compliance, clinical indication and cost all play a role in deciding the most appropriate method, as no single option is ideal in every clinical scenario [28]. Although continuous-wear external monitors and implantable devices with atrial ECG analysis capability are most accurate in arrhythmia detection, alternatives have to be available to provide choice and mitigate high cost, risks and compliance issues with these devices [28].
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with a risk of serious sequelae, including stroke, HF and mortality [29]. It can be asymptomatic or associated with nonspecific symptoms, and is often paroxysmal and short-lived. Accurate and easily accessible methods for detection and monitoring of AF are required [30]. McManus et al. [31] developed a novel iPhone 4S application analyzing pulsatile time series from recordings using the phone’s inbuilt camera placed directly on a finger, and tested it in a prospective cohort study involving 76 hospitalized patients with AF pre- and post-cardioversion. They were able to accurately distinguish between AF and normal sinus rhythm. Although further studies are required to test accuracy, acceptability and feasibility in a real-world environment and include a broader spectrum of arrhythmias, it holds promise as a readily available, user-friendly and cheap tool for assisting in diagnosis and monitoring of AF and eventually other arrhythmias, in real time and on a variety of mobile devices. Similarly, the US FDA-approved AliveCor iPhone ECG device [32], which has two metal electrodes on the back of the phone case that record heart rhythms when held by a user with both hands or placed against their chest, has a demonstrated 98% sensitivity and 97% specificity for automated diagnosis of AF against 12-lead ECG, making it a potential tool for AF screening in the wider population at low cost.
Gussak et al. [28] tested a wireless handheld monitor capable of 12-lead ECG reconstruction for arrhythmia detection rate compared with serial Holter monitoring in 25 patients following AF ablation procedure over a 6-month period. They demonstrated that wireless monitoring was more sensitive than periodic Holter monitoring in detecting AF and atrial flutter (AFL). The system detected both symptomatic and asymptomatic AF and AFL during daily remote monitoring (RM) and enabled differential diagnosis of symptomatic arrhythmias. Patients complied well with the monitoring schedule, suggesting acceptance of the device.
Lin et al. [33] described the development of an ambulatory, real-time and autoalarm intelligent telecardiology system, consisting of a lightweight, power-saving wireless ECG device that communicates via Bluetooth to a mobile device. The mobile device transmits an alert to a remote server if ECG abnormalities are detected and can trigger an emergency medical alarm system when required. In testing, they were able to correctly identify AF, sinus tachycardia, sinus bradycardia, wide QRS complex and cardiac asystole with approximately 94% accuracy in 30 patients tested. Similarly, HeartSaver [34] is a mobile medical device developed for real-time monitoring of a patient’s ECG, which automatically identifies several cardiac pathologies, including AF, MI and atrioventricular block through detection algorithms. When an abnormality is detected via the device’s sensor and ECG processing unit, a microcontroller sends a signal to a cell phone triggering an app to send a text message with the patient’s condition and location to a nominated recipient (e.g., carer or healthcare professional). The inclusion of the patient’s GPS location is a novel idea and important in locating individuals quicker in case of emergency.
In Italy, a study [35] of an emergency medical service (EMS) aimed at reducing Emergency Department (ED) time to diagnosis described the use of prehospital ECG assessment transmitted via mobile phone to a 24/7 telemedicine support hub, where a cardiologist provided ECG interpretation. ECGs were transmitted back to the EMS team or cardiologist on their smartphones. They found that, in 27,841 consecutive EMS patients screened with prehospital ECG for suspected heart disease between October 2004 and April 2006, the rate of at-home AF diagnosis increased from twofold (in 40-year-olds) up to sevenfold (in 70-year-olds). Of the 27,841 EMS patients, syncope was reported in 2,648 patients [36] and, in just under 2% of these patients, serious cardiac rhythm abnormalities requiring urgent cardiology admission were identified, leading to potential reductions in wrong diagnoses and treatment delays. Furthermore, Shacham et al. [37] reviewed medical records on 649 patients (1886 episodes) who contacted a 24/7 subscription telemedical system who had an episode of paroxysmal AF (PAF) over several years. All subscribers to the system were provided with a car-diobeeper capable of transmitting a three- or 12-lead ECG via a mobile phone, thereby giving them virtually unlimited access to the system’s medically staffed call center irrespective of their location. They found that 80% of PAF episodes could be managed successfully out of hospital, thereby ensuring prompt and safe management and reducing unnecessary visits to EDs and hospitalizations.
Cardiovascular implantable electronic devices (CIEDs) are being used for increasingly wider arrhythmia indications and many, including PMs, implantable cardioverter defibrillators (ICDs), implantable cardiac resynchronization therapy (CRT) devices, insertable cardiac monitors (ICMs), can be interrogated remotely [38]. Data downloaded from the device via the built-in transmitter are communicated via a landline or, increasingly, via mobile Global System for Mobile (GSM) communication networks and made available to the treating physician after analysis [38]. With increasing use of these devices comes a rising demand on cardiac services for device surveillance. Improvements in the technology have made RM a viable route to improving patient safety while also enhancing service delivery. RM enables faster clinical response times when abnormalities are detected [39], and also enables the patient to be a more active participant in their own care. Automated device status checks ensures enhanced safety and frees up clinical time as unnecessary clinic visits can be avoided.
Although several of the novel monitoring devices and systems described above have demonstrated potential to monitor AF and related arrhythmias, most require further study to demonstrate safety and efficacy in realworld application. However, they provide a glimpse of future possibilities, where mobility for patients will not be impaired by their health-monitoring devices, where they can receive immediate information about their current clinical status and where the burden on cardiac services can be reduced through early detection and intervention in a wide clinical application and at relatively low cost.
Mobile health in MI
MI remains a leading cause of morbidity and mortality worldwide [40]. Through the application of evidencebased therapies, post-MI survival rates have improved and led to an increase in the population of survivors at risk of a subsequent event and who are therefore candidates for secondary prevention [41]. Most mobile health studies related to MI have looked at acute, prehospital ST-elevation MI (STEMI) care and demonstrated significant improvements in outcomes.
Early reperfusion improves outcomes following STEMI and many initiatives have focused on reducing time-to-treatment or door-to-balloon time (DBT) [42]. In-hospital STEMI care systems have been successfully and widely implemented, and small improvements are unlikely to bring further significant benefit [42]. The prehospital STEMI care system now holds the main opportunity for reducing total ischemic time and time-to- treatment and hence for improving outcomes [42]. In their review, Al-Zaiti et al. [43] found prehospital diagnosis of STEMI via ECG can potentially shorten DBT, decrease infarct size, limit reduction in ejection fraction, improve specialized care access, reduce hospital length of stay (LOS), reduce unnecessary referrals and costs and, in asymptomatic middle-age adults, improve risk stratification. Several telehealth initiatives have trialled prehospital ECG transmission using mobile technologies and similarly found reduced DBT [44]. Brunetti et al. [45] found mobile-transmitted ECGs by EMS improved rates of immediately diagnosed MI, importantly including for atypical presentation, in a large elderly population accessing emergency services, resulting in reduced time to treatment. Recent advances in technology have also enabled accurate interpretation of prehospital ECGs by cardiologists on smartphones in real time [46], thereby enabling appropriately triaged MI transfers directly to percutaneous coronary intervention (PCI) centers and significantly decreasing DBT [47]. The STAT-MI trial [48] used a fully automated wireless network for communicating automatic 12-lead ECGs to offsite cardiologists’ smartphones, which facilitated direct triage of STEMI patients to a cardiac catheterization laboratory. They found patients had shorter DBT, significantly lower peak troponin and creatine phosphokinase, higher left ventricular ejection fractions and shorter hospital LOS compared with control patients. Automated systems such as STAT-MI can go some way to addressing transport delays and inconvenience to EMS caused by ECG transmission issues [43]. Integrating EMR access and historical ECG details to support ECG interpretation on cardiologists’ smartphones [43] can also improve diagnostic accuracy and help resolve potential high false-positive rates of activating PCI laboratories unnecessarily.
There is extensive evidence that secondary prevention programs to reduce CVD risk factors can improve CVD morbidity and mortality in MI survivors [41,49,50], with even modest treatment effects providing costeffective benefits. CR as a recommended approach to secondary prevention is not limited to patients with recent MI or acute coronary syndrome – it is also indicated for those with chronic stable angina, HF and following coronary artery bypass surgery, PCI, valve surgery or cardiac transplantation [51]. Although these programs are traditionally delivered from a hospital outpatient exercise clinic, cost and access constraints have necessitated the development of alternative homecare CR models and the utilization of telemonitoring [41]. Mobile devices offer new opportunities for delivering CR through SMS messaging, journaling apps, connected measurement devices, coaching and continuous and RM and supervision of patients [9].
The Care Assessment Platform (CAP) is a CR model that uses a journaling app on patients’ smartphones to collect health-related information (e.g., physical activity, food intake, weight, BP, stress and amount of sleep), which is then graphically displayed to both the patient and their CR coordinator to monitor the patient’s achievements against individually set CR goals over time [3]. Early findings of this study have been reported to show significantly improved CR completions rates [52]. New innovations like HeartCycle’s guided exercise (GEX) system [53], where patients wear a shirt with embedded sensors that can live-stream health parameters (e.g., ECG, heart rate [HR], breathing rate and activity) via a mobile device, will further support exercise-based CR, independent of location.
Extending the study of the use of mobile technologies to other types of MIs and wider care settings is likely to highlight many new opportunities for improving patient outcomes and service delivery. CR seems a likely candidate for significantly benefiting from the use of these technologies, and publications of outcomes from RCTs evaluating its utilization are eagerly anticipated.
Mobile health in HF
HF is a life-threatening progressive disease and is often associated with dramatically diminished quality of life (QoL) and high levels of comorbidity [54]. It places a huge economic burden on the healthcare system, especially due to high rehospitalization rates [55].
Although some arrhythmia detecting and monitoring initiatives discussed above could apply to HF, the majority of HF mobile health studies have focused on the long-term management of nonpharmacological (e.g., fluid and dietary sodium restriction, physical activity and weight gain) and pharmacological therapies that could be delivered in patients’ homes. This focus has been driven by poor adherence to self-care, with previous studies showing that the least frequently performed self-care behaviors include daily weighing, restricting fluid intake, informing the care team of HF-related symptoms and recognizing weight gain [56].
A few studies have explored the feasibility of portable home-monitoring devices wirelessly connected to a smartphone to monitor HF patients [57–59] and demonstrated high transmitted ECG diagnostic quality and integrity of BP and bodyweight measurements. Another study validated remote echocardiographic interpretation on a website-enabled smartphone utilizing software designed for this purpose against workstation reading by expert echocardiographers, demonstrating minimal loss of diagnostic accuracy when using the smartphone [60].
Piotrowicz et al. [59] demonstrated that home-based telemonitored CR in HF was safe, even when patients felt unwell and had episodes of AF, through monitoring ECG fragments recorded automatically on a telemonitoring device during CR and transmitted to a monitoring center via a mobile phone.
Weight monitoring is particularly important for patients with HF, as rapid weight gain is strongly associated with hospital admission [61] and high mortality [62]. Daily weight monitoring is a key recommendation for self-management of congestive heart failure in major guidelines [63–65].
Despite these recommendations, a review found that only approximately 40% of patients regularly monitored their bodyweight, and approximately a third of patients did not take any action when they gained weight [66]. To address this issue, a major component of mobile health-enabled HF care programs is to assist patients in adhering to weight management.
Seto and colleagues [67] tested a rule-based clinical decision-support system aimed at improved self-care and clinical management that generated alerts and instructions based on patients’ weight, BP, HR and symptoms. Over the 6-–month RCT, 1620 alerts were generated in 50 HF patients, leading to various clinical interventions, including 105 medication interventions. Findings indicated that using the HF rule set improved patients’ QoL and self-care. Although their trial was underpowered to detect differences in hospitalization, mortality and ED visits between groups, the telemonitoring group had high levels of adherence to daily weighing (70% completed at least 80% of possible daily readings) and showed significant improvements in QoL and selfcare maintenance compared with the control group. Another recent study used weight monitoring from a wireless weight scale, transmitted to a mobile phone, combined with an algorithm to improve sensitivity in predicting clinical deterioration in 87 HF patients [68]. The HeartPhone algorithm, based on alerts generated from moving averages of daily weight data deviations above the norm for each individual patient, predicted HF events with significantly more sensitivity than guideline weight change methods. The TEMA-HF 1 RCT [69] evaluated the impact of intensive management of 160 HF patients through a telemonitoring-facilitated collaboration between general practitioners (GPs) and a HF clinic. The intervention group used electronic devices that automatically transmitted daily weight, BP and HR data via mobile phones to a database. Automated alerts to the GP and HF clinic were generated when predefined limits were exceeded to initiate clinical interventions. They found significantly lower all-cause mortality and reduced number of days lost to hospitalization, death or dialysis in the telemonitored HF patients compared with usual care.
However, not all mobile-enabled telemonitoring studies have confirmed these improved outcomes in terms of hospitalization rates, morbidity and mortality. Koehler et al. [70] found that RM had no significant effect on HF hospitalization, cardiovascular death or all-cause mortality in 710 stable HF patients, despite good compliance with daily data transfers, compared with usual care. Further subgroup analyses [71] showed improved outcomes in specific HF patient groups, but further studies are required to identify those who stand to benefit most. Although meta-analyses [72,73] of HF telemonitoring systems have been performed to overcome underpowered RCT studies, their observations have not evaluated the effect of the mobile health components on outcomes of hospitalization, morbidity and mortality. On the other hand, approaches in HF telemedical management and mobile health are based primarily on ongoing monitoring and early detection of clinical deterioration, with corresponding timely interventions, in which case the focus on keeping HF patients maintained and out of hospital is justified.
Application in interventional cardiology
The studies and applications mentioned in this paper provide a glimpse on what these technologies and applications offer interventional cardiology. The educational and networking potential for both cardiologists and patients, including reducing costs, improving access and accelerating learning, is only starting to emerge. Early and accurate triage, realized through interventional cardiologists interpreting remotely transmitted ECGs with reference to patients’ medical records on their smartphones, will lead to further improvements in DBT and better patient outcomes [43–48]. Appropriately triaged patients can be transferred directly to cardiac intervention laboratories, thereby also reducing the load on other acute services and limiting unnecessary laboratory activation [43] with its ensuing costs. Coordination of laboratory services and intraprocedure access to second specialist opinion using mobile applications could further improve efficiency and safety. Postacute monitoring could be carried out remotely, enabling earlier discharge from hospital. Patients’ physiological and other data recorded remotely and displayed through a secure portal can then be overseen by the clinician on their smartphone or tablet, with alerting and decision-support applications providing further assistance. Mobile technology provides opportunities for remote delivery of rehabilitation programs [3] and long-term chronic disease and medication compliance monitoring. Furthermore, it enables remote implantable device surveillance and treatment [38,39]. Mobile applications can also improve efficiency and support health providers in their scheduling, billing and patient communication efforts.
Challenges & considerations in using mobile health applications
Despite the exponential growth in mobile health and the use of, there are currently few guidelines to support organizations in planning, implementation and managing the use of mobile devices, and regulatory frameworks remain immature. Data security, privacy, interoperability and integration standards, as well as medicolegal liability, need to be addressed within the area of policy, to ensure mobile health is not hampered by the same issues that have hindered much of the adoption of eHealth. Furthermore, with the rapid proliferation of health apps, guidance on app development standards, selection criteria and safe usage are urgently required.
Organizations wishing to support the usage of mobile devices by their healthcare professionals need to ensure there have adequate procedures and policies in place to protect patients. The US Department of Health and Human Services [74] recently issued information to help organizations manage the usage of mobile devices, yet legitimate concerns remain about the security of patients’ information when using mobile health technologies. Privacy and data security measures, such as user authentication configuration, encryption, remote wiping/disabling activation and installation of firewall and other security software, are required at all stages of data transmission [74]. Only approved medical devices should be used to ensure data reliability. Furthermore, users need to be educated on the dangers of using file sharing applications and public Wi-Fi connections, as well as the importance of maintaining physical control of their devices and the avoidance of local data storage on mobile devices [74]. Organizations need to safeguard all interactions between mobile technologies and patient health data within their internal networks, and also need to ensure data are appropriately captured and stored within patient records.
Most mobile health apps, despite their increasing popularity among both consumers and health professionals, lack evidence and are developed without involvement of medical professionals [75], thereby potentially compromising safety. To address this, the FDA recently published a draft guideline for medical apps [76], proposing that an app that transforms an accessory or mobile platform for disease prevention, diagnosis, monitoring or management is considered and regulated as a medical device. These apps, unlike wellness apps, would have to demonstrate health benefits, reduced medical errors and maintenance of patient safety during mandatory clinical validation, and would carry FDA certification. However, there are many apps being used in clinical practice that would not qualify as a medical device, yet would still affect patient care, and therefore a requirement for broader quality guidance and official certification remains to support professional and lay users in making better informed app choices. Developments, such as the UK National Health Service’s Library of Health Apps [77], reviewed for clinical safety, and websites providing health professionals the opportunity to suggest and rate medical apps are starting to address this issue. However, in the absence of wide validation and certification, it remains essential for users to be educated about health apps’ potential risks and the importance of researching apps before using them.
Conclusion
Although the normalization of mobile health in cardiac care has been slow, several studies have demonstrated its potential in delivering improved and appropriate personalized care at every stage of the care journey, as well as alleviating some of the healthcare issues resulting from the increasing prevalence of chronic cardiac disease. The main body of evidence found related to the application of mobile health in cardiac arrest, arrhythmias, MI and HF.
There is a need for more evidence around how to optimally structure and organize services around mobile health, as historically studies have focused more on the technology than services. However, several studies have found improvements in effectiveness and efficiency of health services, as well as more efficient health spend. These include improved diagnostic accuracy and speed, shorter time-to-treatment and DBT reductions, improved triage and utilization of specialist facilities, less inappropriate outpatient visits, reduced LOS and rehospitalization rates, and better use of health service resources. RM-utilizing mobile technologies, often supported by automated alerting systems, and ensuing early care interventions, have also been shown to reduce unnecessary ED visits, referrals and hospitalizations.
Studies have also shown patients to potentially benefit significantly from the application of mobile health in cardiology, with improved outcomes such as decreased infarct size, smaller reductions in ejection fractions, lower peak troponin and creatine phosphokinase, improved QoL and self-care, and reduced all-cause and cardiac mortality. Importantly, mobile health offers patients choice and empowers them to be better informed and supported in managing their health in their own environment.
In order to take advantage of these potential benefits of mobile health applications, appropriate regulatory and governance measures need to be integrated within the service delivery model. Healthcare professionals and consumers need to remain vigilant in their selection and usage of mobile applications while the relatively new statutory regulatory frameworks are developed and implemented.
Future perspective
With the high accessibility of mobile technologies and the innovative ways in which they are being utilized, mobile health will continue to transform the delivery of cardiac care. As more evidence emerges about safety and efficacy, and challenges such as data management, privacy issues, and technology interoperability, reliability and governance are addressed, cardiology will continue to be leaders in healthcare’s adoption of mobile health. Although wide and affordable screening may cause a flood of new patients requiring cardiac services, it is also likely to save lives and provide more opportunities for earlier interventions. Patients will experience increasingly individualized care, supported by social health networks, smart automated home-monitoring systems and health record access, thereby empowering them to take better control of their own health.
Adoption of mobile health has been hampered by the lack of a robust evidence base around it, and future processes of clinical validation will need to be faster and flexible to not only keep pace with rapid technological advances, but crucially to provide a framework for mobile healthcare service delivery and identify patient populations who will benefit most from such services. Although aspects of care, such as cardiac interventional procedures and intensive care management, will remain confined to medical institutions with specialized equipment and staff, mobile health will radically alter how cardiac care is delivered to help address some of the pressures on the healthcare system and to provide more patient-centric care. In acute care, mobile health will ensure optimal care is delivered quicker and in the most appropriate facilities. As mobile health service models and organization matures and becomes embedded in routine practice, many services, such as outpatient follow-up, postinterventional care and CR, currently delivered in hospital, will be moved into the community and patients’ homes. With increasingly simple and affordable home monitoring, earlier discharge following interventional procedures becomes safer and another route to freeing up hospital beds. It also enables smarter and more effective monitoring in CR and long-term follow-up of patients with chronic conditions, including HF, arrhythmias and post-MI, thereby enabling early interventions when required and hence reducing readmission rates, as well as providing better patient care. As the technology improves, smaller CIEDs will become more routinely used and for wider indications, with increasingly smart, automated alert systems and appropriate care services implemented in parallel and with real-time feedback provided to patients and their care team on their mobile devices. For interventional cardiologists specifically, future mobile health technologies offer potential to remotely monitor smarter stents, with inbuilt technology to monitor arterial patency longterm. Furthermore, as capacitive touchscreen technologies mature, remote ongoing monitoring of anticoagulation therapies will become the norm, with timely dosage adjustments and SMS prescribing facilitated through smartphones when required.
With appropriate education of the workforce and adequate technical support services, effective and appropriately directed mobile health delivery would relieve the burden on current healthcare systems, provide a more efficient and effective use clinical resources, and enable better patient care.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- Cleland J, Barrett D. Future directions in cardiovascular care. European Cardiology 6(3), 30–32 (2010).
- Prgomet M, Georgiou A, Westbrook JI. The impact of mobile handheld technology on hospital physicians’ work practices and patient care: a systematic review. J. Am. Med. Inform. Assoc. 16(6), 792–801 (2009).
- Walters D, Sarela A, Fairfull A et al. A mobile phone-based care model for outpatient cardiac rehabilitation: the care assessment platform (CAP). BMC Cardiovasc. Disord. 10, 5 (2010).
- WHO. mHealth: New Horizons For Health Through Mobile Technologies. WHO, Geneva, Switzerland (2011).
- WHO. Management of patient information – trends and challenges in member states. In: Global Observatory for eHealth Series – Volume 6. WHO, Geneva, Switzerland (2012).
- The Royal Australian & New Zealand College of Psychiatrists. Technical Specifications for Telepsychiatry. Telepsychiatry, Melbourne, Australia (2013).
- Augestad KM, Lindsetmo RO. Overcoming distance: video-conferencing as a clinical and educational tool among surgeons. World J. Surg. 33(7), 1356–1367 (2009).
- Martinez-Perez B, De La Torre-Diez I, Lopez-Coronado M. Mobile health applications for the most prevalent conditions by the World Health Organization: review and analysis. J. Med. Internet Res. 15(6), e120 (2013).
- Klasnja P, Pratt W. Healthcare in the pocket: mapping the space of mobile-phone health interventions. J. Biomed. Inform. 45(1), 184–198 (2012).
- Kim J, Wang Z, Cai W, Feng D. Multimedia for future health – smart medical home. In: Biomedical Information Technology. Academic Press Elsevier, CA, USA (2008).
- Garcia E, Ding H, Sarela A, Karunanithi M. Can a mobile phone be used as a pedometer in an outpatient cardiac rehabilitation program? Presented at: The 2010 IEEE/ICME International Conference on Complex Medical Engineering. 13–15 July 2010.
- Xia Y, Cheung V, Garcia E, Ding H, Karunaithi M. Development of an automated physical activity classification application for mobile phones. Stud. Health Technol. Inform. 168, 188–194 (2011).
- Van Der Spek S, Van Schaick J, De Bois P, De Haan R. Sensing human activity: GPS tracking. Sensors 9(4), 3033–3055 (2009).
- Hallstrom AP, Ornato JP, Weisfeldt M et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N. Engl. J. Med. 351(7), 637–646 (2004).
- Cummins RO, Ornato JP, Thies WH et al. Improving survival from sudden cardiac arrest: the ‘chain of survival’ concept. A statement for health-professionals from the advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation 83(5), 1832–1847 (1991).
- Kovic I, Lulic I. Mobile phone in the chain of survival. Resuscitation 82(6), 776–779 (2011).
- Wu O, Briggs A, Kemp T et al. Mobile phone use for contacting emergency services in life threatening circumstances. J. Emerg. Med. 42(3), 291–298 (2012).
- Semeraro F, Taggi F, Tammaro G, Imbriaco G, Marchetti L, Cerchiari EL. iCPR: a new application of high-quality cardiopulmonary resuscitation training. Resuscitation 82(4), 436–441 (2011).
- Ahn JY, Cho GC, Shon YD, Park SM, Kang KH. Effect of a reminder video using a mobile phone on the retention of CPR and AED skills in lay responders. Resuscitation 82(12), 1543–1547 (2011).
- Bolle SR, Johnsen E, Gilbert M. Video calls for dispatcherassisted cardiopulmonary resuscitation can improve the confidence of lay rescuers – surveys after simulated cardiac arrest. J. Telemed. Telecare 17(2), 88–92 (2011).
- Yang CW, Wang HC, Chiang WC et al. Impact of adding video communication to dispatch instructions on the quality of rescue breathing in simulated cardiac arrests – a randomized controlled study. Resuscitation 78(3), 327–332 (2008).
- Lee JS, Jeon WC, Ahn JH, Cho YJ, Jung YS, Kim GW. The effect of a cellular-phone video demonstration to improve the quality of dispatcher-assisted chest compression-only cardiopulmonary resuscitation as compared with audio coaching. Resuscitation 82(1), 64–68 (2011).
- Choa M, Cho J, Choi YH, Kim S, Sung JM, Chung HS. Animation-assisted CPRII program as a reminder tool in achieving effective one-person-CPR performance. Resuscitation 80(6), 680–684 (2009).
- Merchant RM, Abella BS, Abotsi EJ et al. Cell phone cardiopulmonary resuscitation: audio instructions when needed by lay rescuers: a randomized, controlled trial. Ann. Emerg. Med. 55(6), 538–543 (2010).
- Low D, Clark N, Soar J et al. A randomised control trial to determine if use of the iResus© application on a smart phone improves the performance of an advanced life support provider in a simulated medical emergency. Anaesthesia 66(4), 255–262 (2011).
- Ringh M, Fredman D, Nordberg P, Stark T, Hollenberg J. Mobile phone technology identifies and recruits trained citizens to perform CPR on out-of-hospital cardiac arrest victims prior to ambulance arrival. Resuscitation 82(12), 1514–1518 (2011).
- Sakai T, Iwami T, Kitamura T et al. Effectiveness of the new ‘Mobile AED Map’ to find and retrieve an AED: a randomised controlled trial. Resuscitation 82(1), 69–73 (2011).
- Gussak I, Vukajlovic D, Vukcevic V et al. Wireless remote monitoring of reconstructed 12-lead ECGs after ablation for atrial fibrillation using a hand-held device. J. Electrocardiol. 45(2), 129–135 (2012).
- Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary care. The National Collaborating Centre for Chronic Conditions, London, UK (2006).
- Lee J, Reyes BA, McManus DD, Mathias O, Chon KH. Atrial Fibrillation Detection using a Smart Phone. Presented at: 34th Annual International Conference of the IEEE Engineering-in-Medicine-and-Biology-Society (EMBS). San Diego, CA, USA, 28 August–1 September 2012.
- McManus DD, Lee J, Maitas O et al. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 10(3), 315–319 (2013).
- Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb. Haemost. 110(2), 213–222 (2013).
- Lin C-T, Chang K-C, Lin C-L et al. An Intelligent Telecardiology System Using a Wearable and Wireless ECG to Detect Atrial Fibrillation. IEEE Trans. Inf. Technol. Biomed. 14(3), 726–733 (2010).
- Sankari Z, Adeli H. HeartSaver: a mobile cardiac monitoring system for auto-detection of atrial fibrillation, myocardial infarction, and atrio-ventricular block. Comput. Biol. Med. 41(4), 211–220 (2011).
- Brunetti ND, De Gennaro L, Pellegrino PL, Dellegrottaglie G, Antonelli G, Di Biase M. Atrial fibrillation with symptoms other than palpitations: incremental diagnostic sensitivity with at-home tele-cardiology assessment for emergency medical service. Eur. J. Prev. Cardiol. 19(3), 306–313 (2012).
- Brunetti ND, De Gennaro L, Dellegrottaglie G, Antonelli G, Amoruso D, Di Biase M. Prevalence of cardiac arrhythmias in pre-hospital tele-cardiology electrocardiograms of emergency medical service patients referred for syncope. J. Electrocardiol. 45(6), 727–732 (2012).
- Shacham J, Birati EY, Malov N et al. Telemedicine for diagnosing and managing paroxysmal atrial fibrillation in outpatients. The phone in the pocket. Int. J. Cardiol. 157(1), 91–95 (2012).
- Crossley GH, Poole JE, Rozner MA et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the Perioperative Management of Patients with Implantable Defibrillators, Pacemakers and Arrhythmia Monitors: Facilities and Patient Management This document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 8(7), 1114–1154 (2011).
- Hauck M, Bauer A, Voss F, Weretka S, Katus HA, Becker R. “Home monitoring” for early detection of implantable cardioverter-defibrillator failure. Clin. Res. Cardiol. 98(1), 19–24 (2009).
- WHO. Global Atlas on Cardiovascular Disease Prevention and Control. WHO, Geneva, Switzerland (2011).
- Suri MFK, Nasar A, Qureshi AI, Kirmani JF, Divani AA. Ineffective secondary prevention in survivors of stroke and cardiovascular events in the US population: report from the National Health and Nutrition Examination Survey 1999–2002. Neurology 64(6), A309–A309 (2005).
- Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N. Engl. J. Med. 369(10), 889–892 (2013).
- Al-Zaiti SS, Shusterman V, Carey MG. Novel technical solutions for wireless ECG transmission & analysis in the age of the internet cloud. J. Electrocardiol. 46(6), 540–545 (2013).
- Drew BJ, Sommargren CE, Schindler DM, Benedict K, Zegre-Hemsey J, Glancy JP. A simple strategy improves prehospital electrocardiogram utilization and hospital treatment for patients with acute coronary syndrome (from the ST SMART study). Am. J. Cardiol. 107(3), 347–352 (2011).
- Brunetti ND, De Gennaro L, Amodio G et al. Telecardiology improves quality of diagnosis and reduces delay to treatment in elderly patients with acute myocardial infarction and atypical presentation. Eur. J. Cardiovasc. Prev. Rehabil. 17(6), 615–620 (2010).
- Gonzalez MA, Satler LF, Rodrigo ME et al. Cellular videophone assisted transmission and interpretation of prehospital 12-lead electrocardiogram in acute ST-segment elevation myocardial infarction. J. Interv. Cardiol. 24(2), 112–118 (2011).
- Sejersten M, Sillesen M, Hansen PR et al. Effect on treatment delay of prehospital teletransmission of 12-lead electrocardiogram to a cardiologist for immediate triage and direct referral of patients with ST-segment elevation acute myocardial infarction to primary percutaneous coronary intervention. Am. J. Cardiol. 101(7), 941–946 (2008).
- Sanchez-Ross M, Oghlakia G, Maher J et al. The STAT-MI (ST-segment analysis using wireless technology in acute myocardial infarction) trial improves outcomes. JACC Cardiovasc. Interv. 4(2), 222–227 (2011).
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 4, CD001800 (2000).
- Taylor Rs, Watt A, Dalal Hm et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int. J. Cardiol. 119(2), 196–201 (2007).
- Kwan G, Balady GJ. Cardiac rehabilitation 2012: advancing the field through emerging science. Circulation 125(7), e369–373 (2012).
- Varnfield M, Karunanithi MK, Ding H et al. Technology based home-care model improves outcomes of uptake, adherence and health in cardiac rehabilitation. Presented at: European Society of Cardiology Congress 2012. Munich, Germany, 25–29 August 2012.
- Skobel E, Martinez-Romero A, Scheibe B et al. Evaluation of a newly designed shirt-based ECG and breathing sensor for home-based training as part of cardiac rehabilitation for coronary artery disease. Eur. J. Prev. Cardiol. doi:10.1177/2047487313493227 (2013) (Epub ahead of print).
- Pagley PR, Beller GA, Watson DD, Gimple LW, Ragosta M. Improved outcome after coronary bypass surgery in patients with ischemic cardiomyopathy and residual myocardial viability. Circulation 96(3), 793–800 (1997).
- Lee WC, Chavez YE, Baker T, Luce BR. Economic burden of heart failure: a summary of recent literature. Heart Lung 33(6), 362–371 (2004).
- Barnason S, Zimmerman L, Young L. An integrative review of interventions promoting self-care of patients with heart failure. J. Clin. Nurs. 21(3–4), 448–475 (2012).
- Scherr D, Zweiker R, Kollmann A, Kastner P, Schreier G, Fruhwald FM. Mobile phone-based surveillance of cardiac patients at home. J. Telemed. Telecare 12(5), 255–261 (2006).
- Winkler S, Schieber M, Lucke S et al. A new telemonitoring system intended for chronic heart failure patients using mobile telephone technology – feasibility study. Int. J. Cardiol. 153(1), 55–58 (2011).
- Piotrowicz E, Jasionowska A, Banaszak-Bednarczyk M, Gwilkowska J, Piotrowicz R. ECG telemonitoring during home-based cardiac rehabilitation in heart failure patients. J. Telemed. Telecare 18(4), 193–197 (2012).
- Choi BG, Mukherjee M, Dala P et al. Interpretation of remotely downloaded pocket-size cardiac ultrasound images on a web-enabled smartphone: validation against workstation evaluation. J. Am. Soc. Echocardiogr. 24(12), 1325–1330 (2011).
- Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 116(14), 1549–1554 (2007).
- Fiutowski M, Waszyrowski T, Krzeminska-Pakula M, Kasprzak JD. Pulmonary edema prognostic score predicts in-hospital mortality risk in patients with acute cardiogenic pulmonary edema. Heart Lung 37(1), 46–53 (2008).
- Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ. 2011 Update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med. J. Aust. 194(8), 405–409 (2011).
- Multidisciplinary Care for People with Chronic Heart Failure. Principles and Recommendations for Best Practice. National Heart Foundation of Australia, Australia (2011).
- McMurray JJ, Adamopoulos S, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 33(14), 1787–1847 (2012).
- Van Der Wal MHL, Jaarsma T, Van Veldhuisen DJ. Noncompliance in patients with heart failure; how can we manage it? Eur. J. Heart Fail. 7(1), 5–17 (2005).
- Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J. Med. Internet Res. 14(1), e31 (2012).
- Ledwidge MT, O’hanlon R, Lalor L et al. Can individualized weight monitoring using the HeartPhone algorithm improve sensitivity for clinical deterioration of heart failure? Eur. J. Heart Fail. 15(4), 447–455 (2013).
- Dendale P, De Keulenaer G, Troisfontaines P et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur. J. Heart Fail. 14(3), 333–340 (2012).
- Koehler F, Winkler S, Schieber M et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the Telemedical Interventional Monitoring in Heart Failure Study. Circulation 123(17), 1873–1880 (2011).
- Koehler F, Winkler S, Schieber M et al. Telemedicine in heart failure: pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int. J. Cardiol. 161(3), 143–150 (2012).
- Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JGF. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur. J. Heart Fail. 13(9), 1028–1040 (2011).
- Clarke M, Shah A, Sharma U. Systematic review of studies on telemonitoring of patients with congestive heart failure: a meta-analysis. J. Telemed. Telecare 17(1), 7–14 (2011).
- HealthIT.gov. Managing Mobile Devices in Your Health Care Organization. www.healthit.gov/sites/default/files/fact-sheet-managing mobile- devices-in-your-health-care-organization.pdf
- Buijink AW, Visser BJ, Marshall L. Medical apps for smartphones: lack of evidence undermines quality and safety. Evid. Based Med. 18(3), 90–92 (2013).
- US FDA. Mobile Medical Applications. www.fda.gov/medicaldevices/ products and medical procedures/connected health/ mobilemedical applications/default.htm
- NHS Choices. Health Apps Library. http://apps.nhs.uk/
• Concludes a strong clinical need to apply modern technologies in cardiovascular care and suggests clinical need is the key driver of using mobile applications in cardiac care.
• Presents an increasing use of mobile devices among healthcare professionals for clinical practice. The trend reflects the broad acceptance of using mobile devices and applications for clinical studies.
• First paper to describe a mobile healthcare delivery model for cardiac rehabilitation.
• Well-recognized paper establishing the mobile health, and providing the prospect of using mobile devices in health/ clinical practice.
• This paper reviews typical mobile applications and relevant intervention strategies in health/clinical practice.
• Demonstrates some novel implantable devices and applications recently adopted to prevent fatal cardiac events.
• This randomized controlled trial (RCT) tested portable monitor-defibrillators equipped with special software for acquiring and automatically transmitting prehospital ECGs in predominantly rural country to the destination hospital. Increased paramedic use of prehospital ECGs and decreased hospital treatment times for ACS are feasible with a simple approach tailored to characteristics of a local geographic region.
• Evaluated a mobile health-based care service for patients with heart failure through a RCT, which is the largest RCT found in the review. Controversially, the evaluation found no significant benefit from its mobile health service in terms of reducing hospitalization, morbidity and mortality.