Research Article - International Journal of Clinical Rheumatology (2016) Volume 11, Issue 6
Molecular signatures in peripheral blood mononuclear cells with osteoarthritis
- Corresponding Author:
- Varsha Gupta
Rheumatology Laboratory, Department of Biotechnology
Chhatrapati Shahu Ji Maharaj University
Kanpur, Uttar Pradesh, India
E-mail: varsha5@yahoo.co.uk
Abstract
Osteoarthritis (OA) is a slow progressive joint disorder in which the joint matrix undergoes dynamic remodeling procedure where the activities of degradative and synthetic enzymes are balanced which in turn maintains the cartilage volume. In OA, the net shift is towards degradation resulting in loss of collagen and proteoglycans from the matrix. However, the etiology of OA remains elusive with inflammation having an important role therefore the present investigation aims to study the gene expression in OA versus control by DNA microarray technology. The study shows upregulated gene expression for apoptotic cell death cascade mediators, inflammatory cytokines, complement components and matrix metalloproteinases (MMPs). Higher expression of anti-inflammatory cytokines and tissue inhibitors of metalloproteinases was observed. Unaltered or lower expression was observed for the components involved in bone metabolism as TGF-β and BMPs. Apoptotic mediators and inflammatory genes are upregulated in OA as compared to genes involved in anabolic and bone turnover pathway, thus shifting the balance towards matrix loss and OA. The higher expression of MMPs, apoptotic death mediators along with cytokines are important to cause cartilage destruction. Therefore therapeutic intervention targeting these may result in better outcome to control the progression of OA.
Keywords
osteoarthritis, hypertension, inflammation, interleukin, complement
Abbreviations
OA, osteoarthritis; TKR, total knee replacement; HTO, high tibial osteotomy; TNF, tumor necrosis factor; IL, interleukin
Introduction
Osteoarthritis (OA) is a common multifactorial disease with unknown etiology characterized by progressive degradation and loss of articular cartilage. Among adults 60 years or above the prevalence of symptomatic knee OA is approximately 10% in men and 13% in women. By 2040, an estimated 78 million Americans ages 18 years or older are projected to have doctor-diagnosed arthritis [1], 49.7% of adults ≥ 65 years reported doctor-diagnosed arthritis from 2010 to 2012 [2]. An estimated 62% of adults with arthritis are <65 years old. An estimated 294,000 children under age 18 have some form of arthritis or rheumatic condition; this represents approximately 1 in every 250 children in the US [3].
OA is generally classified as idiopathic (primary) or secondary. Secondary OA develops in joints with preexisting structural abnormalities. In primary OA, no trauma or other predisposing factors are identified, and intrinsic alterations of the articular tissue, or response to normal cumulative stresses, are presumed responsible.
OA may be a consequence of mechanical and biological events that affects the joints and cause imbalance in the anabolic and catabolic processes within the articular cartilage. The factors which are responsible for increasing susceptibility to OA include obesity, aging, genetics, presence of systemic disorder, trauma etc. In normal people OA might initiate due to minor damage to the joint tissue by physical forces as a single event of trauma or by repeated microtrauma due to altered mechanical loading of the joint [4]. However, pathogenesis of OA is complex where multiple factors are involved in its pathogenesis [5].
Involvement of innate and adaptive immune responses in OA is being accepted in recent epidemiological studies on large number of OA patients because of increased cartilage damage [6], pain and inflammatory synovium/synovitis. Infiltrates of T-cells, B-cells, macrophages [7] and activated complement components [8] are observed in OA synovial tissue. Immunoglobulins and immune complexes against cartilage components are detected in cartilage, synoium and plasma in OA patients. Thus OA shows the pattern which resembles RA [9] but is less aggressive than RA. Increased mononuclear cell infiltration and overexpression of mediators were found to be present in early OA [10]. However, it is not clear how these proinflammatory cytokines, apoptosis mediators, anti inflammatory cytokines interact and orchestrate the onset of progressive disorder as OA.
Therefore the present investigation aims to study the expression of various components for their involvement in OA versus control. This study would help in understanding the diverse factors which leads to pathogenesis of OA for effective therapeutic management.
Materials and methods
Patient selection for gene expression profiling
Three patients (all females) with knee OA and 3 asymptomatic independent controls (all females) were recruited from OPD of Community Health Centre, India. OA patients were screened according to radiological grading [11]. VAS score and ACR classification was followed for classification of OA [12]. Patients with OA were included who had grade II OA, knee pain (asymmetrical) of more than 6 months, stiffness (<30 min), swelling, crepitation, tenderness on medial side of joint, X-ray had >1/3 decrease in joint space and/or presence of osteophytes and decreased range of motion in their knee joint. Their ligament stability (anterior cruciate, posterior cruciate) was normal. The participants who had trauma, any other disease of joint, smokers and obese were excluded. Participants were matched for sex, age, weight and height (body mass index). None of the controls and patients had any comorbid disease.
RNA extraction and microarray analysis
Total RNA was extracted from whole blood with Qiagen RNA extraction kit according to manufacturer’s instructions. Concentration of RNA was measured spectrophotometrically and its integrity was checked by agarose gel electrophoresis. This was used as a starting template to synthesize double-stranded cDNA with random hexamers tagged with T7 promoter sequence. The fragmented DNA was labeled and used for overnight hybridization with Gene ST 1.0 arrays, followed by washing, staining and scanning.
Microarray analysis was done by using Affymetrix Human Gene 1.0 ST arrays. The data QC and RMA normalization was performed for the arrays as recommended by Affymetrix. A fold change of ± 1 was used to select up and down regulated probe sets. The QC analysis was carried out using Affymetrix Expression Console (EC). The statistical analysis was performed using R-programming language and the biological analysis was carried out using GenowizTM software.
Result
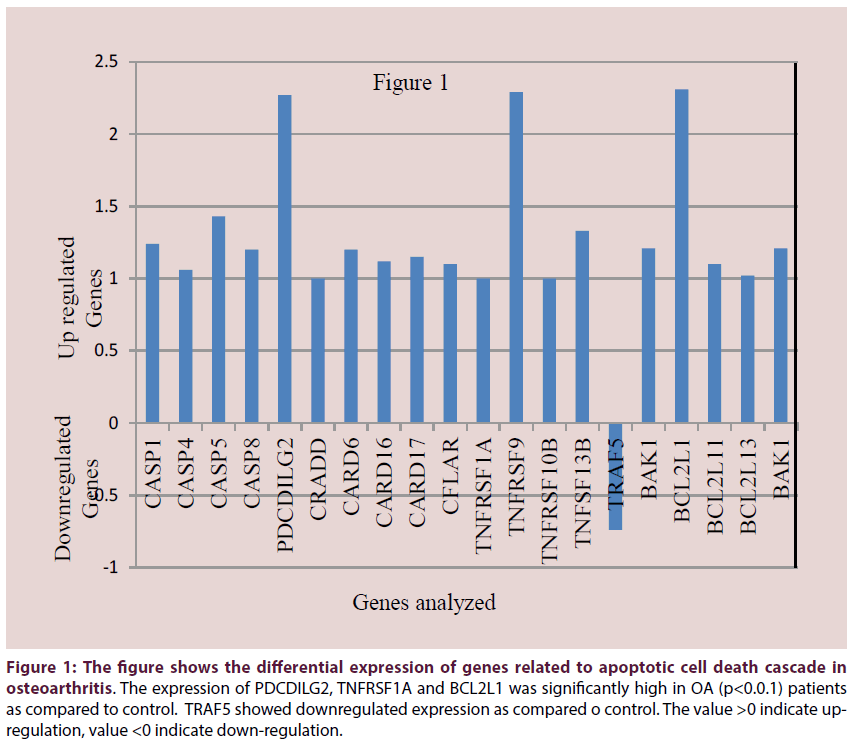
The OA patients and controls were matched for age, BMI and sex. None of the participants had any comorbid conditions. The expression profile obtained by DNA microarray analysis revealed differential expression of apoptotic genes, genes for cytokines, complement components, matrix metalloproteases and genes involved in bone metabolism which have known and suspected role in OA pathogenesis. In our findings, most of the caspases like CASP1, CASP4, CASP5, CASP8 were found to be upregulated. Other genes like CRADD, CARD6, CARD16, CARD17 which are members of caspase recruitment domain were found to be upregulated in OA (Figure 1). Apart from these genes there are members of TNF-receptor family, TNFRSF1A, TNFRSF9 and TNFRSF10B and TNF ligand superfamily member 13B were also found to be upregulated (Figure 1).
Figure 1: The figure shows the differential expression of genes related to apoptotic cell death cascade in osteoarthritis. The expression of PDCDILG2, TNFRSF1A and BCL2L1 was significantly high in OA (p<0.0.1) patients as compared to control. TRAF5 showed downregulated expression as compared o control. The value >0 indicate upregulation, value <0 indicate down-regulation.
Anti-apoptotic gene TRAF which interacts with TRADD and inhibit apoptosis was found to be down regulated. BCL2L1, a proapoptotic regulator had significantly high (p<0.01) expression in OA as compared to control. The expression of PDCDILG2 and TNFRSF1A was significantly high in OA (p<0.01) patients as compared to control. A member of BCL family BAK-1 which is a BCL-anatagonist/killer was also found to be upregulated (Table 1).
| Component | Name of the Gene |
Gene accession | Information about the gene |
|---|---|---|---|
| Apoptosis Tumor necrosis factor | CASP1 | NM_033292 | Caspase1,apoptosis related cysteine peptidase (Interleukin 1,beta,convertase) |
| CASP4 | NM_033306 | Caspase4,apoptosis related cysteine peptidase | |
| CASP5 | NM_004347 | Caspase5,apoptosis related cysteine peptidase | |
| CASP8 | NM_001228 | Caspase8,apoptosis related cysteine peptidase | |
| PDCDILG2 | NM_025239 | Programmed cell death1, ligand 2 | |
| CRADD | NM_003805 | CASP2 and RIPK1 domain containing adapter with death domain | |
| CARD6 | NM_032587 | Caspase recruitment domain family member 6 | |
| CARD16 | NM_052889 | Caspase recruitment domain family member16 | |
| CARD17 | NM_001007232 | Caspase recruitment domain family member 17 | |
| CFLAR | NM_003879 | CASP8 and FADD like apoptosis regulator | |
| TNFRSF1A | NM_001065 | TNF receptor superfamily member 1A | |
| TNFRSF9 | NM_001561 | TNF receptor superfamily member 9 | |
| TNFRSF10B | NM_003842 | TNF receptor superfamily member 10B | |
| TNFSF13B | NM_006573 | TNF ligand superfamily member 13b | |
| TRAF5 | NM_145759 | TNF receptor associated factor5 | |
| BAK1 | NM_001188 | BCL2 antagonist/killer 1 | |
| BCL family member | BCL2L1 | NM_138578 | BCL2 like1 |
| BCL2L11 | NM_138621 | BCL2 like11 | |
| BCL2L13 | NM_015367 | BCL2 like13 | |
| BCL antagonist | BAK1 | NM_001188 | BCL2 antagonist/killer 1 |
| Cytokines | IL1 | NM_000576 | Interleukin 1 |
| IL8 | NM_000584 | Interleukin 8 | |
| IL10 | NM_000572 | Interleukin 10 | |
| IL12A | NM_000882 | Interleukin 12A (natural killer cell stimulatory factor1) | |
| IL13 | NM_002188 | Interleukin 13 | |
| IL17D | NM_138284 | Interleukin 17D | |
| IL18 | NM_001562 | Interleukin 18 (interferon gamma inducing factor) | |
| IL1RA | NM_000877 | Interleukin 1 receptor type1 | |
| Complement components | C1QA | NM_015991 | Complementcomponent1,qsubcomponent,Achain |
| C1QC | NM_001114101 | Complementcomponent1,qsubcomponent,Cchain | |
| C1QB | NM_000491 | Complementcomponent1,qsubcomponent,Bchain | |
| C2 | NM_000063 | Complementcomponent2 | |
| C3 | NM_00064 | Complementcomponent3 | |
| C4A/C4B | NM_007293 | Complementcomponent4A/4B | |
| C5 | NM_001735 | Complementcomponent5 | |
| CFP | NM_002621 | Complement factor properdin | |
| C1QBP | NM_001212 | Complement component 1, q subcomponent binding protein | |
| CFH | NM_000186 | Complement factor H | |
| Matrix metalloproteinases | MMP8 | NM_002424 | Matrix metallopeptidase 8 (neutrophil collagenase) |
| MMP9 | NM_004994 | Matrix metallopeptidase 9 (gelatinase B,92kDa gelatinase 92kDa, typeIV collagenase) | |
| MMP11 | NM_005940 | Matrix metallopeptidase 11 (Stromelysin 3) | |
| MMP17 | NM_016155 | Matrix metallopeptidase 17 (membrane inserted) | |
| ADAMTS1 | NM_006988 | ADAM metallopeptidase with thrombospondin type1, motif 1 | |
| ADAMTS5 | NM_007038 | ADAM metallopeptidase with thrombospondin type1, motif 5 | |
| Tissue inhibitor of metallopeptidase | TIMP1 | NM_003254 | TIMP metallopeptidase inhibitor 1 |
| TIMP3 | NM_000362 | TIMP metallopeptidase inhibitor 3 | |
| Genes involved in bone metabolism | BMP6 | NM_001718 | Bone morphogenetic protein 3 |
| TGFB1 | NM_000660 | Transforming growth factor β1 | |
| TGFB2 | NM_001135599 | Transforming growth factor β 2 | |
| TGFB3 | NM_003239 | Transforming growth factor β 3 | |
| VDR | NM_001017535 | Vitamin D (1,25-dihydroxy vitamin D3) receptor | |
| S100A3,A4 A7,A8 |
NM_002960,019554 ,002963,002964 |
S100 Calcium binding proteinA3,A4,A7,A8 | |
| S100A11,A12 | NM_005620,NM_005621 | S100 Calcium binding proteinA11, A12 |
Table 1: The table shows the genes along with their accessions and their information along with their respective metabolic pathway in which they are implicated. Differential expression is observed for genes involved in apoptosis, regulators of apoptosis, BCL family, cytokines, complement components, matrix metallo proteases, tissue inhibitors of metallo-proteases and genes involved in bone metabolism in OA subjects as compared to control.
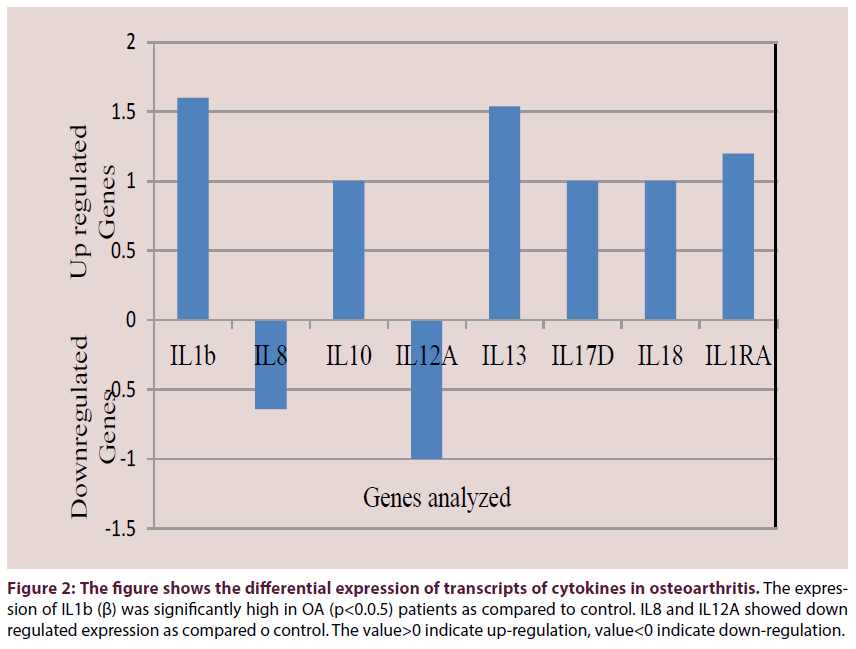
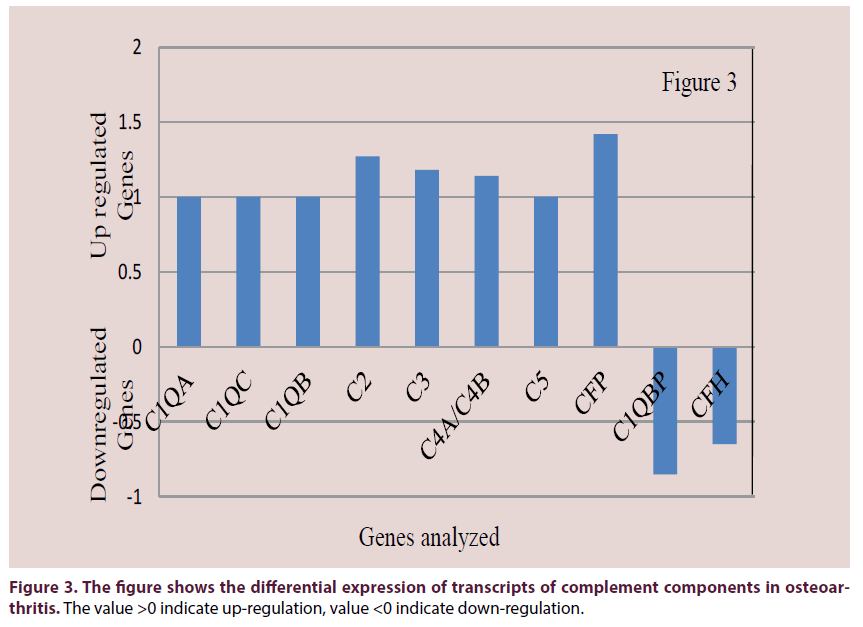
Upregulated gene expression was observed for immune system cytokines and complement components. Upregulated expression was observed for IL-1β (p<0.05), IL-10, IL-13, IL- 17D, IL-18, IL1RA (Figure 2). Downregulated expression was observed for IL-8 and IL-12A. The complement components C1QA, C1QB, C1QC, C2, C3, C4A/C4B, C5, CFP were upregulated (Figure 3). C1QBP and CFH were downregulated.
Figure 2: The figure shows the differential expression of transcripts of cytokines in osteoarthritis. The expression of IL1b (β) was significantly high in OA (p<0.0.5) patients as compared to control. IL8 and IL12A showed down regulated expression as compared o control. The value>0 indicate up-regulation, value<0 indicate down-regulation.
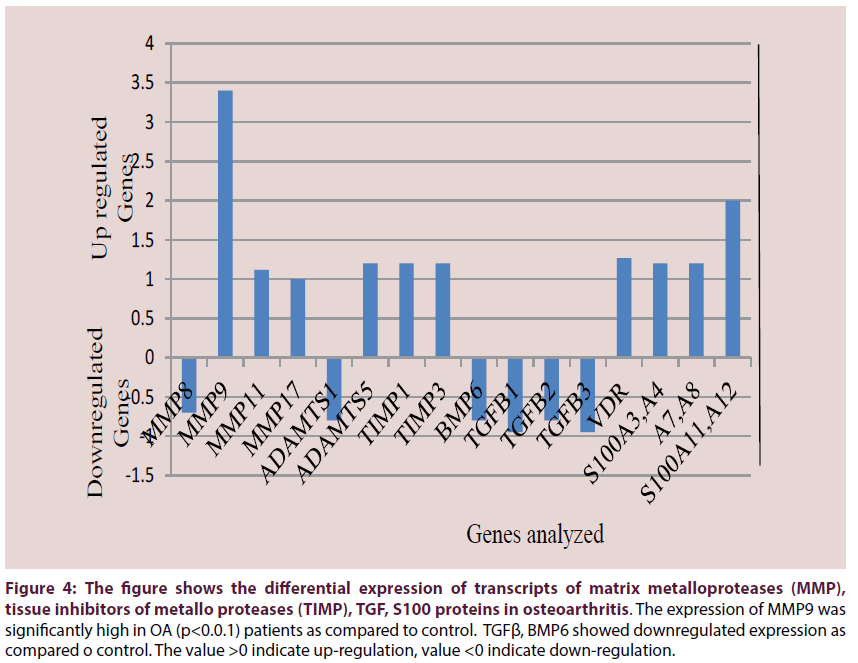
Prominent upregulation was observed for matrix turnover genes like MMPs of which gelatinase MMP-9 was highly upregulated (p<0.01). Stromelysin MMP11 and MMP17 were upregulated but collagenase MMP8 was downregulated (Figure 4). In Aggrecanases, ADAMTS-5 was upregulated and ADAMTS-1 was downregulated (Figure 4). Upregulated expression was observed for TIMPs particularly TIMP-1, and TIMP-3 (Figure 4).
Figure 4: The figure shows the differential expression of transcripts of matrix metalloproteases (MMP), tissue inhibitors of metallo proteases (TIMP), TGF, S100 proteins in osteoarthritis. The expression of MMP9 was significantly high in OA (p<0.0.1) patients as compared to control. TGFβ, BMP6 showed downregulated expression as compared o control. The value >0 indicate up-regulation, value <0 indicate down-regulation.
Interestingly, unaltered expression was observed for many of the candidate susceptibility genes for OA with bone-related functions as COL1A1, ESR1, IGF (data not shown). Bone morphogenetic proteins (BMPs) were all unaltered (data not shown) and BMP6 expression was lower than control (Figure 4). TGFβ1 which plays an important role in bone remodeling as it is a potent stimulator of osteoblastic bone formation, along with TGFβ2 and TGFβ3 which has suppressive effect on IL-2 dependent T-cell growth were also found to be downregulated (Figure 4). S100A proteins which contain calcium binding motif and expressed by macrophages in chronic and acute inflammation were over-expressed. Higher gene expression were observed for vitamin D receptor and S100 protein family as S100P and S100A3, -A4, -A7, -A8, -A11 and -A12 (Figure 4). All the genes and their metabolic pathways along with accession numbers are listed in Table 1.
Discussion
OA is the most frequent disease of muscoskeletal system. Burden of OA is rising consistently due to progressive aging and obesity. Nearly 1 in 2 people may develop symptomatic knee OA by age 85 years [13]. In recent years, there are ever increasing evidences in favor of inflammatory changes in OA. Chronic inflammation has been reported not only in cartilage tissue but also within synovium. Present study shows considerable changes in expression of genes related to apoptotic proteins and inflammatory cytokine, interleukins, and MMPs.
We have found increased expression of apoptotic genes in PBMCs. In the recent studies, increased cell death has been reported to be a feature of OA cartilage in humans and in animal models [14-16]. Cell density is found to be reduced in aging and OA cartilage [17]. In OA cartilage cell death and matrix degradation correlate with one another [18] as matrix degradation results in the loss of survival mechanisms [19] and cell death can contribute to matrix degradation [20] and calcification. It has been reported that cell death in OA cartilage has certain features of apoptosis [14] which is mediated by aspartate specific cysteine proteases or caspases [21]. They must be playing an important role in OA pathogenesis as in in vitro studies caspase inhibitors are able to prevent cell death and maintain chondrocyte function [22].
The study be Lima et al. [23] showed that the broad spectrum caspase inhibitor was able to reduce the size of OA cartilage lesions, however, it was not able to bring improvement in grade of OA lesion. In our study high gene expression was observed for some caspases and positive regulators of apoptosis. These support the role of cell death mediators contribute to OA pathogenesis. Lima et al. [23] have reported high CASP1 and CASP3 activities in animal model.
However, pathophysiology of OA is complex in which many biochemical factors, enzymes and immune mediators are involved. Different cytokines are produced by activated synoviocytes, mononuclear cells or by articular cartilage which in turn upregulate gene expression of many metalloproteinases. In our study IL-1β and IL-18 are upregulated which have a role in promoting joint inflammation and cartilage degradation [24]. Thus OA can have pathologic changes which may involve inflammation with varying degree of severity. As reported by Wojdasiewicz et al. [25] that interactions between proinflammatory and anti-inflammatory process occur which are driven by cytokine networks.
Minor wear and tear of the joint may be due to obesity, systemic disease, postural causes or infections. Therefore minor wear and tear response in load bearing joint may release chemotactic factors which attract immune cells at the target site. Regular tear response due to mechanical load bearing of the joint might result in release of cytokines and further many other components are involved which may result in OA. The cytokines disrupt the catabolic and anabolic processes, which are important in mechanical load bearing joints [26] resulting in progressive degeneration of articular cartilage. These collectively are mediators of inflammatory, degradative and production processing leading to gradual loss of joint function and onset of pain.
Inflammatory cytokines involved in disease pathogenesis include IL-1β, TNF-α, IL-6, -15, -17 and -18 whereas cytokines with antagonistic effects involved are IL-4, -10 and -13. The proinflammatory cytokines upregulated in our study as IL-1β, IL-17D, IL-18 along with TNFSF and its helper components may contribute to disease response. Of these IL-1β is considered key cytokine involved in pathogenesis of OA. Patients with OA have an elevated levels of IL- 1β in both synovial fluid, synovial membrane, cartilage and subchondral bone layer [27,28]. IL- 1β is produced as precursor (pro IL-1β) which is cleaved by caspase -1 (IL-1β converting enzyme). Once IL-1β is bound to its receptor it increased various effector expressions.
Study by Kubota et al. [29] showed that IL- 1β levels in synovial fluid of temporomandibular joints have positive correlation with OA changes. Shlopov et al. [30] have reported high IL-1β binding due to increased expression of its receptor on chondrocyte located in cartilage proximal to macroscopic OA site. There is a possibility that upregulation of IL-1R1 on the chondrocytes makes these cells sensitive to the effects of IL- 1β. Higher IL-1R1 has been reported in OA synovial cells [31]. IL-1 ligand cluster also increases susceptibility to knee OA [32]. IL- 1β has been reported to enhance expression of MMPs as MMP1, -3, -13 and ADAMTS-4 in OA chondrocytes [33]. Bondeson et al. reported ADAMTS-4 and ADAMTS-5 were upregulated by IL-1β in human OA synovial fibroblasts [34]. Dai et al. reported upregulation of IL-18 by IL-1β and upregulation of ADAMTS-5 by IL-18 [35]. Thus IL-1βmay mediate its catabolic effects through other factors in the system. ADAMTS are responsible for proteolysis of aggrecan molecule, one of their member ADAMTS-4 production is stimulated by both IL-1β and TNF-α. However in our study ADAMTS-4 expression did not showed any variation and ADAMTS-5 and ADAMTSL-1 were upregulated and ADAMTS-1 expression was downregulated.
Collagen, the major articular joint protein is another target whose synthesis is suppressed by IL-1β. In our study also the gene expression involved in anabolic metabolism of bone as BMP6, TGFβ1, β2 and β3 are downregulated and other members do not show any variation as compared to control. Yudoh et al. [36] have reported significant reduction in the production of type-II collagen by rabbit chondrocytes incubated in the presence of 10 ng/mL of IL-1β. Similar inhibition of type II collagen mRNA was observed in human chondrocyte cell lines [37].
IL-1β has also an important role in apoptotic cell death. In our study key apoptotic family members along with their accessory proteins showed higher expression as compared to control (Figure 1). Lopez-Armada et al. reported depolarization of mitochondria and upregulation of proapoptotic Bcl-2 family proteins in human articular chondrocytes treated with IL-1β [38]. Heraud et al. reported 18–21% of human OA cartilage chondrocytes exhibit apoptotic features and IL-1β is capable of increasing the percentage of apoptotic cells in both normal and OA cartilage in a dose dependent manner [39]. Thus IL-1β has multiple effects on the cartilage whereby it inhibits its resoration possibility, increases its deterioration by other factors and has direct adverse effects on chondrocytes. Studies have reported that IL-1β stimulation of articular cells such as chondrocytes leads to expression of tumor necrosis factor-a (TNF-α), IL-8, complement factors, and prostaglandin E2, each having the capacity to induce hematopoietic cell infiltration and propagate local inflammation and tissue damage.
In our study TNFRSF1A, TNFRSF10B and TNFSF13B are upregulated (Table 1). TNF may bind to membrane receptor located on every nucleated cell and mediate its effects. Another cytokine IL-17 is high in the serum and the synovial fluid of patients with OA and is positively correlated with the radiographic image of lesions in OA [40]. IL17 is upregulated in our study also. It inhibits the synthesis of proteoglycans by chondrocytes and promotes the production of enzymes of the MMP group [41].
Like IL1β, IL18 stimulates the expression of MMP1,-3, -13 [35] and is upregulated in our study. It increase the concentration of cartilage degrading enzymes, and inhibition of production of proteoglycans, aggrecan and type II collagen and these chondrocytes exhibit morphological changes of apoptotic cells [42].
In our study high expression were observed for complement components C1QA, QB, QC, C2, C3, C4A/B, C5, CFP and factor D in OA patients. Study by Wang et al. [43] showed higher transcript for complement effectors C7, C4A, factor B, C9 and C5 and markedly lower expression of the transcripts encoding the complement inhibitors clusters as factor H, C4-BP and C1 inhibitor in OA as compared to healthy synovial membrane. They reported that effector complement component membrane attack complex (MAC) was able to induce expression of proinflammatory and degradative enzymes in OA [43]. Cultured chondrocytes coated with sublytic levels of MAC, increased the expression of multiple genes as those encoding cartilage degrading enzymes [44] (MMPs and ADAMTS) and inflammatory cytokines [45] which are implicated in OA. MAC deficient mice were protected against (OA). Thus complement cascade is crucial to the pathogenesis of OA. Where dysregulation of gene expression in joint tissues may contribute to a local preponderance of complement effectors over inhibitors in OA. Complement activation in turn results in the formation of MAC on chondrocytes, which kills the cells or triggers release of MMPs, inflammatory mediators etc.
The expression of S100A3, S100A4, S100A7, S100A8, S100A11, S100A12 is upregulated in our patients. Articular ECM has been reported to be a target of catabolic activities of cytokines especially IL1β. IL1β upregulated the major extracellular proteolytic enzymes in cartilage degradation, as matrix metalloproteinases (MMPs) and ADAMTS. The protein levels of MMP-1, MMP2 and MMP9 were higher in patients with OA than those in the control group [46]. In our study we could not observe differences in gene expression of MMP1 and MMP2 in OA versus control group but observed significantly high expression of MMP9 and high MMP11 and MMP17 in OA patients than control. High MMP9 protein along with others is implicated in pathogenesis of OA. MMP9 along with MMP1 and MMP2 is very efficient in the turnover of the extracellular matrix due to their protease activities against their target proteins and contribute to the process of tumor invasion and metastasis in some diseases through tissue remodeling [47]. Osteoclasts constitutively express MMP2 and synthesize MMP9, MMP3 and TIMP1 in response to IL1 stimulation, and during OA the increased levels of osteoclast-derived MMPs might contribute to osteoclast lacunar resorption [48]. Masuhara et al. [49] have demonstrated higher plasma levels of MMP-9 in patients with rapidly destructive hip OA in comparison with patients with OA or normal controls. There is a possibility of enhanced production of MMP9 by synovial cells of patients with destructive OA [49]. A direct route into the bloodstream via the subchondral microcirculatory system and an indirect route from synovial fluid into circulation may account for high MMP9 in OA [50]. Regulatory mechanism of gelatinase expression has been proposed. The increase of gelatinases in OA may be due to abnormal mechanical pressure applied to the articulation. The cyclic compression on osteoblasts from OA subchondral bone increases the expression of genes coding for MMP9. TGFβ1 protects articular cartilage by downregulating the expression of MMP9 of chondrocytes and synoviocytes in OA, which may delay the biological behavior of this disease. Negative correlation was observed between MMP9 expression and TGFβ1 protein [51]. In our study TGF1, β2 and β3 are downregulated along with BMP6. For other members of these families no difference in the gene expression was observed. We also observed high expression of anti inflammatory cytokines as IL-10 and IL13. These anti-inflammatory cytokines modulate an inflammatory response and act protectively on joint tissue. Chondrocytes express both the cytokine IL10 and the receptor IL10R [52]. It has been reported that IL10 is involved in stimulating the synthesis of type II collagen and aggrecan. Following the administration of IL10 in in vitro conditions, both healthy articular cartilage and one in the course of OA demonstrated an increase in proteoglycan synthesis. IL10 has also been shown to inhibit MMP levels [53] and apoptosis of chondrocytes. The anti-inflammatory and chondroprotective effects of IL13 on the cells of the immune system, articular cartilage and synovium in OA have been well documented [54]. IL13 affects the levels of IL1β, TNFα, MMP3 and increases the levels of IL1Ra [25].
In our preliminary study on analysis of gene expression conducted on OA patients with differing grades, MMP9, IL13, TNFα, CCR5 were highly upregulated in grade II OA (data not shown). Expression of MMP9 remained high throughout (data not shown). The expression of TGF-β was inconsistent and TIMP-1 was expressed in all grades of OA (data not shown).
In conclusion inflammation along with apoptotic death mediators deteriorates articular cartilage in the joint. However, simultaneous anabolic changes are not sufficient to control the process of degradation. Therefore management with appropriate inflammatory controls may reduce the morbidity of OA with prolonging time for HTO or TKR.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethical committee and written informed consent was obtained from all the participants.
Funding
This study was funded by Department of Science and Technology, Government of India (SR/FT/L-101/2006) and Indian Council of Medical Research (67/15/2013- IMM/BMS).
Conflict of interest
Authors declare that there are no conflicts of interest. Author 1 declares that he has no conflict of interest. Author 2 declares that he has no conflict of interest. Author 3 declares that she has no conflict of interest. Author 4 declares that he has no conflict of interest. Author 5 declares that he has no conflict of interest. Author 6 declares that he has no conflict of interest. Author 7 declares that she has no conflict of interest.
Author contribution
Authors 1, 2, 3, 5, 7 planned and did all the experimental work. Authors 4, 5, 6, 7 contributed to work planning and finalization of the manuscript.
References
- Hootman JM, Helmick CG, Barbour KE, et al. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults. 2015-2040. Arthritis Rheumatol. 2015-2040 (2016).
- Barbour KE, Helmick CG, Theis KA, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation -United States, 2010–2012. Morb. Mortal. Wkly. Rep. 62(44), 869-873 (2013).
- Sacks JJ, Helmick CG, Luo Y, et al. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Rheum. 57(8), 1439-1445 (2007).
- Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med. Clin. North. Am.93(1), 1–24 (2009).
- Haseeb H, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin. Immunol. 146(3), 185-196 (2013).
- Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis-results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 13(5), 361–367 (2005).
- Nakamura H, Yoshino S, Kato T, et al. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cartilage. 7(4), 401–402 (1999).
- Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17(12), 1674–1679 (2011).
- Mishra R, Singh A, Chandra V, et al. A comparative analysis of serological parameters and oxidative stress in osteoarthritis and rheumatoid arthritis.Rheumatol. Int.32(80, 2377-82 (2012).
- Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64(9), 1263–1267 (2005).
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann. Rheum. Dis. 16(4), 494-502 (1957).
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 29(8), 1039-1049 (1986).
- Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 59(9), 1207–1213 (2008).
- Blanco FJ, Guitian R, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis, a possible pathway for osteoarthritis pathology. Arthritis Rheum. 41(2), 284–289 (1998).
- Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 27(2), 455–462 (2000).
- Mistry D, Oue Y, Chambers MG, Kayser MV, Mason RM. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage. 12(2), 131–141 (2004).
- Vignon E, Arlot M, Vignon G. The cellularity of fibrillated articular cartilage, a comparative study of age-related and osteoarthritic cartilage lesions from the human femoral head. Pathol. Biol. 25(1), 29–32 (1975).
- Kim HA, Suh DI, Song YW. Relationship between chondrocyte apoptosis and matrix depletion in human articular cartilage. J. Rheumatol.28(9), 2038–2045 (2001).
- Frisch SM, Screaton RA.Anoikis mechanisms. Curr. Opin. Cell. Biol. 13(5), 555–562 (2001).
- Lo MY, Kim HT. Chondrocyte apoptosis induced by collagendegradation, inhibition by caspase inhibitors and IGF-1. J. Orthop. Res. 22(1), 140–144 (2004).
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 281, 1312–1316 (1998).
- D’Lima DD, Hashimoto S, Chen PC, Lotz MK, Colwell CW Jr. Prevention of chondrocyte apoptosis. J. Bone. Joint. Surg. Am. 83A Suppl. 2(1), 25–26 (2001).
- D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 54(6), 1814-1821 (2006).
- Lai YC, Shaftel SS, Miller JN, et al. Intraarticular induction of interleukin-1β expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 54(4), 1184–1197 (2006).
- Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The Role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators.Inflamm. 1-19 (2014).
- Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PMR. 3(6), S3–S11 (2011).
- Massicotte FD, Lajeunesse M, Benderdour, et al. Can altered production of interleukin-1, interleukin-6, transforming growth factor- and prostaglandin E2 by isolated human subchondral osteoblasts identity two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 10(6), 491–500 (2002).
- Sohn DH, Sokolove J, Sharpe O, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 14(1), R7 (2012).
- Kubota E, Imamura H, Kubota T, et al. Interleukin 1 beta and stromelysin (MMP3) activity of synovial fluid as possible markers of osteoarthritis in the temporomandibular joint. J. Oral.Maxillofac. Surg. 55(1), 20–27 (1997).
- Shlopov BV, Gumanovskaya ML, Hasty KA. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 43(1), 195–205 (2000).
- Sadouk MB, Pelletier JP, Tardif G, et al. Human synovial fibroblasts coexpress IL-1 receptor type I and type II mRNA. The increased level of the IL-1 receptor in osteoarthritic cells is related to an increased level of the type I receptor. Lab. Invest. 73(3), 347–355 (1995).
- Loughlin J, Dowling B, Mustafa Z, Chapman K. Association of the interleukin-1 gene cluster on chromosome 2q13 with knee osteoarthritis. Arthritis Rheum. 46(6), 1519-1527 (2002).
- Fan Z, Bau B, Yang H, Soeder S, Aigner T.Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1β.Arthritis Rheum. 52(1), 136–143 (2005).
- Bondeson J, Lauder S, Wainwright S, et al. Adenoviral gene transfer of the endogenous inhibitor IKBa into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kBdependent. J. Rheumatol. 34(3), 523-533 (2007).
- Dai SM, Shan ZZ, Nishioka K, Yodoh K. Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivol. Ann. Rheum. Dis. 64(5), 735-742 (2005).
- Yudoh K, Shishido K, Murayama H, et al. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocytes catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis Rheum. 56(10), 3307-3318 (2007).
- Goldring MB, Birkhead J, Sandell LJ, Kimura T, Krane SM. Interleukin I suppress expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J. Clin. Invest. 82(6), 2026-2037 (1988).
- Lopez-Armada MJ, Carames B, Lires-Dean M. et al. Cytokines, tumor necrosis factor-a and interleukin-1b, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 14(7), 660-669 (2006).
- Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritis human articular cartilage. Ann. Rheum. Dis. 59(12), 959-965 (2000).
- Chen B, Deng Y, Tan Y, Qin J, Chen LB. Association between severity of knee osteoarthritis and serum and synovial fluid interleukin 17 concentrations. J. Int. Med. Res. 42(1), 138–144 (2014).
- Benderdour M, Tardif G, Pelletier JP et al. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1. J. Rheumatol.29(6), 1262–1272 (2002).
- Inoue H, Hiraoka T, Hoshino, et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone. 42(6), 1102-1110 (2008).
- Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17(12): 1674-1679 (2011).
- Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol.90(5), 463–479 (2009).
- Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr.Opin. Rheumatol. 20(5), 565–572 (2008).
- Zeng GQ, Chen AB, Li W, Song JH, Gao CY. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genetics. Mol. Res.14(4), 14811-822 (2015).
- Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 27(31), 5287-5297 (2009).
- Seibel MJ, Duncan A, Robins SP. Urinary hydroxypyridinium crosslinks provide indices of cartilage and bone involvement in arthritic diseases. J. Rheumatol.16(7), 964–970 (1989).
- Masuhara K, Nakai T, Yamaguchi K, Yamasaki S, Sasaguri Y. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 46(10), 2625–2631 (2002).
- Masuhara K, Lee SB, Nakai T, Sugano N, Ochi T, et al. Matrix metalloproteinase in patients with osteoarthritis of the hip. Int. Orthop. 24(2), 92–96 (2000).
- Guo J, Zhang W, Li Q, Gan H, Wang Z. Significance of expressions of matrix metalloproteinase 9 mRNA, transforming growth factor beta1, mRNA and corresponding proteins in osteoarthritis. Chinese. J.Repar. Recon. Surg. 25(8): 992–997 (2011).
- Iannone F, De Bari C, F. Dell’ Accioet al. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin. Exp.Rheumatol.19(2), 139–145 (2001).
- Wang Y, Lou S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chinese. Med. J. 114(7), 723–725 (2001).
- Hart PH, Ahern MJ, Smith MD, Finlay-Jones JJ. Regulatory effects of IL-13 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Clin. Exp. Immunol.99(3), 331–337 (1995).