Review Article - Imaging in Medicine (2010) Volume 2, Issue 3
MRI of bone tumors: advances in diagnosis and treatment assessment
Virna Zampa†, Giuliana Roselli1 and Giovanni Beltrami2
1 Diagnostic Imaging, Radiology 1 CTO- AOUC Firenze, Italy
2 Department of Orthopaedic Oncology & Reconstructive Surgery CTO-AOUC, Largo Palagi, 1 50139 Firenze, Italy
- *Corresponding Author:
- Virna Zampa
Diagnostic & Interventional Radiology
University of Pisa, Via Paradisa
2 56100 Pisa, Italy
Tel.: +39 050 995 551
Fax: +39 050 573 905
E-mail: virnazampa@hotmail.com
Abstract
MRI is a unique imaging technique that allows direct visualization of bone marrow with high spatial resolution and is considered the best tool for local staging of bone tumors. Owing to its high natural contrast resolution, it can depict specific tissue components useful for tumor characterization. Moreover, there is great interest in MRI capability to assess post-chemotherapy changes in order to identify unresponsive tumors early in the course of therapy. To increase the accuracy in the assessment of tumor response to treatment, new and more sophisticated approaches using fast and ultrafast MRI are under evaluation. This article presents an overview of the state-of-the-art of MRI in staging bone lesions, discusses the role that this technique plays in tumor characterization and provides a quick look at future technological advancements.
Keywords
bone tumor staging; diffusion imaging; dynamic study; follow-up; magnetic resonance imaging; MRI; MR spectroscopy; whole body
The evaluation of bone tumors often requires more than one imaging modality, including radiography, bone scintigraphy, CT, MRI and PET. Conventional radiography is usually the first step in the diagnostic process, representing an essential tool in the evaluation of the aggressiveness of the lesion and the response of the host bone. It provides important information regarding lesion site and edges, bone matrix mineralization, cortical involvement and periosteal reaction; thus, it remains the cornerstone for differential diagnosis of skeletal tumors and tumor-like lesions [1].
A bone scan is a nonspecific but sensitive modality for assessing abnormalities in bone formation and bone perfusion, helpful in the identification of active versus indolent lesions and in screening multiple skeletal lesions. This technique is less important in assessment of solitary lesions but can add valuable information in the case of polyostotic involvement.
Multidetector CT allows quick and accurate assessment of bone lesions. CT is particularly useful when lesions are located in areas of complex anatomy (e.g., the spine or pelvis), where conventional radiographs fail owing to limited contrast resolution, complex skeletal anatomy and/or superposition of skeletal elements. It is superior to MRI for the visualization of fine bony details or small calcifications. In fact, CT is the best modality in depicting cortical alteration, periosteal reaction and subtle matrix calcifications. Finally, abdominal and chest CT remains the fundamental tool in the staging of patients with bone tumors.
Owing to its exquisite evaluation of bone marrow, MRI is considered the best tool for local staging of bone tumors, providing an accurate depiction of bone marrow and soft tissue involvement.
By applying the various techniques, MRI can identify specific tissue components that are useful for the characterization of a bone lesion. However, it must be stressed that magnetic resonance (MR) images should only be interpreted with concurrent radiographic correlation.
In summary, many benign bone tumors and tumor-like lesions, including most connectivetissue and fibro-osseous lesions of bone, can reliably be diagnosed by radiography and do not require further imaging. However, if a lesion is not characterized or shows an aggressive behavior, a cross-sectional modality may be helpful or even necessary. Compared with radiography and CT, MRI has proven to be especially advantageous in identification of non-mineralized chondroid matrix, vascular tissue, cysts and hemosiderotic tissues.
Using tailored sequences, MRI can assess postchemotherapy changes, as many of these tumors are treated before surgery. To increase the accuracy in the assessment of treatment response, new and more sophisticated approaches using fast and ultrafast MRI are under evaluation.
FDG PET represents a noninvasive means of estimating tumor aggressiveness and can be used to detect progression or regression of disease. Such information, in combination with anatomic imaging modalities such as CT and MRI, can be used to optimize staging, restaging and assessment of therapy response [2,3].
Staging
TNM and Enneking’s are two major systems of bone tumor staging. TNM is applicable to most bone tumors, except malignant lymphoma, multiple myeloma, periosteal and other surface osteosarcomas (OS), and parosteal chondrosarcoma. The T factor represents the tumor extent: T1 represents tumors 8 cm or less in greatest dimension; T2, tumors over 8 cm in greatest dimension; and T3, discontinuous tumors in the primary bone site. The N factor is not as important in bone tumors as lymph node metastases are rare. If lymph node metastasis exists, the prognosis is poor and it is regarded as distant metastasis. The M factor is divided into two grades: M0 for the absence of metastases; and M1 for the presence of metastases. The G factor, the histological grade, is divided into four grades: G1, highly differentiated; G2, moderately differentiated; and G3 and G4, poorly differentiated. G4 is the highest grade and includes Ewing’s sarcoma and malignant lymphoma.
Enneking’s surgical grading system is similar to the TNM system with some modifications; it is only applicable to mesenchymal tumors and not hematopoietic ones. The T factor is divided into two grades: T1, limited to one compartment; and T2, transcompartmental extension. The N factor is regarded as metastasis (M factor) in this system. The G factor includes histological, radiological and clinical features. Three grades include imaging features: G0, benign; G1, low-grade malignant; and G2, high-grade malignant. Surgeons prefer this system since it includes the concept of compartments, an important element for planning tumor resection and reconstruction. A compartment is an anatomic region surrounded by a natural barrier; for example, the synovial capsule, articular cartilage, bone cortex, periosteum, major fasciae and tendinous origins represent natural boundaries. A lesion confined to one of these spaces is called intracompartmental. The regions without natural boundaries (e.g., paraspinal, periclavicular, axillary regions and the dorsum of the hands and feet) are designated as extracompartmental spaces.
The staging of hematopoietic tumors deserves a brief mention. Staging of malignant lymphoma involving bone is the same as that of other organs and is based on the disease extent evaluated by clinical and imaging studies. According to the Cotswolds modification of the Ann Arbor staging classification, lymphoma of bone without regional nodal involvement or disseminated disease is classified as stage 1E, whereas lymphoma of bone with regional nodal involvement and without disseminated disease is classified as stage 2E [4].
Multiple myeloma has a different grading system from that of malignant lymphoma. The Durie–Salmon grading system includes a simple, plain radiographic scoring system, but is not as accurate for determining the disease extent. As the value of MRI for a sensitive detection and the prognostic significance of marrow infiltration, Durie and coworkers introduced the Durie–Salmon PLUS grading system, which includes whole-body (WB)â�?�?MRI and PET as diagnostic tools [5,6]. In this system, stage IA represents a single plasmacytoma and/or limited disease on imaging (smoldering or indolent myeloma); stage IB, less than five focal lesions or mild diffuse disease; stage IIA/B, between five and 20 lesions or moderate diffuse disease; and stage IIIA/B, over 20 lesions or severe diffuse disease (when the signal intensity [SI] reaches the SI of the intervertebral disc on T1-weighted spin-echo [T1w SE] images). The letter A indicates normal renal function and B indicates abnormal renal function.
Local staging
MRI is a unique imaging technique that allows direct visualization of bone marrow with high spatial resolution (Figure 1). Its high soft tissue contrast enables a precise assessment of bone marrow infiltration and surrounding tissue involvement at high sensitivity, even in the absence of evident osteolytic or metabolic changes. Therefore, MRI is undoubtedly the best modality for the detection of bone tumors of the peripheral skeleton and is routinely employed for its accuracy in defining origin, anatomic extent and margins of a bone lesion [7]. The advances in software, resolution and faster imaging times have increased the capability of MRI to accurately stage bone tumors preoperatively.
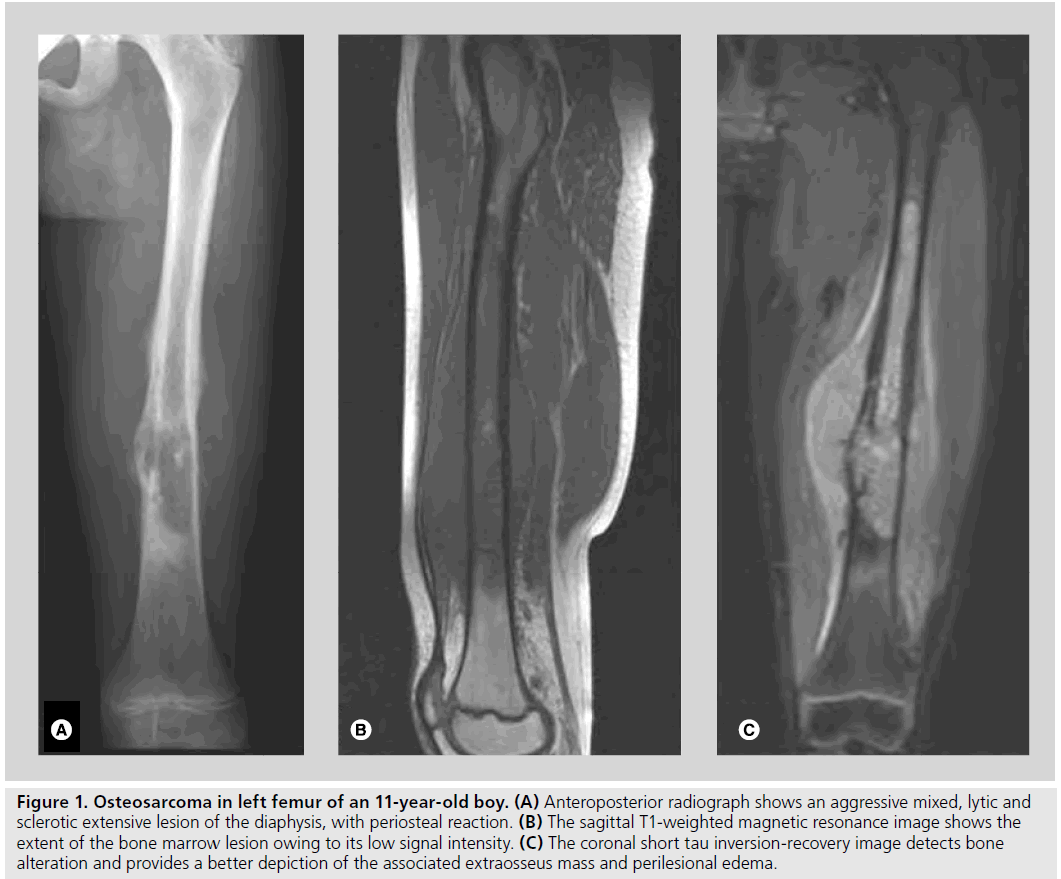
Figure 1. Osteosarcoma in left femur of an 11-year-old boy. (A) Anteroposterior radiograph shows an aggressive mixed, lytic and sclerotic extensive lesion of the diaphysis, with periosteal reaction. (B) The sagittal T1-weighted magnetic resonance image shows the extent of the bone marrow lesion owing to its low signal intensity. (C) The coronal short tau inversion-recovery image detects bone alteration and provides a better depiction of the associated extraosseus mass and perilesional edema.
Local staging should be performed prior to biopsy because postbiopsy hemorrhage or edema may interfere with the assessment leading to over staging; furthermore, MRI can guide the surgeon in choosing the best biopsy path and site.
Staging malignant primary tumors requires the evaluation of both intra- and extra- medullary involvement. Intramedullary extension includes an assessment of longitudinal extent, epiphyseal involvement and skip metastases; these factors will determine the site of bone resection [7]. Extramedullary extension includes an assessment of muscle compartments and joint involvement, and the relationship with the neurovascular bundle; these factors will determine the feasibility and type of limb salvage performed. Therefore, they represent invaluable information for surgical planning.
Radiologists have an important role in ensuring the appropriate execution of the imaging and analysis of the lesion in all its extent. Technically, two different approaches can be chosen: either a single examination, using the proper coils and large field of view to entirely cover the segment, or a two-step examination, performing exploration of all the bone segment followed by a more detailed study tailored to the lesion, with a dedicated coil. For example, when a bone tumor is encountered in skeletally immature children, evaluation of the growth plate and epiphysis involvement must be accurately assessed (Figure 2). Indeed, physeal sparing may be significant to the very young, in whom growth is essential; moreover, the preservation of the natural joint has a better functional outcome. Therefore, it is advisable to image the lesion with a dedicated coil that assures a higher spatial resolution, using both sagittal and coronal planes, since focal abnormalities of the physis can be underestimated if only one plane is imaged. In addition, employment of surface coils enables a more accurate depiction of the neurovascular bundle and joint anatomical structures.
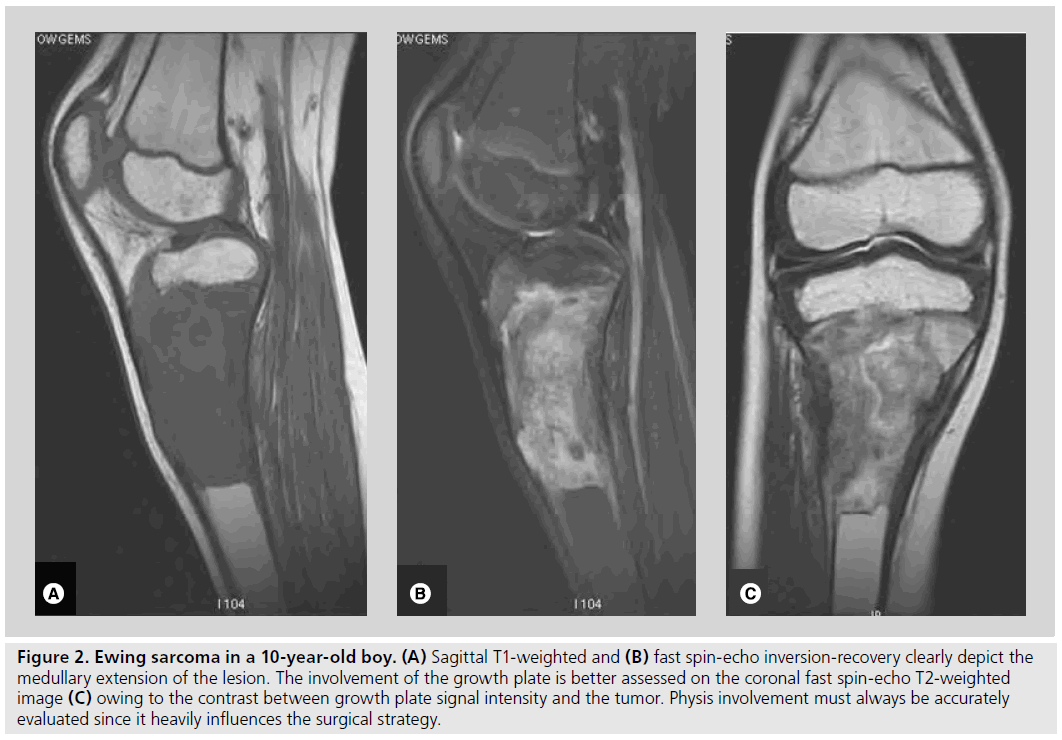
Figure 2. Ewing sarcoma in a 10-year-old boy. (A) Sagittal T1-weighted and (B) fast spin-echo inversion-recovery clearly depict the medullary extension of the lesion. The involvement of the growth plate is better assessed on the coronal fast spin-echo T2-weighted image (C) owing to the contrast between growth plate signal intensity and the tumor. Physis involvement must always be accurately evaluated since it heavily influences the surgical strategy
The intramedullary longitudinal extent of a tumor can be assessed by considering an anatomic palpable landmark as a reference point for the surgeon; for example, if the lesion is in the distal femur, a plane crossing through the knee joint may be used as the reference point from which to measure the longitudinal extent of the tumor.
The evaluation of bone marrow edema (BME) extension is another significant aspect in staging primary bone tumors [8]; it is fundamental to differentiate the true tumor margins from the peritumoral marrow edema. BME exhibits a poorly defined, patchy appearance with homogeneous intermediate SI on T1w and high SI on T2w fat-suppressed SE or short tau inversionrecovery (STIR) sequences. STIR is more accurate than the T1w sequence in depicting BME but can overestimate the degree of intraosseous macroscopic extent of the tumor.
Differentiating BME from the underlying lesion may be problematic as both may demonstrate similar signal characteristics. It is useful to examine the T1w images to identify the lesion margin and then compare this with the watersensitive sequence. This will enable the assessment of the volume of edema versus tumor size. Moreover, the peritumoral BME is often homogeneous in appearance, whereas the underlying lesion usually has a more heterogeneous signal pattern. Finally, dynamic contrast MRI can improve the depiction of the true tumor margin owing to the differing enhancement curves of the BME and the adjacent tumor [8].
MRI is considered the best technique to visualize skip lesions (Figure 3). Skip lesions are simultaneous, secondary foci of disease that reflect the hematogenous spread of the tumor in the affected bone. They are correlated with a higher incidence of distant metastases and local recurrence and, consequently, with a worse prognosis [9]. The presence of skip lesions determines the level of surgical resection; hence, the importance of examining the entire bone segment. However, not all marrow abnormalities are necessarily skip metastases. In fact, marrow SI changes can be caused by edema, infarction, focal hyperplasia and marrow reconversion. Differential diagnosis between these entities is essential for surgical planning; FDG PET or even biopsy can be helpful in ambiguous cases.
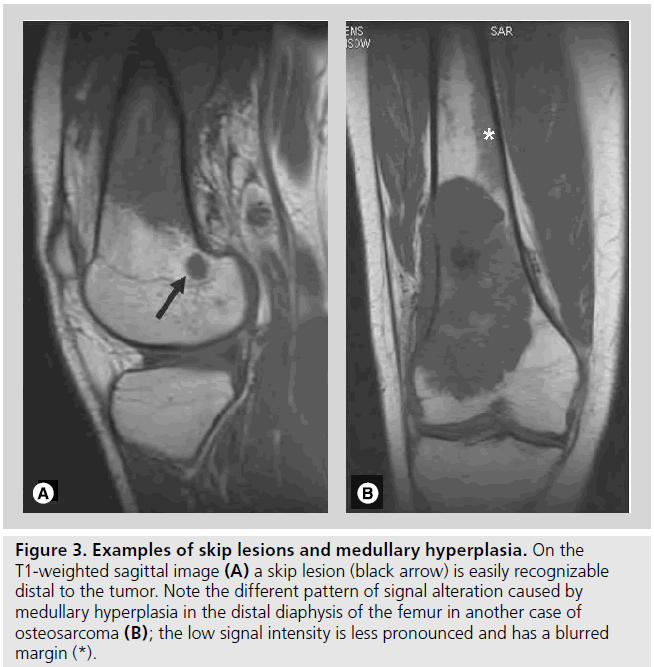
Figure 3. Examples of skip lesions and medullary hyperplasia. On the T1-weighted sagittal image (A) a skip lesion (black arrow) is easily recognizable distal to the tumor. Note the different pattern of signal alteration caused by medullary hyperplasia in the distal diaphysis of the femur in another case of osteosarcoma (B); the low signal intensity is less pronounced and has a blurred margin (*).
Assessment of the relationship between the tumor and neurovascular bundle is another crucial point for surgical planning. To evaluate this feature, the axial plane is preferable since it enables the visualization of adipose tissue around the neurovascular bundle. The absence of adipose tissue can represent a mere contact between tumor and neurovascular bundle or an initial infiltration; this latter aspect is hard to define by imaging and requires histological correlation.
Joint involvement requires extra-articular resection; therefore, it is essential to assess the presence of intra-articular masses or focal replacement of intrasynovial fat. Finally, a pathological fracture is taken to confirm joint contamination, as it is equivalent to an extracompartmental extension.
Despite all the technological advances in MRI, T1w SE or fast-SE images still represent the most accurate sequences for imaging bone marrow, and are considered the best way to detect skip lesions and epiphysis involvement [7]. This simple sequence must, therefore, be included in any protocol when staging a bone tumor.
MRI protocol must also include T2w sequences, with and without fat saturation, as these can be helpful for evaluating cartilage, joint and soft tissue involvement but also to characterize the different tissue components.
By careful evaluation, MRI is also able to catch findings suggestive of the tumor growth pattern. The presence of transtrabecular or transcortical infiltration and periosteal extension can help to assess the aggressiveness of a bone lesion and cannot be defined by other imaging modalities [10]. For example, transcortical infiltration is a frequent finding in the Ewing/PNET group and in bone lymphomas. The tumor growth pattern was found to be an independent prognostic factor to help predict oncologic outcome. Kim et al. reported a better survival in patients with stage IIB longitudinally growing OS (that usually have a diaphyseal location), compared with concentric ones [11].
The administration of contrast media should always be performed in the staging phase using both dynamic MRI study and T1w sequences in the static phase. In fact, contrast enhancement enables the detection of areas of tumor viability prior to biopsy and allows differentiation between the tumor and adjacent edema.
Finally, postcontrast MRI, as well as diffusion-weighted sequences, can be useful to monitor the response to neoadjuvant chemotherapy and to identify postsurgical recurrences.
Characterization
The primary goal of imaging should be the specific diagnosis of a bone lesion, thus eliminating the need for biopsy or narrowing of the differential diagnosis, in order to decide whether biopsy, surgical intervention or simple observation are required for further management.
Despite the availability of advanced imaging methods such as CT and MRI, the diagnosis of a tumor or tumor-like lesion of the bone still depends on the conventional radiograph. The diagnosis of a bone tumor can frequently be suggested on the basis of age, location and classical radiographic findings. In fact, the findings derived from x��?rays allow formulation of a reasonable hypothesis regarding the histological nature and possible differential diagnosis of a lesion.
Lytic bone lesions can only be detected on a standard radiogram when the tumor has determined 30–50% of trabecular bone destruction. Therefore, MRI may be useful in any case of neoplastic infiltration without bony destruction. Moreover, in specific cases, cross-sectional imaging represents an additional helpful and even necessary modality.
Although MRI may have a limited additional role in the characterization of bone tumors, contrast resolution can be manipulated by varying the signal parameters. Therefore, a wide variety of images can be obtained, and allow characterization of some tissue components based on their signal characteristics. The careful use and interpretation of these studies can lead to a reasonable differential diagnosis prior to biopsy [12]. A detailed analysis of some specific features can allow the diagnosis of some entities with good reliability.
SI analysis
During analysis of the SI of a lesion it is possible to recognize specific tissue components. High SI on T1w images that persists on fatsuppressed sequences, indicates the presence of meta-hemoglobin and can be found in telangiectatic OS, aneurysmal bone cyst (ABC), giant cell tumor (GCT), fibrous dysplasia or, rarely, in chondroblastoma or osteoblastoma. This finding can also be observed in malignant tumors after radiotherapy or chemotherapy.
Similarly, the presence of fat tissue in a bone lesion (e.g., intraosseous lipoma, periosteal and parosteal lipoma, and hemangioma) displays high SI on T1w images, which will be annulled when fat saturation is applied.
Low SI on T2w sequences can be caused by hemosiderin deposition, fibrous tissue, calcification or mineralized osteoid matrix. It may be encountered in GCT, fibrous dysplasia, parosteal or osteoblastic OS and cartilaginous tumors. Fibrous tumors may also exhibit low SI on T2w images caused by a dense fibrous component within the collagenous matrix. In these lesions, the SI depends on the ratio between cellularity and collagenous matrix.
On T2w images and especially on gradientecho T2* and fat-saturated sequences, nonmineralized chondroid tissue has a high SI; when a mineralized matrix is present, areas of low SI can be displayed with punctate, flocculent or ring morphology. Owing to its accurate depiction of cartilage, MRI is the most reliable method for the measurement of cartilage cap thickness in osteochondroma, a very important finding to assess the malignant transformation of these lesions.
Unfortunately, most skeletal tumors demonstrate nonspecific SI behavior on MRI; heterogeneous SI with significant overlap on T1w and T2w sequences is frequently encountered in both benign and malignant lesions.
Margins & morphology
Conventional radiography remains the prime study for the definition of margins. This feature, referred to as the appearance of the lesion on conventional radiographs, can also be applied to CT. Overall, CT can give a better evaluation of peripheral margins of the lesion and provides the assessment of the interface between lesion and normal bone. It also improves the depiction of the patterns of osteolysis, which is useful for assessing the biologic activity of a bone neoplasia. Conversely, these features cannot be applied to MRI. In fact, some benign lesions can be overestimated as a result of bone marrow and soft tissue edema; furthermore, MRI has less potential to show different patterns of bone destruction. Nevertheless, MRI can have a specific role in the characterization of some bone tumors that have particular morphological aspects.
It is well known that cortical penetration, periosteal reaction and soft tissue involvement are signs of biologically aggressive lesions, and are usually encountered in malignant tumors.
Identification of fluid-fluid levels in cystic cavities within a well-defined lesion is a characteristic of ABC, although it can also be found in GCT (Figure 4), fibrous dysplasia, plasmacytoma, telangiectatic OS, chondroblastoma and some bone metastases.
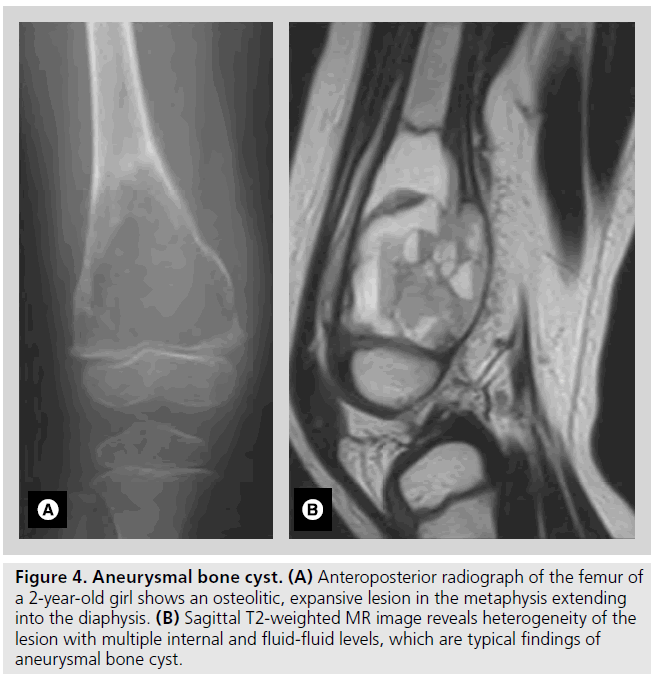
Figure 4.Aneurysmal bone cyst. (A) Anteroposterior radiograph of the femur of a 2-year-old girl shows an osteolitic, expansive lesion in the metaphysis extending into the diaphysis. (B) Sagittal T2-weighted MR image reveals heterogeneity of the lesion with multiple internal and fluid-fluid levels, which are typical findings of aneurysmal bone cyst.
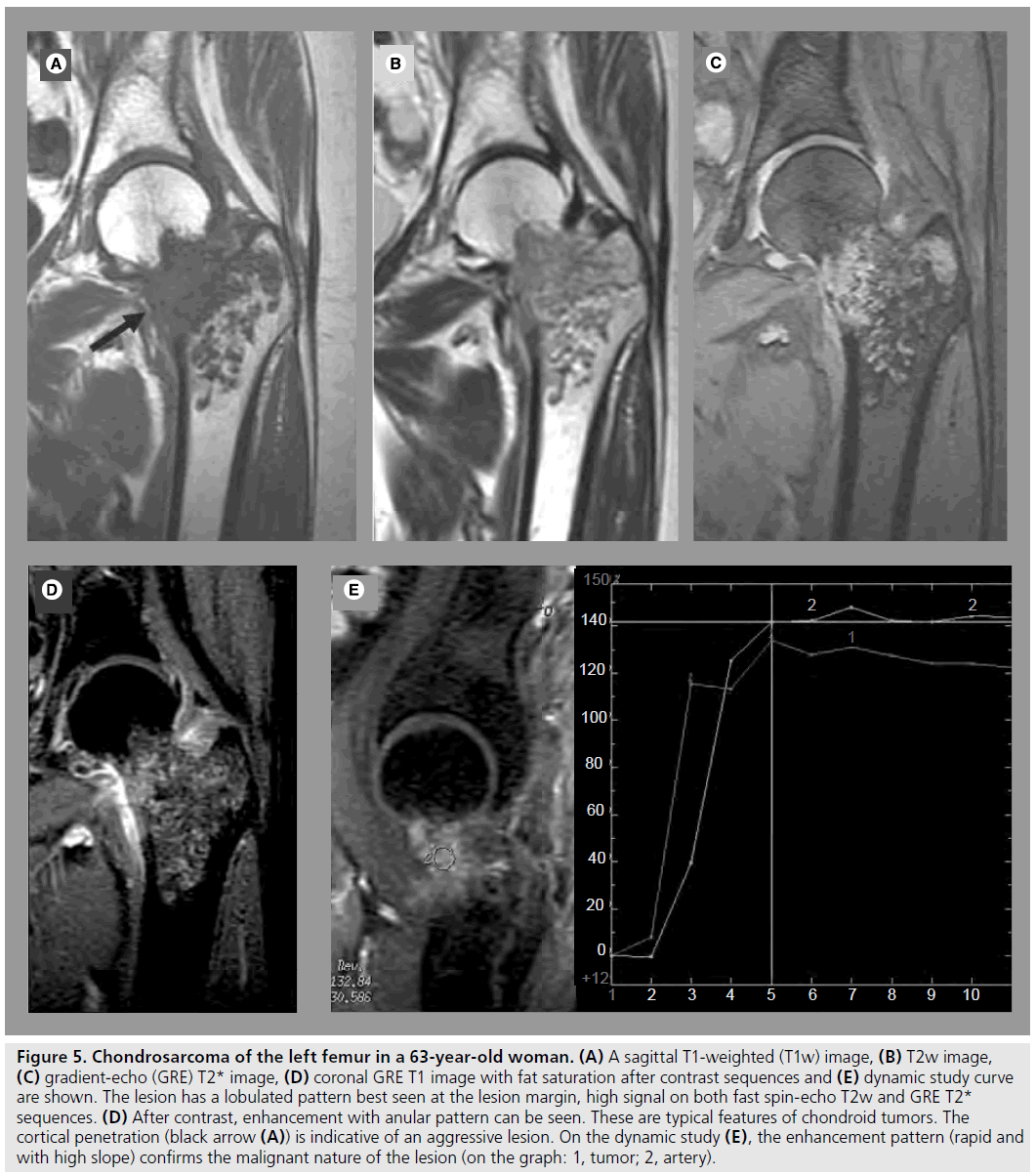
Benign and low-grade cartilaginous tumors are characterized by their lobulated morphology representing cartilage nodules separated by septa (Figure 5). On the other hand, the features of high-grade and dedifferentiated chondrosarcoma can be nonspecific, and resemble other aggressive tumors. Dedifferentiation is the development of a new high-grade malignancy in association with a preexisting lowgrade malignancy or a benign tumor. Typical signs are cortical involvement and the presence of a soft tissue mass. MRI has an important role in delineating the extent of the intraosseous and soft tissue involvement preoperatively [13]. A third of dedifferentiated chondrosarcoma are characterized by bimorphic features visible by radiography, most commonly secondary to a dominant lytic area within or adjacent to a mineralized tumor. In these cases, the high-grade noncartilaginous component usually exhibits a lower SI than the conventional chondrosarcomatous component and prominent diffuse enhancement is seen after contrast administration on MRI [14].
Figure 5.Chondrosarcoma of the left femur in a 63-year-old woman. (A) A sagittal T1-weighted (T1w) image, (B) T2w image, (C) gradient-echo (GRE) T2* image, (D) coronal GRE T1 image with fat saturation after contrast sequences and (E) dynamic study curve are shown. The lesion has a lobulated pattern best seen at the lesion margin, high signal on both fast spin-echo T2w and GRE T2* sequences. (D) After contrast, enhancement with anular pattern can be seen. These are typical features of chondroid tumors. The cortical penetration (black arrow (A)) is indicative of an aggressive lesion. On the dynamic study (E), the enhancement pattern (rapid and with high slope) confirms the malignant nature of the lesion (on the graph: 1, tumor; 2, artery).
Dedifferentiation into a more aggressive lesion is also frequent in parosteal OS, a lowgrade well-differentiated malignant tumor normally characterized by low SI on both T1w and T2w images. When present, dedifferentiation is usually visible as a lytic area in a sclerotic mass. Although the radiolucent area can be absent, the high-grade component of the tumor is characterized by T2w hyperintensity on MRI; owing to its hypervascularity, it can be detected after contrast administration [15]. Differentiation of parosteal OS into a telangiectatic OS has been reported; in this case, a purely lytic mass, partially composed of fluid cavities, was easily detected by CT and MRI [16].
Bone marrow edema
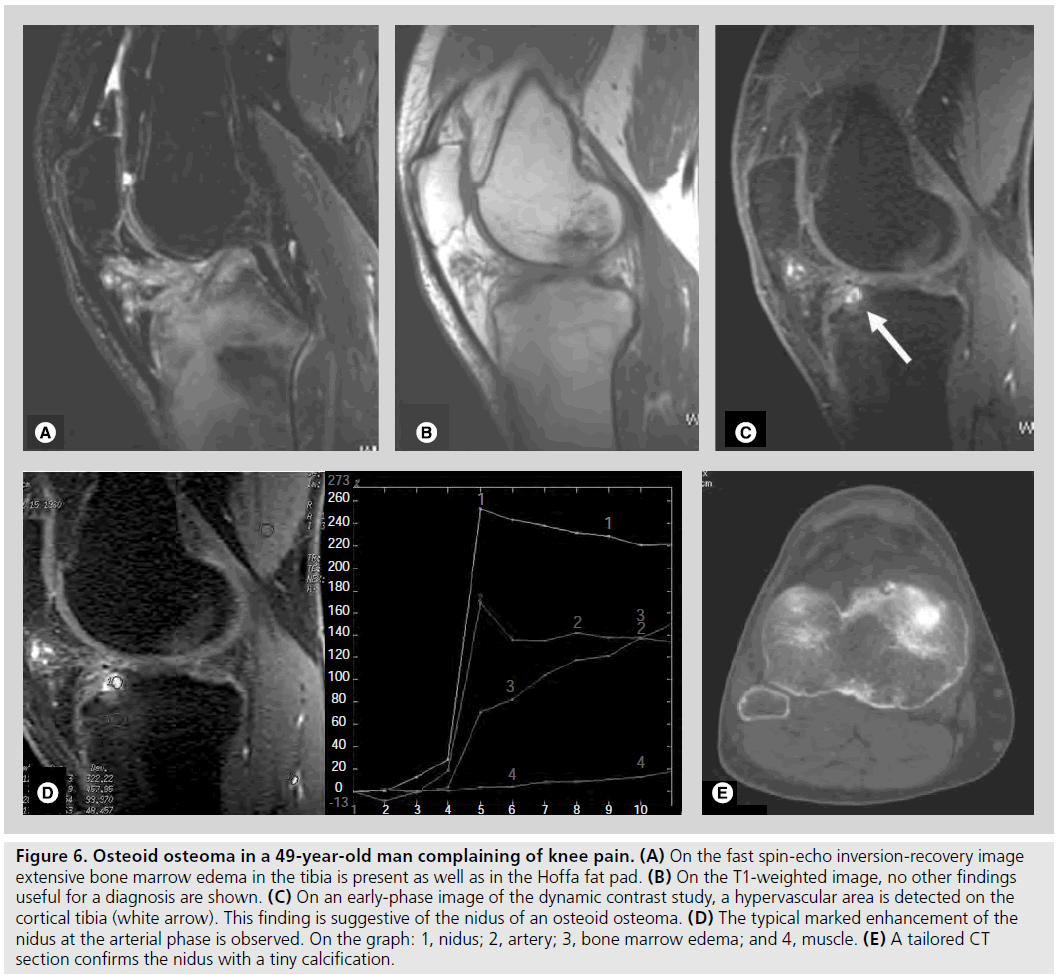
MRI is the best technique in depicting BME, which can be associated with both benign and malignant lesions [8]. Extensive BME related to small-sized lesions is often predictive of benign tumors and is typically associated with osteoid osteoma (Figure 6), osteoblastoma, chondroblastoma, Langheran’s cell histiocitosis and, less frequently, ABC or GCT.
Figure 6.Osteoid osteoma in a 49-year-old man complaining of knee pain. (A) On the fast spin-echo inversion-recovery image extensive bone marrow edema in the tibia is present as well as in the Hoffa fat pad. (B) On the T1-weighted image, no other findings useful for a diagnosis are shown. (C) On an early-phase image of the dynamic contrast study, a hypervascular area is detected on the cortical tibia (white arrow). This finding is suggestive of the nidus of an osteoid osteoma. (D) The typical marked enhancement of the nidus at the arterial phase is observed. On the graph: 1, nidus; 2, artery; 3, bone marrow edema; and 4, muscle. (E) A tailored CT section confirms the nidus with a tiny calcification.
Conversely, minimal BME surrounding a large lesion is more likely to be associated with malignant lesions and is often found in Ewing’s sarcoma, chondrosarcoma, OS and, particularly, in bone metastases.
Dynamic contrast-enhanced study
In the characterization step, the dynamic contrast-enhanced (DCE) study can give additional diagnostic information, providing a noninvasive assessment of microcirculatory characteristics of a lesion. The curves obtained by plotting the SI changes over time provide qualitative and semiquantitative information, which reflect the passage of the contrast agent within the target tissue.
Some lesions exhibit specific enhancement patterns useful for their characterization. The marked enhancement of the nidus in the arterial phase makes the diagnosis of osteoid osteoma very specific even when it is located in atypical sites (Figure 6) [17]. An early enhancement with a steep slope followed by an early and rapid washout, has been proved to characterize GCT [18].
A DCE study can help to distinguish benign from malignant cartilaginous tumors. Benign chondromas do not enhance or enhance slowly, while chondrosarcomas display early enhancement (Figure 5) [19]. These rapidly enhancing areas can be very small and must be looked for very carefully on dynamic images.
Although some tumors can be characterized by the pattern of enhancement, the slope of the time–intensity curve (TIC) in dynamic sequences is not always reliable for differentiating benign from malignant lesions, because the overlap is too large. Therefore, TIC and slope values can help to formulate a reasonable diagnosis, only when taking into account all the other imaging features as well as the clinical data.
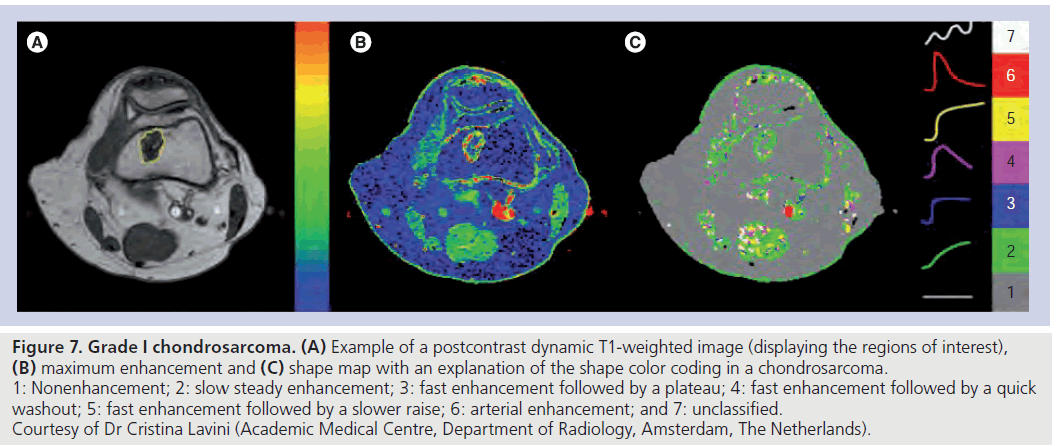
Interestingly, Lavini et al. proposed a new way to look at TICs, such that these are not averaged over a selected region of interest, but rendered pixel-by-pixel [20]. The authors applied this method to chondroid tumors, comparing the two approaches, and proposed the pixel-by-pixel analysis to be a more appropriate tool to classify the tumors (Figure 7). Owing to its simplicity, this method can be implemented in routine practice when a quantitative analysis of tissue permeability is not essential.
Figure 7.Grade I chondrosarcoma. (A) Example of a postcontrast dynamic T1-weighted image (displaying the regions of interest), (B) maximum enhancement and (C) shape map with an explanation of the shape color coding in a chondrosarcoma. 1: Nonenhancement; 2: slow steady enhancement; 3: fast enhancement followed by a plateau; 4: fast enhancement followed by a quick washout; 5: fast enhancement followed by a slower raise; 6: arterial enhancement; and 7: unclassified. Courtesy of Dr Cristina Lavini (Academic Medical Centre, Department of Radiology, Amsterdam, The Netherlands).
A more sophisticated approach using DCE imaging and deconvolution analysis allows the measurement of tumor blood flow (TBF); however, the role of this parameter in the characterization of bone tumors still needs to be clarified [21].
In summary, MRI has high specificity when a bone lesion presents particular features, specific morphology or a well-defined enhancement pattern. Some entities, such chondrogenic tumors, solitary cysts and ABC, GCT, lesions containing fatty tissue and, to a certain extent, osteoid osteomas, can be diagnosed with good reliability by MRI.
MR spectroscopy
Magnetic resonance spectroscopy (MRS) is an interesting application of MRI that can give additional information for tumor characterization by revealing the presence or absence of water-soluble choline metabolites. Choline is a component of phospholipid metabolism of cell membranes. The composition of membrane phospholipids in tumor tissue is an important indicator of tumor cellularity, proliferative capacity and differentiation state.
Increased choline probably reflects increased membrane synthesis or an increased number of cells, and can be detected in malignant bone and soft tissue tumors [22]. Nevertheless, in a study focused on the evaluation of the MRS features of GCT, the results indicated that this particular bone tumor may show raised choline levels on proton MRS. Therefore, this finding is not always an indicator of malignancy [23].
Therefore, although radiography remains the first modality able to characterize bone tumors, MRI may represent an adjunctive tool for characterization of bone lesions by evaluating SI, enhancement characteristics and growth patterns. It must be kept in mind that its major role is local staging, surgical planning and follow-up.
Post-treatment survey
To monitor the effect of chemotherapy or radiotherapy, the ideal technique should be reliable, reproducible, noninvasive and able to identify nonrespondent tumors early in the course of therapy. This could help to reduce unnecessary toxicity and optimize patient management. There is also a need to identify patients at higher risk of relapse for strategic therapeutic choices [24].
MRI is the conventional method used to monitor the response to therapy of bone tumors. The traditional method of measuring tumor size has proved to be of limited value as it does not reflect changes in tumor viability induced by therapy. Moreover, osseous tumors may respond well to therapy without substantially changing in size [25].
WHO and RECIST criteria take into consideration only the measurement of the maximum tumor diameter in the axial plane of CT and/ or MRI; for this reason they are not routinely used in the clinical practice.
The Japanese Orthopaedic Association (JOA) has conducted a retrospective validation study of the JOA criteria of preoperative chemotherapy for bone and soft tissue sarcomas, modifying the WHO/RECIST criteria. In malignant bone tumors, the JOA criteria are only directed to extraosseous involving bone lesions. In this method, the maximal distance from the surface of affected bone to the top of the extraosseous mass lesion is measured on CT and/or MRI images. The tumor reduction ratio is then calculated according to the following formula [26]:
Pretreatment MD - post-treatment MD/ pretreatment MD x 100%
Owing to the aforementioned limitations of the morphologic study, new methodological approaches have been evaluated. In particular, measuring changes in tumor perfusion might allow discrimination between responders and nonresponders, irrespective of volumetric response.
In an attempt to increase the accuracy of MRI in identifying treatment response, functional imaging techniques, such as DCE study and diffusion-weighted imaging (DWI), are being investigated.
DCE study
Dynamic contrast-enhanced study provides a method of quantifying TBF that better reflects tumor viability. Therefore, it may help monitor treatment response. It consists of a rapid acquisition associated with paramagnetic contrast media injection. High temporal resolution (<15 s) is mandatory to differentiate viable tumor zones from the avascular necrotic areas and fibrosis. Recent advances in MR systems and ultrafast MRI sequence provide dynamic sequences with higher temporal and spatial resolution. Usually, a 2D or 3D fast multiplanar spoiled gradient-echo or turbo-FLASH sequence is used; parallel imaging can be helpful to increase temporal resolution.
For interpretation of the DCE study data, different approaches have been proposed. A standard and simple approach is the qualitative assessment of the curve’s shape and its empirical semiquantitative analysis. The main parameters include peak enhancement, rate of enhancement and terminal slope. Post-treatment changes cause decreased vascular permeability of the tumor vessels, reflected by a decrease in the rate of enhancement. Moreover, wash-in accumulation can be encountered, caused by reduced diffusibility related to repair mechanisms [27]. Although these parameters are simple and straightforward, they lack reproducibility and comparability; in fact, they vary greatly depending on the dose and flow rate of contrast agent injection, acquisition protocols and scanners.
To overcome these limitations, a more sophisticated method of analysis has been developed, the so-called pharmacokinetic model. After converting the dynamic signal to contrast agent concentration, several pharmacokinetic parameters can be assessed on the basis of pharmacokinetic modeling. This analysis is able to define tumor microvascularization in a more precise way, thus allowing the prediction of the accessibility of the tumor to drugs before treatment and the assessment of the viability of the residual tumor after treatment.
By means of this model, some authors estimated the necrotic fraction that represents a predictor of good response to therapy in patients with OS and Ewing’s sarcoma. Furthermore, tumor size and the pharmacokinetic parameter kep (rate of contrast between the plasma and the tumor extravascular fluid space), calculated at the end of therapy, were significantly associated with the disease-free survival in pediatric OS [28,29]. The authors observed that the pharmacokinetic model was able to assess important parameters related to vascular permeability, perfusion and extravascular volume.
Another quantitative parameter that can be extracted by DCE study using deconvolution analysis is TBF. TBF maps are able to demonstrate heterogeneity of blood flow in tumors; a substantial decrease of TBF after chemotherapy has been reported [30]. Kajihara et al. calculated TBF and the steepest slope – determined from the time–intensity curve – in a group of patients with musculoskeletal lesions. They demonstrated a possible role of TBF in evaluating the preoperative treatment response in malignant tumors. Kajihara et al. also reported that TBF reflects therapeutic changes better than steepest slope, since the latter parameter is affected by factors other than tumor vascularity [31].
Despite the many research papers in this field, a clear consensus on the exact kinetic model to be used for analyzing DCE-MRI is still lacking and the empirical quantification of DCE data is still widely applied. This was highlighted in an interesting review that also reported the lack of any validation of the absolute perfusion parameters provided by DCE data [32]. Moreover, DCE-MRI data analysis is complex and influenced by several factors (choice of pharmacokinetic model, selection of artery input function and accuracy of the intrinsic baseline T1 measurement) that can significantly interfere with the accuracy and repeatability of DCE results [33–35].
Therefore, new approaches to analyze the data from DCE-MRI are required. Guo et al. proposed a novel method called curve pattern analysis. It automatically generates quantitative curve pattern analysis parameters, which are less sensitive to acquisition protocols, without requiring aortic input function and baseline T1 measurement [36]. This relatively simple method is able to characterize permeability and flow in the tissue according to the curve pattern. However, further studies are needed to verify its diagnostic accuracy.
In summary, the implementation of pharmacokinetic model analysis for the interpretation of the DCE study data in daily clinical practice has been hampered by the lack of a standardized methodology. The heterogeneity in scan acquisition and data analysis complicates the interpretation of study results; moreover, data analysis can be complex and time consuming. For these reasons, simpler methods are under investigation.
Diffusion imaging
Diffusion imaging relies on selective excitation of the water resonance and generation of a contrast image that depends on differential nuclear relaxation times and self-diffusion coefficients. Thus, DWI reflects the microstructural characteristics and physiological state of the tissues.
Post-therapeutic changes, such as cell membrane injury, cell death or a reduction in cell density, cause an expansion of the extracellular diffusion space and a greater degree of unrestricted extracellular water motion, leading to a high apparent diffusion coefficient (ADC). Therefore, viable and necrotic tumor tissue can be differentiated by means of ADC. In particular, necrotic tissue shows rapid diffusion, and thus higher ADC values, as a result of loss membrane integrity.
In 2006, two studies investigated whether DWI can be useful for monitoring the therapeutic response of primary bone tumors. The authors demonstrated that ADC changes in good responders were significantly higher than in nonresponders, thus making ADC value an interesting tool [37]. In particular, Uhl et al. correlated DCE study and DWI results, demonstrating that both methods allowed the recognition of tumor necrosis induced by chemotherapy in OS. By evaluating microvessel density, vascular permeability, local blood volume and flow, the authors hypothesized that DWI correlates directly with tumor necrosis, while DCE indirectly reflects tumor necrosis [38].
In a recent study of patients with OS treated with chemotherapy, the authors assumed that the minimum ADC reflects the highest cellular component in the tumor, providing more valuable information. By means of the minimum ADC calculated in the solid tumor components, they demonstrated a significant difference between good and poor responders, while no statistically significant difference was observed in the average ADC ratio. Based on their results, the minimum ADC is considered preferable for evaluating the histological response to chemotherapy in OS than the average ADC [39].
MR spectroscopy
Magnetic resonance spectroscopy can also have a role in monitoring patients with bone tumor by identifying the early metabolite changes after chemotherapy. Decline of choline after treatment in malignant bone tumors has been reported. MRS results correlate with the DCEMRI data and tumor size. Therefore, proton MRS could improve the diagnostic specificity of MR examination in the follow-up of tumor treatment [40].
In conclusion, MRI shows promising results in assessing the response to neoadjuvant chemotherapy and may be useful in the early differentiation of good and poor responders. This distinction is fundamental since it heavily influences subsequent therapeutic choices. In fact, based on this element, it is possible to modify the treatment plan in nonresponders, either by introducing different pharmaceutical drugs or by anticipating surgical intervention.
Future perspective
WB-MRI & higher field magnets
Significant improvements in hardware, with the introduction of a multireceiver channel scanner with automated free table movement, have made WB��?MRI clinically feasible. Moreover, innovation in sequence design and image acquisition, such as the parallel strategy, have reduced overall examination time.
In the field of oncologic imaging, various useful application have been proposed for WB��?MRI [41]. In particular, the STIR and DWI sequences have been demonstrated to be more sensitive than bone scintigraphy for the detection of cortical bone and bone marrow metastasis [42]. Therefore, patients might benefit from early accurate staging and improved therapeutic options.
As a WB bone marrow screening method, WB��?MRI is a useful tool for the precise assessment of the total skeletal status and is highly effective in staging specific malignant bone marrow diseases, such as multiple myeloma, lymphoma or early bone metastasis.
In particular, WBâ�?�?MRI has been demonstrated to be a sensitive tool in the initial work-up of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. In the study of Bäuerle et al., a half of all observed lesions would have been missed by standard spinal MRI. The authors also reported that clinical parameters could not exclude the presence of extra-axial lesions [6].
In childhood lymphoma, in which marrow involvement is usually focal and can be missed at blind biopsy, STIR WB��?MRI represents a complementary imaging tool to detect otherwise unrecognized marrow involvement. However, it is important to note that the abnormal findings may be unspecific and must be interpreted in the context of the clinical setting and other imaging results [43].
Recently approved clinical WB��?MRI scanners with a field strength of 3 T have become commercially available. This has opened the way for a migration of multiorgan and WB protocols to a higher field strength. Thus, 3 T imaging has the potential to improve small lesion evaluation and tumor staging, and it can be used for WB screening for metastasis. The gain of signal-to-noise ratio (SNR) reduces the overall examination time without compromising contrast resolution, especially for the acquisition of T2w imaging. Furthermore, the increased SNR at 3 T allows the recording of highly resolved isotropic 3D data sets with shorter acquisition times. High SNR and the ability to acquire high-resolution thin section images are major advantages of 3 T that benefit musculoskeletal imaging [44]. Furthermore, 3 T MRI provides good quality images even for small field of views, which can be an advantage in children. The maximum contrast- to-noise ratio also depends on the magneticfield strength and it has been demonstrated that the contrast-to-noise ratio between muscle and bone, bone and cartilage, and cartilage and fluid is higher at 3 T than at 1.5 T [45].
The implementation of hyperechoes and variable flip angle techniques can keep specific absortion rate (radiofrequency energy absorbed by the body tissues) levels within a tolerable range.
Concerning MRS, sensitivity for metabolite detection and quantification are expected to improve at 3 T owing to the increased SNR and resolution compared with lower-fieldstrength scanners. In fact, the increased absolute chemical-shift separation at 3.0 T may be advantageous since it results in a better resolution of distinct resonances in MRS and tends to cause spin systems to be less strongly coupled. Thus, more metabolites and coupling patterns can be identified and quantification is enabled [45,46].
In a recent paper, MRS at 3 T has been used to characterize musculoskeletal lesions. Lee et al. assessed whether the concentration of choline correlated with the pathologic degree of malignancy. They concluded that MRS is a helpful method with high specificity, but relatively low sensitivity [47].
MR thermography & MR-guided interventions
Recent advances in MRI technology have provided noninvasive imaging for guiding focused ultrasound treatment, because of its capability to monitor tissue temperature elevation and thermal coagulation. The integration of MRI and focused ultrasound surgery has resulted in realtime, image-controlled, noninvasive thermal ablation systems.
Using MRI as a guiding method, it is possible to accurately delineate the tumor, allowing precise 3D treatment planning. Moreover, the continuous temperature mapping in the treatment zone (MR thermography) avoids thermal injury of normal tissues and offers the unique potential for closed-loop feedback control of tissue heating and for direct measurement of the deposited thermal dose. This can significantly enhance the safety and efficacy of focused ultrasound surgery [48]. In a recent study the authors reported their successful results using MRI-guided focused ultrasound treatment in patients with bone metastases. The lack of adverse events, short treatment duration and the ability to perform the procedure on an outpatient basis make MRIguided focused ultrasound an effective palliative treatment in selected patients with painful bone metastases [49,50].
In conclusion, the role of MRI in the local staging of bone tumors is well established, as is its capability in characterizing some specific pathologic entities.
The more recent studies are focused on interpreting perfusion parameters as possible predictors of post-treatment response and survival. Despite strong efforts and the interesting results concerning DCE study and diffusion imaging, up to now, the gold standard in post-treatment survey is still resection specimen histology, which influences the therapeutic strategy. Nevertheless, this fact should not impede the search for newer, more reliable methods of analysis.
For example, a correlation between VEGF expression, assessed in biopsy and resected specimens by immunohistochemistry in OS, and vascular permeability, detected by DCE, is reported in a noteworthy paper. This study suggests an important role of DCE-MRI as a noninvasive imaging surrogate of tumor angiogenesis in OS [51].
Any discussion of the monitoring of post-treatment changes must include PET. FDG PET has opened a new avenue in the evaluation of response to treatment. Although the method is not without limitations, it has some important advantages in the case of tumors. One particular advantage of FDG PET imaging is that it is a quantitative study from which statistical measurements can be determined. The ability to distinguish viable from nonviable tumors is perhaps the most powerful attribute of FDG PET imaging and explains its increasing use among clinicians in recent years [52]. Several studies have demonstrated the capability of FDG PET to determine the response after neoadjuvant chemotherapy for primary tumors of bone, although discrepancies in the results are reported. In the study by Igara et al., the pathologically determined degree of necrosis postneoadjuvant chemotherapy was only concordant with PET in 57.1% of cases, while a significant number of patients had discrepancies [53]. These discrepancies may, in part, be explained by chemotherapy-induced inflammation, an effect that can potentially cause increased FDG uptake. This element should be considered during post-therapy PET interpretation in bone and soft tissue sarcoma.
Nowadays, the role of FDG PET in some pathologies is well defined. In patients with lymphomas, FDG PET has been established as the imaging modality of choice for staging and treatment monitoring. MRI maintains a role only in selected cases, such as those with a high risk of bone marrow involvement, equivocal findings on PET, extracompartmental tumor growth or to evaluate potential treatment complications. Already established in many institutions, future developments will almost certainly include ‘fusion’ or ‘hybrid’ imaging methods, such as PET-CT and PET-MR in the treatment monitoring of patients. This fascinating technique opens up new research horizons and offers potential for the development of more specific radiotracers.
Higher field magnets (up to 7 T) will further increase the potentials of MRI in the diagnostic and screening phase. The increasing availability of 3 T equipment will reveal the high field’s actual advantages and the proper indications for its use. Much of the literature mainly describes the application concerning joint structure evaluation, while little has been written on the neoplastic pathology of the musculoskeletal system. The performance of MRI perfusion and diffusion sequences as well as spectroscopy could probably be improved at higher magnetic fields. In particular, WBâ�?�?MR diffusion imaging is a novel and promising technique that may contribute to superior sensitivity in the detection of tumor manifestations.
In the assessment of distant metastatic spread WB��?MRI is highly sensitive and has advantages over PET/CT, especially in tumors that frequently spread to the liver, bone or brain. WB��?MRI is also very attractive as a radiationfree alternative for imaging pediatric tumor patients who may require multiple follow-up examinations. It allows for precise assessment of the bone marrow and has been proven to be highly accurate for the staging of hematologic diseases, such as multiple myeloma, and in screening for metastasis.
Finally, minimally invasive surgery will potentially greatly benefit from MR guidance. To date, the only reported applications are bone metastases and in patients treated with external beam radiation therapy and hyperthermia for high-grade extremity soft tissue sarcomas [54].
In the literature, reports dedicated to the application of ultrasonographically guided highintensity focused ultrasound in the salvage of limbs in patients with OS can be found [55]. Therefore, we can expect an expansion of this field in the future and a possible new application for MR thermography.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as: * of interest
*of considerable interest
References
- Miller TT: Bone tumors and tumor-like conditions: analysis with conventional radiography. Radiology 246(3), 662–674 (2008).
- Cheon GJ, Kim MS, Lee JA et al.: Prediction model of chemoteraphy response in osteosarcoma by 18F-FDG PET and MRI. J. Nucl. Med. 50(9), 1435–1440 (2009). & Analyzes combined information on 18F-FDG PET and MRI scans – acquired before and after chemotherapy – to predict histologic response to neoadjuvant chemotherapy in osteosarcoma.
- Hamada K, Tomita Y, Inoue A et al.: Evaluation of chemotherapy response in osteosarcoma with FDG PET. Ann. Nucl. Med. 23, 89–95 (2009).
- Hwang S: Imaging of lymphoma of the musculoskeletal system. Radiol. Clin. North Am. 46, 379–396 (2008).
- Baur-Melnyk A, Buhmann S, Becker C et al.: Whole-body MRI versus whole-body MDCT for staging of multiple myeloma. AJR Am. J. Roentgenol. 190, 1097–1104 (2008).
- Bäuerle T, Hillengass J, Fechtner K et al.: Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imaging. Radiology 252, 477–485 (2009).
- Saifuddin A: The accuracy of imaging in the local staging of appendicular osteosarcoma. Skeletal Radiol. 31, 191–201 (2002).
- James SLJ, Panicek DM, Davies AM: Bone marrow oedema associated with benign and malignant bone tumors. Eur. J. Radiol. 67, 11–21 (2008).
- Sajadi KR, Heck RK, Neel MD et al.: The incidence and prognosis of osteosarcoma skip metastases. Clin. Orthop. Relat. Res. 426, 92–96 (2004)
- Ehara S: MR imaging in staging bone tumors. Cancer Imaging 6, 158–162 (2006).
- Kim MS, Lee SY, Cho WH: Growth patterns of osteosarcoma predict patient survival. Arch. Orthop. Trauma Surg. 129, 1189–1196 (2009). & Retrospective study in which the authors examine whether tumor growth patterns correlate with clinicopathologic variables.
- Alyas F, James SL, Davies AM, Saifuddin A: The role of MR imaging in the diagnostic characterization of appendicular bone tumors and tumor-like conditions. Eur. Radiol. 17, 2675–2686 (2007).
- Gelderblom H, Hogendoorn PC, Dijkstra SD et al.: The clinical approach towards chondrosarcoma. Oncologist 13(3), 320–329 (2008)
- Littrell LA, Wenger DE, Wold LE et al.: Radiographic, CT, and MR imaging features of dedifferentiated chondrosarcomas: a retrospective review of 174 de novo cases. Radiographics 24, 1397–1409 (2004).
- Bertoni F, Bacchini P, Staals EL, Davidovitz P: Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer 103(11), 2373–2382 (2005).
- Azura M, Vanel D, Alberghini M, Picci P, Staals E, Mercuri M: Parosteal osteosarcoma dedifferentiating into telangiectatic osteosarcoma: importance of lytic changes and fluid cavities at imaging. Skeletal Radiol. 38(7), 685–690 (2009).
- Zampa V, Bargellini I, Ortori S, Faggioni L, Cioni R, Bartolozzi C: Osteoid osteoma in atypical locations: the added value of dynamic gadolinium-enhanced MR imaging. Eur. J. Radiol. 71(3), 527–535 (2009).
- Libicher M, Bernd L, Schenk JP, Mädler U, Grenacher L, Kauffmann GW: Characteristic perfusion pattern of osseous giant cell tumor in dynamic contrast-enhanced MRI. Radiologe 41(7), 577–582 (2001).
- Geirnaerdt MJ, Hogendoorn PC, Bloem JL, Taminiau AH, van der Woude HJ: Cartilaginous tumors: fast contrast-enhanced MR imaging. Radiology 214(2), 539–546 (2000).
- Lavini C, Pikaart BP, de Longe MC, Schaap GR, Maas M: Region of interest and pixel-by-pixel analysis of dynamic contrast enhanced magnetic resonance imaging parameters and time–intensity curve shapes: a comparison in chondroid tumors. Magn. Reson. Imaging 27, 62–68 (2009). & Proposes a new approach for analyzing the dynamic study and applied this simpler method for evaluating the behavior of chondroid tumors.
- Kajihara M, Sugawara Y, Sakayama K , Kikuchi K, Mochizuki T, Murase E: Evaluation of tumor blood flow in musculoskeletal lesions: dynamic contrastenhanced MR imaging and its possibility when monitoring the response to preoperative chemotherapy – work in progress. Radiat. Med. 25, 94–105 (2007).
- Wang CK, Li CW, Hsieh TJ, Chien SH, Liu GC, Tsai KB: Characterization of bone and soft-tissue tumors with in vivo 1H MR spectroscopy: initial results. Radiology 232, 599–605 (2004).
- Sah PL, Sharma R, Kandpal H et al.: In vivo proton spectroscopy of giant cell tumor of the bone. AJR Am. J. Roentgenol. 190(2), 133–139 (2008).
- Lewis VO: What’s new in musculoskeletal oncology. J. Bone Joint Surg. Am. 91, 1546–1556 (2009).
- McCarville MB: New frontiers in pediatric oncologic imaging. Cancer Imaging 8, 87–92 (2008).
- Ueda T, Naka N, Araki N et al.: Validation of radiographic response evaluation criteria of preoperative chemotherapy for bone and soft tissue sarcomas: Japanese Orthopaedic Association Committee on Musculoskeletal Tumors Cooperative Study. J. Orthop. Sci. 13, 304–312 (2008).
- Knopp MV, von Tengg-Kobligk H, Choyke PL: Functional magnetic resonance imaging in oncology for diagnosis and therapy monitoring. Mol. Cancer Ther. 2, 419–426 (2003).
- Dyke JP, Panicek DM, Meyers PA et al.: Osteogenic and Ewing sarcoma: estimation of necrotic fraction during induction chemotherapy with dynamic contrastenhanced MR imaging. Radiology 228, 271–278 (2003).
- Reddick WE, Wang S, Xiong X et al.: Dynamic magnetic resonance imaging of regional contrast access as an additional prognostic factor in pediatric osteosarcoma. Cancer 91(12), 2230–2237 (2001).
- Sugawara Y, Murase K, Kikuchi K et al.: Measurement of tumor blood flow using dynamic contrast-enhanced magnetic resonance imaging and deconvolution analysis: a preliminary study in musculoskeletal tumors. J. Comput. Assist. Tomogr. 30, 983–990 (2006).
- Kajihara M, Sugawara Y, Sakayama K, Kikuchi K, Mochizuki T, Murase K: Evaluation of tumor blood flow in musculoskeletal lesions: dynamic contrastenhanced MR imaging and its possibility when monitoring the response to preoperative chemotherapy – work in progress. Radiat. Med. 25(3), 94–105 (2007).
- De Langen AJ, Van de Boogaart VEM, Marcus JT, Lubberink M: Use of H2 15O-PET and DCE-MRI to measure tumor blood flow. Oncologist 13, 631–644 (2008). & Interesting comparison between H2 15O-PET and dynamic contrast-enhanced (DCE)-MRI. The authors discuss the principles of perfusion imaging with H2 15O-PET and DCE-MRI and compare the differences and limitations of the two methods, and review publications on the use of both methods in monitoring response to anticancer therapy.
- Huang W, Wang Y, Panicek DM, Schwartz LH, Koutcher JA: Feasibility of using limited-population-based average R10 for pharmacokinetic modeling of osteosarcoma dynamic contrast-enhanced magnetic resonance imaging data. Magn. Reson. Imaging 27, 852–858 (2009).
- Wang Y, Huang W, Panicek DM, Schwartz LH, Koutcher JA: Feasibility of using limited-population-based arterial input function for pharmacokinetic modeling of osteosarcoma dynamic contrast-enhanced MRI data. Magn. Reson. Med. 59(5), 1183–1189 (2008).
- Guo JY, Reddick WE, Rosen MA, Song HK: Dynamic contrast-enhanced magnetic resonance imaging parameters independent of baseline T10 values. Magn. Reson. Imaging 27(9), 1208–1215 (2009).
- Guo JY, Reddick WE: DCE-MRI pixel-bypixel quantitative curve pattern analysis and its application to osteosarcoma. J. Magn. Reson. Imaging 30, 177–184 (2009). & A novel method to characterize and quantify signal curves from the DCE-MRI data without any prerequisites, such as aortic input function or T1 measurement, is presented.
- Hayashida Y, Yakushiji T, Awai K et al.: Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur. Radiol. 16, 2637–2643 (2006).
- Uhl M, Saueressig U, van Buiren M et al.: Osteosarcoma: preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusionweighted magnetic resonance imaging. Invest. Radiol. 41(8), 618–623 (2006).
- Oka K, Yakushiji T, Sato H, Hirai T, Yamashita Y, Mizuta H: The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol. 39, 141–146 (2010).
- Hsieh TJ, Li CW, Chuang HY, Liu GC, Wang CK: Longitudinally monitoring chemotherapy effect of malignant musculoskeletal tumors with in vivo proton magnetic resonance spectroscopy: an initial experience. J. Comput. Assist. Tomogr. 32(6), 987–994 (2008).
- Schmidt GP, Reiser MF, Baur-Melnyk A: Whole-body MRI for the staging and follow-up of patients with metastasis. Eur. J. Radiol. 70, 393–400 (2009).
- Nakanishi K, Kobayashi M, Nakaguchi K et al.: Whole body MRI for detecting metastatic bone tumor: diagnostic value of diffusion-weighted images. Magn. Reson. Med. Sci. 6(3), 147–155 (2007).
- Kellenberger CJ, Epelman M, Miller SF, Babyn PS: Fast STIR whole-body MR imaging in children. Radiographics 24(5), 1317–1330 (2004).
- Kuo R, Panchal M, Tanenbaum L, Crues JV 3rd: 3.0 Tesla imaging of the musculoskeletal system. J. Magn. Reson. Imaging 25, 245–261 (2007).
- Bolog N, Nanz D, Weishaupt D: Muskuloskeletal MR imaging at 3.0 T: current status and future perspectives. Eur. Radiol. 16, 1298–1307 (2006).
- Fayad LM, Barker PB, Jacobs MA et al.: Characterization of musculoskeletal lesions on 3-T proton MR spectroscopy. AJR Am. J. Roentgenol. 188, 1513–1520 (2007).
- Lee CW, Lee JH, Kim DH et al.: Proton magnetic resonance spectroscopy of musculoskeletal lesions at 3 T with metabolite quantification. Clin. Imaging 34(1), 47–52 (2010).
- Jolesz FA, Hynynen K, McDannold N, Tempany C: MR imaging-controlled focused ultrasound ablation: a noninvasive imageguided surgery. Magn. Reson. Imaging Clin. N. Am. 13, 545–560 (2005).
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M: Palliative treatment of painful bone metastases with MR imaging – guided focused ultrasound. Radiology 249(1), 355–363 (2008).
- Liberman B, Gianfelice D, Inbar Y et al.: Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann. Surg. Oncol. 6(1), 140–146 (2009). & Clinical results of a multicenter study using magnetic resonance-guided focused ultrasound for palliation of bone metastases.
- Bajpai J, Gamanagatti S, Sharma MC et al.: Noninvasive imaging surrogate of angiogenesis in osteosarcoma. Pediatr. Blood Cancer 54(4), 526–531 (2010).
- Biswal S, Resnick DL, Hoffman JM, Gambhir SS: Molecular imaging: integration of molecular imaging into the musculoskeletal imaging practice. Radiology 244(3), 651–671 (2007).
- Iagaru A, Masamed R, Chawla SP et al.: F-18 FDG PET and PET/CT evaluation of response to chemotherapy in bone and soft tissue sarcomas. Clin. Nucl. Med. 33, 8–13 (2008).
- Craciunescu OI, Stauffer PR, Soher BJ et al.: Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med. Phys. 36(11), 4848-4858 (2009).
- Li C, Wu P, Zhang L, Fan W, Huang J, Zhang F: Osteosarcoma: limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound. Cancer Biol. Ther. 8(12), 1102–1108 (2009).