Review Article - Imaging in Medicine (2010) Volume 2, Issue 2
MRI of the colon
Marije P van der Paardt†, Frank M Zijta and Jaap Stoker
University of Amsterdam, Meibergdreef 9, 1100 AZ, Amsterdam, The Netherlands
- *Corresponding Author:
- Marije P van der Paardt
Department of Radiology, Academic Medical Center
University of Amsterdam, Meibergdreef 9, 1100 AZ
Amsterdam, The Netherlands
Tel: +31 205 662 432
Fax:+31 205 669 119
E-mail: m.p.vanderpaardt@amc.uva.nl
Abstract
Magnetic resonance imaging of the colon is widely used for the diagnosis and treatment of anorectal disorders, but other applications can be considered as well. In this article we discuss the application of MRI in colorectal cancer, inflammatory bowel disease (Crohn’s disease and ulcerative colitis) and acute abdominal pain. The latter concerns MRI without a specific preparation while the other two applications concern the use of bowel preparation and colon distension (magnetic resonance colonography). CT is now the principal technique for these applications. The major advantage of MRI of the colon over CT is the lack of ionizing radiation exposure. The high soft-tissue contrast might be another advantage. Research over the last decade has demonstrated that MRI is a valuable alternative to CT, with accuracy values equal or superior to CT. Colorectal diffusion-weighted imaging has been increasingly studied with promising results.
Keywords
3 T MRI; acute abdominal pain; colorectal cancer screening ; diffusion-weighted imaging; inflammatory bowel disease ; magnetic resonance colonography
Magnetic resonance imaging is increasingly used in the management of patients with colorectal diseases. MRI is an established technique for the evaluation and staging of anorectal diseases (e.g., rectal cancer, perianal fistulas) and readers are referred to current available literature for information on anorectal MRI [1]. In this clinically orientated article we discuss the application of MRI of the colon in several new fields: colorectal cancer, inflammatory bowel disease (Crohn’s disease and ulcerative colitis) and acute abdomen.
Colorectal cancer
To date, conventional colonoscopy is considered the most accurate diagnostic tool for diagnosing colorectal cancer (CRC) and precursors of CRC (adenomas) [2]. Colonoscopy combines detection of lesions with polypectomy and histopathology, which makes it a powerful technique. Yet, the relatively invasive nature of the procedure leads to high procedural discomfort, which ultimately results in poor participation in screening populations for CRC. At this point a noninvasive alternative would be valuable, especially as the risk of significant lesions (and thus the need for colonoscopy) is relatively low in screening (0.5–1.0% for colon cancer and 5–10% for advanced neoplasia) [3]. Aside from the invasive character, colonoscopy is characterized by an additional drawback, concerning the high rates of incomplete examinations. A retrospective study reported that 81% of conventional colonoscopies in a large population were completed (4304 patients and 5145 colonoscopies); failures were mainly due to the presence of stenosis, abdominal adhesions, elongated bowel segments and inadequate bowel preparation [4]. In addition, a prospective study of colonoscopy practice in the UK demonstrated that only 56.9% of colonoscopies could be objectively confirmed as complete [5]. Nonetheless, efforts have been made to decrease the number of incomplete colonoscopies presently resulting in high completion rates [6,7]. Alternative methods to visualize the colon have been studied. Double-contrast barium enema has been surpassed by CT colonography. During the last decade, CT colonography and, to a lesser extent, using magnetic resonance (MR) has been a focus of research and major impetus for studying these modalities were the limited invasiveness and the additional imaging of extraluminal structures. CT colonography has been studied extensively for this purpose and its diagnostic value has been proven [8,9]. The results and availability have resulted in the recommendation of CT colonography as a screening tool for CRC by the American Cancer Society [2].
A drawback of CT colonography is the lifetime cancer risk associated with the radiation exposure to CT colonography, which was estimated to be 0.14% for 50 year olds. Efforts have been made to further decrease the radiation exposure by optimizing scan protocols, including the use of dose-reduction strategies [2,10,11]. However, the risk is not negligible and thus a noninvasive alternative without ionizing radiation exposure would be valuable.
The use of MRI of the colon with bowel preparation and colon distension (MR colonography) offers a potential instrument for evaluation of the colon, comparable to CT colonography and colonoscopy. As advances in MR technology have resulted in reduced acquisition times and reduced artifacts related to peristalsis and respiration, MR colonography has been increasingly studied over the last decade [12,13]. The lack of ionizing radiation and high soft-tissue contrast are considerable advantages of MRI over CT colonography. MR colonography is comparable to CT colonography with respect to noninvasiveness [14,15] and assessment of extracolonic structures [16,17]. However, the lack of consensus concerning bowel preparation and acquisition methods [18], and the higher costs and more limited availability have hampered the use of MRI [12].
Detection of polyps & colorectal carcinoma
The potential risk of patients developing CRC from colorectal adenomas is related to both size and histology [19]. Importantly, at colonography no histological distinction can be made between the two principal categories of colorectal polyps: adenomas and hyperplastic polyps. Thus, measurement is the most important criterion for estimating the risk of malignancy at colonography. For this purpose, colorectal polyps can be stratified into three generally accepted size thresholds reflecting the potential risk to contain or progress into cancer: polyps with a size of 10 mm or larger, intermediate polyps (6–9 mm) and polyps smaller than 6 mm [19]. Polyps with a size of 10 mm or larger are almost always adenomas and the risk of malignancy is substantial (>10% and increasing with size) [19]. Intermediate-sized polyps are generally adenomas and hyperplastic polyps. Although the risk of malignancy in these adenomas is low (<1%), it is not negligible. Hyperplastic polyps are considered to have very low risk of malignant transformation (serrated adenoma). Although diminutive polyps (<6 mm) are mostly hyperplastic polyps and can be disregarded [19], it has to be taken into account that some hyperplastic polyps may not be benign [20].
Scan basics
To ensure high-signal homogeneity and low imaging distortion, the magnet should have high homogeneity for the total scanned volume. A high magnetic field is required for an adequate signal-to-noise ratio. In this respect, field strengths of 1.5 T are preferred since the prevalence of artifacts is low and acquisition times are short [21], which permits data collection under acceptable breath-hold conditions.
To assure high spatial resolution for MRI of the colon, short repetition and echo times are required to perform fast imaging with balanced steady-state precession sequences and to obtain T1‑weighted images. In addition, short acquisition times allow coverage of a large imaging volume during one single breath-hold.
Since the integrated body coil is not sufficient for a high frequency signal reception, phased array coils are necessary to ensure a high signalto- noise ratio and covering of the anatomical area of interest. Furthermore, when available, parallel imaging should be used, enabling an increase of spatial resolution or decreased scan times.
Patient preparation
Magnetic resonance colonography is highly dependent on adequate colonic distension and bowel preparation, the latter either by bowel cleansing or fecal tagging. These are key elements for sufficient visualization of colorectal polyps and cancer. Optimal differentiation between the bowel wall and lumen is essential for identification of pathology. Either a dark- or bright-lumen strategy can be used for this purpose, referring to the signal intensity of the bowel contents at T1‑weighted sequences (see sections on fecal tagging and bright and dark lumen). Residual stool can mimic or mask colorectal pathology, which may lead to false-positive and false-negative findings [22].
Bowel cleansing
Bowel cleansing can be achieved by substances used for bowel purgation prior to colonoscopy or CT colonography. Polyethylene-glycolelectrolyte solutions and sodium phosphate solutions are generally applied for bowel purgation. The latter, however, is hyperosmolar and can lead to electrolyte imbalances and might, ultimately, lead to renal damage [23]. When extensive bowel preparation is used for MR colonography, the examination should preferably be done in the morning after bowel preparation in order to reduce patient discomfort.
While bowel purgation is generally accepted in clinical practice, patients consider cleansing as burdensome and one of the most unpleasant elements of colonoscopy [14,15,24]. Similar to CT colonography, no visual inspection of the bowel is performed with MR colonography and, consequently, an adequate contrast between bowel wall (and pathology) and bowel content is sufficient for diagnostic evaluation. This allows the application of a limited bowel preparation, which can be regarded as an important advantage over colonoscopy [15]. For this purpose, tagging of bowel content (fecal tagging) is mandatory.
Fecal tagging
Fecal tagging in MR colonography is used to homogenously label fecal material with the ingestion of oral contrast media that modify the signal intensity of the bowel contents. This increases the ability to adequately differentiate the colonic lumen from the colonic wall and can be used to decrease the burden of bowel preparation [15]. In addition to the modification of signal characteristics of the bowel content, fecal tagging contrast agents should mix well with the bowel content, should not be reabsorbed and should be easy to ingest. For both dark- and bright-lumen approaches, several fecal tagging strategies have been proposed in recent MR colonography literature. In dark-lumen MR colonography, large volumes of highly concentrated barium sulfate were traditionally studied as a tagging agent for a low signal intensity of the bowel lumen, which resulted in excellent lumen– wall differentiation on T1‑weighted imaging if applied in conjunction with contrast enhancement of the bowel wall [15]. MR colonography demonstrated high sensitivity (91%) for any sized colorectal lesion and a specificity of 91.7% using a 200 ml barium-based contrast agent with each meal starting 36 h prior to the exam [25]. However, the initial encouraging results were tempered by the study of Goehde et al. where poor patient acceptance was found for a similar barium-sulfate tagging preparation (6 × 150 ml barium sulfate) [26]. The barium-based tagging was rated more uncomfortable than the bowel cleansing for conventional colonoscopy. Painful constipation and stool thickening were reported as the most disturbing factors. Furthermore, the barium-based tagging in this study did not provide sufficient stool darkening, resulting in a poor image quality in 18% of the scans. As a consequence, moderate results in lesion detection were found [26].
To date, various barium-based fecal tagging approaches have been proposed in dark-lumen MR colonography, in order to improve both lumen homogeneity and patient acceptance. In a prospective study [27] an alternative bariumbased fecal tagging strategy was performed for the assessment of patient acceptance using a solution containing 5% gastrografin, 1% barium and 0.2% locust bean gum. In this study the fecal tagging strategy was considered significantly less burdensome than bowel purgation. However, no significant difference in overall acceptance for both procedures was noted [27]. Diagnostic accuracy for detecting patients with colorectal polyps larger than 10 mm was 70% and specificity was reported as 100% in a study with an identical fecal tagging strategy [28]. Furthermore, Achiam et al. proposed the use of ferumoxsil, a negative contrast tagging agent, owing to its paramagnetic features resulting in decreased stool intensity, even at short echo time [29]. In this study, the tagging efficiency proved to be significantly better using barium sulfate/ferumoxsil compared with barium sulfate alone [29]. Based on the results of the initial study, Achiam and colleagues implemented this combined tagging strategy in a prospective study in 56 patients and reported acceptable per-patient sensitivity and specificity rates of 100 and 91.4%, respectively, for detecting patients with polyps larger than 10 mm [30].
In a prospective feasibility study [31], performed in our center, three different fecal tagging preparation strategies were applied and compared with respect to image quality and patient acceptance in a surveillance group. Two dark-lumen strategies were applied with barium-based contrast agents and one brightlumen strategy with gadolinium in combination with a low-fiber diet. The gadolinium-based strategy was rated best and demonstrated better diagnostic confidence, although no valid conclusions could be drawn regarding polyp detection given the limited number of patients [31]. The latter bright-lumen approach was subsequently evaluated in a prospective study comparing the MR colonography preparation with full preparation for colonoscopy in 209 surveillance patients. Significantly less discomfort was demonstrated for the MR colonography bowel preparation in comparison to the colonoscopy bowel preparation. Furthermore, MR colonography, without extensive preparation, was preferred to colonoscopy [15]. In this cohort the per-patient sensitivity was 75% (9/12) for polyps of 10 mm or larger and per-patient specificity was 93% (175/188). Per-polyp sensitivity was 77% (17/22) for polyps of 10 mm or larger [32].
A limitation of this bowel preparation fecal tagging strategy is that no immediate colonoscopy following MR colonography is feasible as the colon is insufficiently cleansed.
Spasmolytic agents
Intravenously administered spasmolytic agents contribute to bowel distension and spasmolysis providing a higher patient acceptance of rectally administered enemas and a decrease of artifacts [33]. The half-life of spasmolytic agents is short; therefore, administration should be carefully planned. Glucagon and butylscopolamine are frequently used agents. Glucagon relaxes smooth muscles, although the colon is less sensitive to the effects compared with, for instance, small bowel. Butylscopolamine is not approved by the US FDA but is regularly used in Europe. It also relaxes smooth muscle and is believed to distend the colon more effectively than glucagon [33].
Colonic distension
Under physiological circumstances, colonic bowel loops are collapsed. Whereas a collapsed segment may mimic pathological bowel wall thickening, this might contribute to false-positive findings of tumor or inflammation. Furthermore, unfolded bowel segments may mask polyps or even colonic masses [34]. Consequently, sufficient distension is a prerequisite for detection of (precursors of) CRC.
In MR colonography, water-based enemas are common bowel distending methods, consisting of either warm tap water [25,28,35–37] or a mixture of gadolinium and water [15]. Additionally, insufflation of air or CO2 is also reported for this purpose [38], which is common practice in CT colonography [8]. Yet, susceptibility artifacts hampered the use of gaseous media for insufflation in initial studies [39], but improved techniques enabled MR colonography distension by gaseous insufflation [40]. Water-based enemas give a constant distension of the colon, while in the case of manual insufflation, the intracolonic pressure might vary due to gas incontinence and ileocecal reflux.
In MR colonography, using water-based enemas, usually 1–3 l of tap water is administered [18] by a rectal canule under hydrostatic pressure. Water has a biphasic signal enabling colonic lumen differentiation from the low-signal bowel wall on T2‑weighted series. Preliminary MRI of the colon was performed with water-based barium solutions [12], but was abandoned as water alone demonstrated similar imaging quality on dark-lumen MR colonography T1‑weighted sequences [36]. Furthermore, water can be labeled with a gadolinium-containing contrast agent [41] allowing bright-lumen imaging (see bright lumen section).
Studies using gaseous agents for colonic distension in MR colonography are fairly limited; nonetheless insufflation of the colon with CO2 or room air has been evaluated in a few studies [38,40,42]. Bowel distension by insufflation results in low signal intensity of the bowel lumen at T1- and T2‑weighted sequences. Similarly to CT colonography, automated CO2 insufflation can be used. Here the intracolonic pressure is monitored, with additional administration in case of ileocecal reflux and gas incontinence preventing decreased distension. Importantly, an enema is considered the most burdensome part of a MR colonography examination, as patients feel uncomfortable preventing spill of the large volume of fluid in the colon [15]. Leakage of air or CO2 is considered far less problematic and, moreover, an additional advantage of CO2 is the better absorption in the colonic wall, resulting in less discomfort after the procedure as compared with room air. Until now, no MRI compatible CO2 insufflator is available and, thus, a CT colonography insufflator with long tubing to the MRI suite is needed for this purpose. Nonetheless, so far, no studies have reported on the use of automated CO2 insufflation in MR colonography.
In seven patients with identified colon carcinoma the diagnostic accuracy of MR colonography for depiction of colon carcinoma was evaluated with appliance of CO2 enemas [38]. The results were promising as all carcinomas were correctly detected. Distension with room air, however, demonstrated less encouraging results in a study with 156 patients, at average or increased risk for CRC, where only four out of 31 patients with colorectal polyps were identified due to physiological artifacts, moderate distension and fecal residue [43]. Conversely, Ajaj et al. compared room air insufflation with waterbased enemas with respect to patient acceptance and image quality [40]. No significant differences were found and conclusions were drawn that both techniques perform equally well in colonic distension and signal-to-noise ratios.
Patient acceptance for the aforementioned distension methods vary considerably. MR colonography with air-based distension compared with colonoscopy was found to be equally burdensome in 165 patients at high risk for CRC [43], but colonoscopy was preferred, most likely due to sedation and shorter examination times.
Ajaj et al. demonstrated comparable discomfort for water-based enemas compared with airbased distension in MR colonography, randomly performed in 50 patients and both examinations completed in five volunteers. However, patient acceptance compared with colonoscopy was not mentioned [40].
As previously mentioned, the use of CO2 in MR colonography colonic distension is not yet thoroughly explored. One study demonstrated that in colonoscopy both air and CO2 performed equally well in distension, but CO2 resulted in better patient acceptance [44]. The use of an automated insufflator with controlled administration of CO2 has an advantage over manual administration. Currently, better patient acceptance of one of the aforementioned methods in MR colonography is not apparent.
Bright & dark lumen
Colonic distension in MR colonography can be achieved by the use of a negative or a positive contrast agent, depending on the specific characteristics of the contrast agent and the applied MRI sequences. In literature, bright-lumen imaging often refers to the high signal intensity of the bowel lumen on T1‑weighted sequences, whereas the colonic wall remains low in signal intensity, enabling visualization of filling defects. In this approach, the colonic lumen is prepared with a mixture of water and gadolinium, which is rectally administered [45]. This results in a relatively high signal intensity of colonic lesions and a low signal intensity of colonic lumen at T2‑weighted sequences. Luminal pseudolesions can also be produced by nonpathological causes – residual air and fecal material – that hamper the diagnostic accuracy of MR colonography [32]. To overcome this problem data acquisition has to be performed in supine and prone position, as these false-positive findings are subjected to gravity.
Two initial studies applied this bright-lumen technique for the detection of colorectal polyps and CRC, demonstrating high diagnostic accuracy for detection of lesions exceeding 10 mm. However, the diagnostic accuracy for smaller lesions varied [41,46]. Other studies demonstrated limited sensitivity and specificity for the detection of polyps and colorectal masses due to false-positive and false-negative findings [32,37]. Although preliminary studies used the bright-lumen approach, a change in luminal acquisition method is observed owing to the costs of the contrast agent and the less conspicuous enhancement following intravenous contrast medium administration.
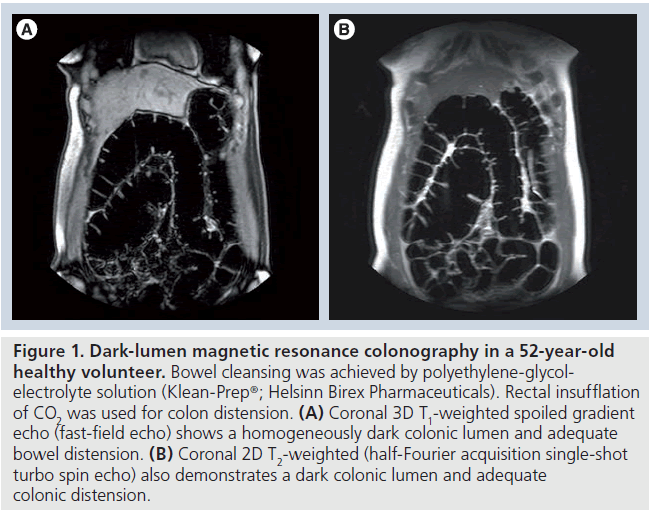
Dark-lumen MR colonography does not primarily focus on low signal intensity filling defects, but is based on contrast enhancement of the bowel wall, wall-related pathology and a homogeneously dark colonic lumen during T1‑weighted sequences. A water- or air-based enema enables bowel wall distension and is characterized by low signal intensity on T1‑weighted sequences (Figure 1) [36]. The bowel wall enhancement is obtained by intravenously administered paramagnetic contrast and allows depiction of abnormalities of the bowel wall. Pre- and postcontrast imaging is performed to avoid false-positive and false-negative findings. The 3D acquisition is recommended to be repeated in the coronal plane following a delay of 70 and 120 s.
Figure 1.Dark-lumen magnetic resonance colonography in a 52-year-old healthy volunteer. Bowel cleansing was achieved by polyethylene-glycolelectrolyte solution (Klean-Prep®; Helsinn Birex Pharmaceuticals). Rectal insufflation of CO2 was used for colon distension. (A) Coronal 3D T1-weighted spoiled gradient echo (fast-field echo) shows a homogeneously dark colonic lumen and adequate bowel distension. (B) Coronal 2D T2-weighted (half-Fourier acquisition single-shot turbo spin echo) also demonstrates a dark colonic lumen and adequate colonic distension.
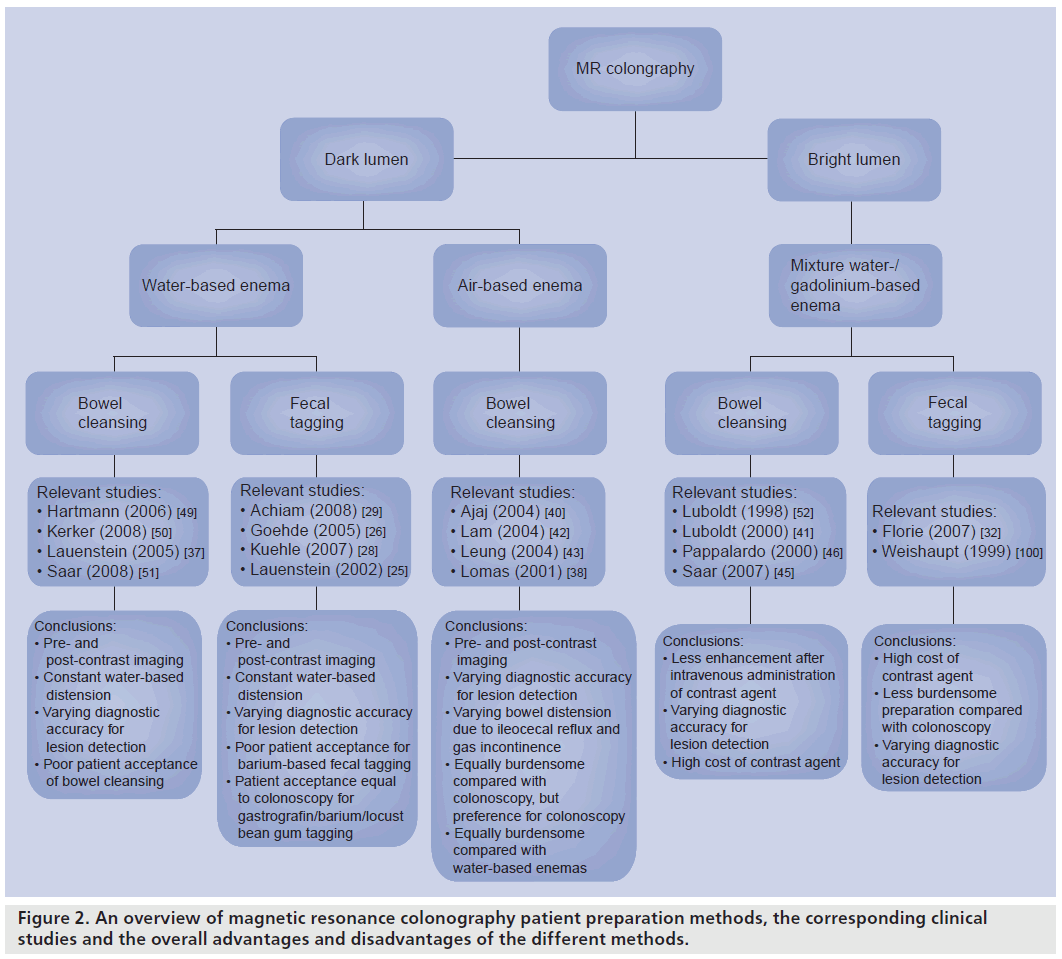
The lesion enhances when it concerns a truepositive finding (e.g., polyp or CRC) if not it represents stool. A plain T1‑weighted sequence is performed prior to intravenous contrast medium administration to allow identification of insufficiently dark stool mimicking lesions. Prone and supine positioning obviates false-positive findings from air residue, but is especially valuable for obtaining distension of all segments. As in CT colonography, optimal distension of some bowel segments is obtained in the supine position (e.g., transverse colon) and others in the prone position (e.g., rectum) [47,48]. An overview on the different bright- and dark-lumen techniques can be found in Figure 2.
Figure 2.An overview of magnetic resonance colonography patient preparation methods, the corresponding clinical studies and the overall advantages and disadvantages of the different methods.
Accuracy
The first published article on the use of darklumen MR colonography was published in 2001 [36]. The imaging was performed after bowel cleansing, administration of a water-based enema and pre- and post-contrast administration. The study demonstrated promising results regarding acquisition time, review time and diagnostic accuracy; no false-negative findings. Since 2001 several studies have been performed with MR colonography using the dark-lumen approach.
A recently published systematic review by Zijta et al. demonstrated good results for the detection of colorectal lesions with MR colonography for both dark- and bright-lumen strategies [18]. A total of 13 studies were included with a total study population of 1285 patients. In nine studies symptomatic and/or asymptomatic patients at increased risk of CRC were included [25,26,32,37,43,45,49–51], and in three studies indications for colonoscopy were unclear [30,41,52]. One study described a population of 315 asymptomatic individuals with a normal risk profile for CRC [28].
The systematic review demonstrated that the sensitivity for the detection of CRC, observed in five studies, was 100% [25,37,45,49,51]. Furthermore, per-patient sensitivity of MR colonography was 88% (95% CI: 63–97%) and specificity was 99% (95% CI: 95–100%) for the detection of large polyps (≥10 mm). On a per-polyp basis, polyps of 10 mm or larger were detected with a sensitivity of 84% (95% CI: 66–94) [18].
As indicated, only one study has focused on asymptomatic individuals with a normal risk profile for CRC [28] and nine studies have evaluated MR colonography in high-risk populations [25,26,32,37,43,45,49–51], which leads to a higher prevalence of abnormalities and will ultimately result in better diagnostic outcomes [28]. The screening study demonstrated an overall patient-based sensitivity of 36.4% and specificity of 90.2%. For lesions of 5–10 mm, sensitivity was 60% and increased to 70 and 100% specificity for lesions larger than 10 mm in size. Importantly, extensive variability between study results in sensitivity and specificity values of MR colonography and detection of colorectal lesions was demonstrated. This might be a consequence of variations in technical and imaging aspects [18].
As previously mentioned, MRI has the advantage of additional imaging of extracolonic structures. The reported data on extracolonic findings in MR colonography are limited and only two studies had the main objective to evaluate extracolonic structures [17,53]. A recent study from Yusuf et al. demonstrated the prevalence of extracolonic findings in 210 patients at an increased risk for CRC who underwent brightlumen MR colonography [17]. The study demonstrated extraluminal findings in 125 patients (59.5%) with a wide range of findings (e.g., lymphadenopathy, aortic aneurysm, gall bladder stones, hepatic and renal cysts). A total of ten findings (4.8%) were potentially important (scored according to the CRADS reporting system [16]), of which two revealed to be malignant (1.0%) [17]. Yusuf et al. concluded that in bright-lumen MR colonography extracolonic findings are common, but the majority have low clinical importance [17]. Ajaj et al. demonstrated extracolonic findings in 69% of 375 subjects who underwent dark-lumen MR colonography for suspected colonic disease [53]. A total of 12% (31 subjects) of the findings were therapeutically relevant and, therefore, 27 of 31 patients were subjected to additional examination. In all cases the therapeutically relevant findings were confirmed. Therefore, Ajaj et al. concluded that dark-lumen MR colonography has a high accuracy for the assessment of extracolonic findings [53].
Inflammatory bowel disease
Crohn’s disease and ulcerative colitis are the two major inflammatory bowel diseases (IBD). Crohn’s disease can affect any part of the GI tract, but has a predilection for the terminal ileum and proximal colon. Ulcerative colitis affects the colon with a predilection for the distal colon and rectum. IBD is characterized by remissions and exacerbations while symptoms are poor indicators of disease activity. Work-up is necessary to differentiate between active and inactive disease and to establish alternative diagnoses. Disease activity has to be monitored during treatment, as treatment is associated with side effects and considerable costs. Thus, treatment should be adjusted as soon as possible when there is no benefit of treatment.
Imaging is an important technique in the work up and for monitoring treatment response in IBD. Ultrasound, CT and MRI can be used for diagnosing IBD. The accuracy of each of these examinations is comparable but each technique has its strength and limitations [54]. Since patients with Crohn’s disease are often young and typically require multiple examinations, the cumulative dose of multiple CT examinations is substantial [55]. Accordingly, the use of an accurate technique without ionizing radiation is preferable. The lack of ionizing radiation exposure, coupled with a comparable accuracy, favors the use of either ultrasound or MRI. Ultrasound gives real-time information on peristalsis in addition to the morphological features. Contrast-enhanced ultrasound correlates well with colonoscopic disease activity [56]. This technique, however, is operator dependent. By contrast, MRI has an unlimited field of view and good reproducibility, which are principal advantages over ultrasound. As previously mentioned, the lack of the availability and cost of MRI in some locations may, however, limit the use of the technique.
Most research in MRI in IBD concerns evaluation of the small bowel in Crohn’s disease. This is because of a longstanding need for assessment of the small bowel where conventional enteroclysis has limitations (no assessment of mural and extramural abnormalities and limited evaluation of disease activity) and drawbacks (radiation exposure). Initially, there was no obvious need for colonic evaluation as the colon is evaluated with colonoscopy as standard of care. However, the burden of colonoscopy with bowel preparation hampers frequent application and MRI may also play a role here; although the body of evidence is limited and does not give univocal evidence.
Technique
Most likely, distension of the colon is mandatory for a good evaluation of the colon in IBD. In early studies, high sensitivities were reported for differentiating type and severity of IBD. Without reported use of bowel preparation methods and the sole use of intravenous contrast medium, the authors concluded a comparable diagnostic accuracy of MRI as compared with endoscopy [57]. More recent studies, evaluating the colon without rectal administered distension, tend to support [58] or refute [59] these findings.
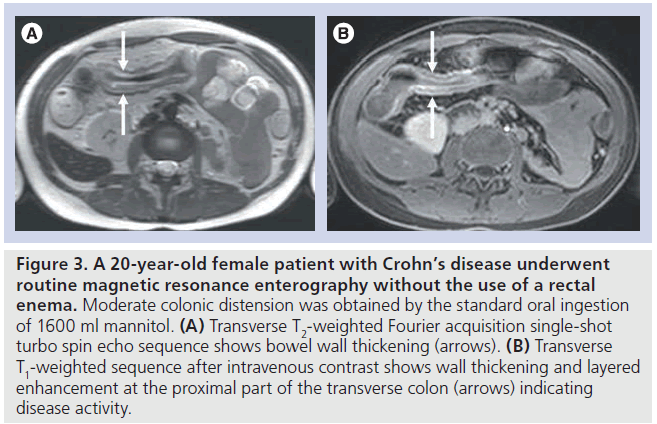
As in small-bowel evaluation, colonic luminal distension can be expected to lead to more optimal evaluation [60,61]. Distension is obtained by a rectal enema, sometimes combined with oral administration of contrast material. A series in 15 normal subjects and 23 patients with suspected IBD of the colon demonstrated that MR colonography with a rectal enema is feasible and leads to a good colonic distension [35]. As in CRC, either a dark- or bright-lumen strategy can be used for distension, often concerning a biphasic strategy (opposite signal intensity on T1‑weighted sequences and T2‑weighted sequences) [62]. In MRI of the small bowel a dark-lumen strategy is preferred with lowsignal intensity at T1‑weighted sequences and high-signal intensity at T2‑weighted sequences. An advantage of a strategy with a dark colonic lumen at T1‑weighted sequences is the optimal contrast between bowel wall and lumen with the use of intravenous contrast medium. This is important as bowel wall enhancement is used as an indicator of disease activity (Figure 3) [63].
Figure 3.A 20-year-old female patient with Crohn’s disease underwent routine magnetic resonance enterography without the use of a rectal enema. Moderate colonic distension was obtained by the standard oral ingestion of 1600 ml mannitol. (A) Transverse T2-weighted Fourier acquisition single-shot turbo spin echo sequence shows bowel wall thickening (arrows). (B) Transverse T1-weighted sequence after intravenous contrast shows wall thickening and layered enhancement at the proximal part of the transverse colon (arrows) indicating disease activity.
Whether or not bright- or dark-lumen colonography should be used is not established for colonography in IBD and conflicting results are reported. In a series of 22 consecutive patients suspected for or with known IBD a bright-lumen strategy was used for MR colonography [64]. Bowel preparation comprised a gadolinium– water enema for luminal distension and additional intravenous administration of gadolinium. Bowel-wall contrast enhancement and bowelwall thickening were used as MRI features of inflammation. The sensitivity for detecting segmental inflammation in patients with Crohn’s disease was 31.6% and was 58.8% for ulcerative colitis. The same group also studied dark-lumen MR colonography in another cohort with only slight improvement in overall segmental inflammation detection [65]. In a series of 15 healthy volunteers and 23 patients with known IBD there was a high sensitivity (87%) for identifying segmental IBD changes with dark-lumen MR colonography [35]. In this series no histopathology was obtained of endoscopically normal mucosa that might influence the results. It remains to be demonstrated whether the differences are related to the strategy used or that other factors, such as disease spectrum and reference standard, are more important. Further developments concern studying the possibility of colonic distension by oral contrast material to decrease patient discomfort.
Disease activity
Apart from diagnosing IBD, the level of disease activity is important for management. A limited number of series studied this in patients with Crohn’s disease of the bowel. These studies concerned the small bowel and colon, and were summarized in a systematic review [63]. MRI correctly graded 91% of the patients with frank disease, 62% with mild disease and 62% in remission. Inaccurate grading primarily concerned grading patients in remission as having mild disease. Most commonly used imaging features indicative of disease activity were thickened bowel wall and enhancement, although other features are used as well. However, the optimal imaging features have not been fully established. A recent study comparing MRI of small bowel Crohn’s disease to histopathology of 18 resection specimens reported that increased bowel wall thickness, high mural signal intensity at T2‑weighted sequences and a layered pattern of enhancement indicate active disease [66]. These findings may apply to the colon as well, but this needs to be substantiated. Results until now are equivocal. In a study using MR colonography in 29 patients with IBD some of the features similar to small-bowel evaluation were used [67]. These concerned increased bowel-wall contrast enhancement, bowel-wall thickening, presence of mesenterial lymph nodes and the absence of the normal haustral pattern. The sensitivity was low (32%) and the specificity good (88%), while the barium fecal tagging regime resulted in a poor patient acceptance. Others primarily studied the enhancement of the colonic wall. One group demonstrated a significant correlation with colonoscopy [68]. Another research group reported on 37 patients and used an enhancement index taking into account the presence of adequate colonic distension [69]. This approach resulted in a moderate sensitivity (63%) and adequate specificity (80%). In both studies, bowelwall attenuation was subjectively assessed, in the absence of predefined criteria. Others used MR enterography in conjunction with a water-based enema to assess disease activity in 50 patients with established Crohn’s disease of the colon and terminal ileum [70]. This produced good results, with a significant correlation (r = 0.82, p = 0.001) between MRI and the Crohn’s disease endoscopic index of severity. MRI had a high accuracy for the detection of disease activity with a sensitivity of 81% and a specificity of 89%. Relative contrast enhancement and wall thickness were independent predictors of disease activity.
At this time point, the optimal MRI features predictive for disease activity of the colon are not established. However, increased wall thickness, increased enhancement and layered enhancement are obvious candidates and may be considered when reading MRI of the colon in IBD.
Acute abdominal pain
Urgent acute abdominal pain, defined as conditions necessitating treatment within 24 h, is frequently caused by colonic inflammatory conditions [71]. Acute appendicitis and diverticulitis are the most frequent urgent causes of acute abdominal pain. Imaging is mandatory for establishing the diagnosis or determining an alternative diagnosis. Plain radiography plays no role as it does not have any impact on the diagnosis and management of patients [72]. Ultrasound with graded-compression technique and multidetector CT are the primary imaging techniques in these patients. The advantage of ultrasound is that it entails a widely available, real-time examination. Limitations are that it is operator dependent, the field of view can be limited by air or bony structures and it can be difficult to interpret in obese patients. CT gives a good overview and is not hampered by air or bony structures.
Limitations are the ionizing radiation exposure and the risk of contrast medium-induced nephropathy as iodinated contrast medium is used. A recent meta-analysis demonstrated that CT is the most accurate technique for diagnosing acute appendicitis [73]. In diverticulitis there is no significant difference in accuracy, although CT is more likely to identify alternative diseases [74]. This may favor an approach using CT in all patients suspected for acute appendicitis of diverticulitis. However, this is associated with the risk of cancer induction by radiation exposure and contrast medium-induced nephropathy.
To overcome this, different approaches should be considered. Recently, a study demonstrated that the most effective approach in patients with acute abdominal pain is initial ultrasound with subsequent CT in inconclusive and negative cases [72]. This leads to the highest sensitivity for urgent conditions and leads to reduced radiation exposure and costs when compared with CT in all individuals. However, 6% of urgent conditions are missed and 49% of the patients still have to undergo CT. An alternative approach is to use MRI.
Technique
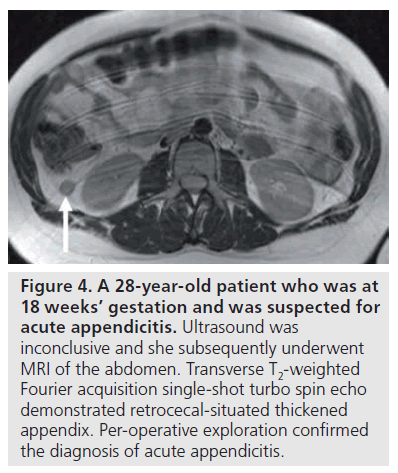
MRI of the abdomen is often thought of as a lengthy procedure, which is, however, not the case in acute abdominal pain. This MRI procedure comprises standard breath-hold MRI sequences. Single-shot half-Fourier rapid acquisition with relaxation enhancement (e.g., half- Fourier acquisition single-shot turbo spin echo) and balanced true steady-state free precession (e.g., true fast imaging with steady state precession) are mainstay, leading to a room time of 10–15 min. No intravenous contrast medium is administered. Oral administration of a contrast agent (e.g., ferumoxsil and barium sulfate) seems unnecessary (Figure 4) [75–79]. Diffusionweighted imaging (DWI) might prove helpful to identify inflammation.
Figure 4.A 28-year-old patient who was at 18 weeks’ gestation and was suspected for acute appendicitis. Ultrasound was inconclusive and she subsequently underwent MRI of the abdomen. Transverse T2-weighted Fourier acquisition single-shot turbo spin echo demonstrated retrocecal-situated thickened appendix. Per-operative exploration confirmed the diagnosis of acute appendicitis.
Results
Initial research on MRI in acute abdominal pain concerned abdominal pain in pregnancy. This has a variety of diagnoses, of both obstetric and non-obstetric causes. Acute appendicitis is one of the most common non-obstetric causes in pregnancy and indication for surgical exploration. Although surgical intervention is recommended, owing to high rates of fetal loss in perforated appendicitis, almost 25–50% of cases of surgical exploration are negative for acute appendicitis [80–82]. The changed position of the appendix during pregnancy makes the differential diagnosis more difficult [81]. Although surgical intervention is recommended owing to high rates of fetal loss in perforated appendicitis, almost a quarter to a half of the cases of surgical exploration is negative for acute appendicitis [76,80]. Imaging is mandatory to diagnose or exclude acute appendicitis [83]. Ultrasound is the initial imaging technique for diagnosing acute appendicitis in pregnancy, but the diagnostic accuracy of ultrasound varies widely in pregnancy [80]. CT is not appropriate in this setting owing to the ionizing radiation exposure and use of iodinated contrast medium. MRI has been demonstrated to be a valuable alternative. A systematic review by Basaran et al. found no significant difference between performance of CT and MRI when ultrasound was either normal or inconclusive [80]. With pooled estimates of sensitivity and specificity of 85.7 (95% CI: 63.7–97%) and 97.4% (95% CI: 86. 2–99.9%) for CT and 80 (95% CI: 44–98%) and 99% (95% CI: 94–100%), respectively, for MRI. This makes MRI a good alternative to CT, not only in pregnancy, as good results were also reported in nonpregnant patients [84]. With respect to the lack of ionizing radiation, this especially benefits younger individuals. The lack of the risk of contrast medium-induced nephropathy primarily benefits middle-aged and older individuals.
The body of evidence on MRI in acute abdominal pain almost exclusively concerns acute appendicitis. Further studies should evaluate the accuracy of MRI in diagnosing other diseases before the technique can be fully applied. A study evaluated MRI in 55 patients suspected to have acute diverticulitis [85]. The results were good (sensitivity 94%; specificity 88%), but concerned a limited series with a high prevalence of disease. For acute conditions not originating from the colon, MRI has almost exclusively been studied for diseases of the liver, biliary tree, gallbladder and pancreas [86]. To our knowledge, no study evaluated MRI in acute abdominal pain in general. Neither are there series concerning challenging diagnoses, such as mesenteric ischemia and perforation.
3 T MR colonography
In the last decade, high-field imaging has become increasingly studied and the application is widely implemented [87]. In 3 T MRI the high-field strength can be used to increase spatial resolution and decrease acquisition times [21,88]. This can improve image quality considerably. In MR colonography this implicates improved depiction of colonic segments with increased enhancement of contrast agents. However, direct adoption of sequences used at 1.5 T is not feasible. At high-field strength, tissue T1 and T2 relaxation parameters are different than at 1.5 T, as well as specific absorption rate and changes in susceptibility and chemical shift effects [21,89]. Susceptibility artifacts in MR colonography are especially seen at soft tissue– air interfaces (e.g., residual gas in the colonic lumen) [90]. Field heterogeneities are frequently observed at the boundaries of the field of view, resulting in different signal intensities [21]. The increase in signal-to-noise ratio can be of help in a reduction of artifacts. In addition, parallel and fast-imaging techniques can reduce noise and decrease acquisition times [89,90]. Although high-field imaging is promising, there is limited evidence concerning the role of 3 T MRI in MR colonography [21]. A study in 40 patients demonstrated no significant difference in image quality at 3 T compared with 1.5 T in two sequences (T1‑weighted fat-suppressed gradient echo and T2‑weighted single-shot fast spin echo) [91]. Wessling et al. demonstrated no significant difference in detection of polyps larger than 6 mm in a phantom at 1.5 and 3 T [92]. Florie et al. performed MR colonography at 1.5 and 3 T but did not assess the difference in performance for both imaging modalities [32]. However, a study performed by Saar et al. demonstrated a sensitivity of 100% for colorectal lesions larger than 6 mm [51]. For staging disease activity in IBD, high-field imaging was well-matched to 1.5 T [54].
Diffusion-weighted imaging
Diffusion-weighted imaging provides information on the diffusion of water molecules throughout the body. Diffusion of water molecules outside the body is characterized as an unrestricted random movement of water molecules [93]. In the body, the diffusion of water molecules is restricted by cellular membranes and intra- and extra-cellular elements, such as macromolecules. Therefore, diffusion in vivo can be extrapolated to the assessment of tissue cellularity; in tumor tissue the cellularity is high, consequently movement of water molecules is restricted by abundant cell membranes and, therefore, diffusion of water molecules is restricted to a large extent [93]. By contrast, damaged cell membranes provide an increased diffusion of water molecules. Therefore, DWI can provide valuable information on tissue perfusion, tumor cellularity and vascular leakage [93,94].
In DWI, a standard T2‑weighted spin-echo sequence is modified by applying an asymmetric pair of diffusion-sensitizing (bipolar) gradients around the 180° refocusing pulse [93]. The first gradient provides phase information as does the second gradient; however, as a consequence of movement of the molecules, the signal will not be entirely rephased by the second gradient, resulting in a reduction of signal intensity. DWI is based on the attenuation of signal intensity by the movement of water molecules [93]. The sensitivity to molecule motion is defined by the amplitude, duration and time interval of the gradient pulses, known as b‑values. Large b‑values result in signal attenuation for slow-moving molecules and vice versa. DWI is generally performed at multiple b‑values to observe attenuation of the signal and variation in diffusion. The signal intensity (y‑axis) can be plotted against the b‑values (x‑axis) as a logarithm. The slope of the fitted line through the plots represents the apparent diffusion coefficient (ADC). The ADC differs for different tissues describing the degree of attenuation of diffusion [95]. Low ADC values demonstrate high cellular areas and vice versa [93].
Diffusion-weighted imaging is extensively used in neuroimaging, while its application in colorectal imaging is primarily studied in the field of oncology [96–98]. Colorectal use is hampered by susceptibility and motion artifacts caused by residual air, respiration, peristalsis and blood flow [98]. Several studies demonstrated the valuable potential of DWI for the detection of CRC [96–98]. Ichikawa et al. demonstrated a mean sensitivity and specificity for the detection of colorectal adenocarcinoma of 90.9 (30/33; 95% CI: 74.5–97.6%) and 100% (15/15; 95% CI: 74.6–100%) [98]. High b‑value DWI demonstrated a sensitivity of 100% (15/15) and specificity of 65% (13/20) for the detection of rectal cancer in 35 patients [97]. Rao et al. demonstrated an increase of receiver-operating characteristics analysis with the addition of DWI to T2‑weighted imaging [96].
One study has focused on DWI in Crohn’s disease [99]. The study demonstrated a sensitivity, specificity and accuracy of 85.7, 75.7 and 77.3%, respectively, for the detection of active disease segments in the large bowel with DWI [99]. Although standardization of techniques, quantification and analysis have to be further explored and improved, DWI is increasingly evolving as a clinically valuable tool for colorectal imaging [94].
Conclusion
MRI of the colon is a topic of ongoing research. The technique has not been established, although some aspects become clear (e.g., bowel distension is mandatory for MRI for CRC and IBD). The body of evidence for MRI of the colon is not extensive and for some indications limited (acute abdominal pain), but considerable efforts are made to fill this gap.
In patients with symptoms of CRC, MR colonography has been demonstrated to be accurate in detecting clinically relevant lesions. However, the technique is not fully established and further studies should optimize the technique. At this moment, MR colonography can be used as an alternative to CT colonography when the latter is contraindicated. However, sufficient experience should be present for performing and reading the MR colonography examination.
In IBD, MRI is as accurate as other techniques and lacks ionizing radiation exposure. This favors the use of MRI. Accuracy of MRI for grading disease activity is good for frank disease but mediocre for inactive or limited disease activity. The optimal technique for performing MRI of the colon is not yet established. Furthermore, identification of features indicative for disease activity is mandatory.
MRI in patients with acute abdominal pain is performed as a short MRI examination without preparation. The results of MRI in acute appendicitis are good and justify the use of this technique rather than CT. In acute diverticulitis the data is very limited, although encouraging. Limitation is that the accuracy of MRI for many other causes of acute abdominal pain is not known yet.
Future perspective
Over the last decade, MR colonography for (precursors of ) CRC has experienced a high degree of development and amelioration. Nevertheless, extensive variability between study results in sensitivity and specificity values of MR colonography and detection of colorectal lesions was demonstrated. This might be a consequence of variety in technical and imaging aspects. To further optimize the diagnostic accuracy of MR colonography, different patient-preparation strategies are being studied, with special regard for patient acceptance. A strategy with limited bowel preparation and the use of automated CO2 insufflation might be advantageous.
In patients at an increased risk or with symptoms for CRC, future developments might entail MR colonography as a screening tool. However, first MR colonography must be studied in sizeable cohorts and compared with colonoscopy (and CT colonography). Furthermore, other important aspects, such as participation rate and cost–effectiveness must be studied.
In IBD, MRI can play an important role in the work up and for monitoring treatment response. Patients with IBD are often young individuals in their reproductive age and assessment of the disease activity requires frequent evaluation. In addition, the optimal technique for performing MRI in this field is yet to be established. We believe that future research in IBD will focus on improving imaging techniques with respect to noninvasiveness. Further developments concern studying the possibility of colonic distension by oral contrast material alone to decrease patient discomfort. The optimal combination of imaging features for disease activity has to be determined.
Since MRI for acute abdominal pain is mainly studied in pregnant patients with suspected appendicitis, further studies should evaluate the accuracy of MRI in diagnosing other diseases. As CT has proven its diagnostic ability in the acute setting when examination with ultrasound is inconclusive, we believe that MRI offers a valuable alternative, especially in young individuals in their reproductive age and patients at risk of contrast medium-induced nephropathy.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
*of interest
* of considerable interest
References
- Lambregts DMJ, Maas M, Beets-Tan RGH: MRI of the rectum. In: Magnetic Resonance Imaging of the Gastrointestinal Tract. Stoker J (Ed.). Springer Verlag, Berlin, Germany, 205–227 (2010).
- Levin B, Lieberman DA, McFarland B et al.: Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi- Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 134(5), 1570–1595 (2008).
- Lieberman D: Colon cancer screening and surveillance controversies. Gastroenterology 25, 422–427 (2009).
- Dafnis G, Granath F, Pahlman L, Ekbom A, Blomqvist P: Patient factors influencing the completion rate in colonoscopy. Dig. Liver Dis. 37(2), 113–118 (2005).
- Bowles CJ, Leicester R, Romaya C, Swarbick E, Williams CB, Epstein O: A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 53(2), 277–283 (2004).
- Sarwar S, Anwar MM, Ryan B, O’Morain C: Colonoscopy completion rates – are we prepared for a national screening programme? Ir. Med. J. 100(9), 585–587 (2007).
- Regula J, Rupinski M, Kraszewska E et al.: Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N. Engl. J. Med. 355(18), 1863–1872 (2006).
- Halligan S, Altman DG, Taylor SA et al.: CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 237(3), 893–904 (2005).
- Johnson CD, Chen MH, Toledano AY et al.: Accuracy of CT colonography for detection of large adenomas and cancers. N. Engl. J. Med. 359(12), 1207–1217 (2008).
- Brenner DJ, Georgsson MA: Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 129(1), 328–337 (2005).
- Liedenbaum MH, Venema HW, Stoker J: Radiation dose in CT colonography – trends in time and differences between daily practice and screening protocols. Eur. Radiol. 18(10), 2222–2230 (2008).
- Lauenstein T, Holtmann G, Schoenfelder D, Bosk S, Ruehm SG, Debatin JF: MR colonography without colonic cleansing: a new strategy to improve patient acceptance. AJR Am. J. Roentgenol. 177(4), 823–827 (2001).
- Luboldt W, Bauerfeind P, Steiner P, Fried M, Krestin GP, Debatin JF: Preliminary assessment of three-dimensional magnetic resonance imaging for various colonic disorders. Lancet 349(9061), 1288–1291 (1997).
- van Gelder RE, Birnie E, Florie J et al.: CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology 233(2), 328–337, (2004).
- Florie J, Birnie E, van Gelder RE et al.: MR colonography with limited bowel preparation: patient acceptance compared with that of full-preparation colonoscopy. Radiology 245(1), 150–159 (2007).
- Zalis ME, Barish MA, Choi JR et al.: CT colonography reporting and data system: a consensus proposal. Radiology 236, 3–9 (2005).
- Yusuf E, Florie J, Nio CY et al.: Incidental extracolonic findings on bright lumen MR colonography in a population at increased risk for colorectal carcinoma. Eur. J. Radiol. (2009) (Epub ahead of print).
- Zijta FM, Bipat S, Stoker J: Magnetic resonance (MR) colonography in the detection of colorectal lesions: a systematic review of prospective studies. Eur. Radiol. (2009) (Epub ahead of print). & Systematic overview of prospective studies on the diagnostic accuracy of magnetic resonance (MR) colonography for the detection of colorectal polyps and cancer.
- Iafrate F, Hassan C, Pickhardt PJ et al.: Portrait of a polyp: the CTC dilemma. Abdom. Imaging 35(1), 49–54 (2008).
- Goldstein NS, Bhanot P, Odish E, Hunter S: Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am. J. Clin. Pathol. 119(6), 778–796 (2003).
- Lauenstein TC, Saar B, Martin DR: MR colonography: 1.5 T versus 3 T. Magn. Reson. Imaging Clin. N. Am. 15(3), 395–402, vii (2007).
- Kinner S, Lauenstein TC: MRI of the colon (MR-colonography): technique. In: Magnetic Resonance Imaging of the Gastrointestinal Tract. Stoker J (Ed.). Springer Verlag, Berlin, Germany, 173–184 (2010). & State-of-the-art overview of the technique of MR colonography.
- Rex DK: Dosing considerations in the use of sodium phosphate bowel preparations for colonoscopy. Ann. Pharmacother. 41(9), 1466–1475 (2007).
- Jensch S, Bipat S, Peringa J et al.: CT colonography with limited bowel preparation: prospective assessment of patient experience and preference in comparison to optical colonoscopy with cathartic bowel preparation. Eur. Radiol. 20(1), 146–156 (2010).
- Lauenstein TC, Goehde SC, Ruehm SG, Holtmann G, Debatin JF: MR colonography with barium-based fecal tagging: initial clinical experience. Radiology 223(1), 248–254 (2002).
- Goehde SC, Descher E, Boekstegers A et al.: Dark lumen MR colonography based on fecal tagging for detection of colorectal masses: accuracy and patient acceptance. Abdom. Imaging 30(5), 576–583 (2005).
- Kinner S, Kuehle CA, Langhorst J et al.: MR colonography vs. optical colonoscopy: comparison of patients’ acceptance in a screening population. Eur. Radiol. 17(9), 2286–2293 (2007).
- Kuehle CA, Langhorst J, Ladd SC et al.: Magnetic resonance colonography without bowel cleansing: a prospective cross sectional study in a screening population. Gut 56(8), 1079–1085 (2007). & Only prospective cross-sectional study on MR colonography in a screening population.
- Achiam MP, Chabanova E, Løgager VB, Andersen LP, Thomsen HS, Rosenberg J: MR colonography with fecal tagging: barium vs. barium ferumoxsil. Acad. Radiol. 15(5), 576–583 (2008).
- Achiam MP, Logager VB, Chabanova E, Eegholm B, Thomsen HS, Rosenberg J: Diagnostic accuracy of MR colonography with fecal tagging. Abdom. Imaging 34(4), 483–490 (2009).
- Florie J, van Gelder RE, Haberkorn B et al.: Magnetic resonance colonography with limited bowel preparation: a comparison of three strategies. J. Magn. Reson. Imaging 25(4), 766–774 (2007).
- Florie J, Jensch S, Nievelstein RA et al.: MR colonography with limited bowel preparation compared with optical colonoscopy in patients at increased risk for colorectal cancer. Radiology 243(1), 122–131 (2007).
- Rogalla P, Lembcke A, Ruckert JC et al.: Spasmolysis at CT colonography: butyl scopolamine versus glucagon. Radiology 236(1), 184–188 (2005).
- Kinner S, Lauenstein TC: MR colonography. Radiol. Clin. North Am. 45(2), 377–387 (2007).
- Ajaj WM, Lauenstein TC, Pelster G et al.: Magnetic resonance colonography for the detection of inflammatory diseases of the large bowel: quantifying the inflammatory activity. Gut 54(2), 257–263 (2005).
- Lauenstein TC, Herborn CU, Vogt FM, Goehde SC, Debatin JF, Ruehm SG: Dark lumen MR-colonography: initial experience. Rofo 173(9), 785–789 (2001).
- Lauenstein TC, Ajaj W, Kuehle CA, Goehde SC, Schlosser TW, Ruehm SG: Magnetic resonance colonography: comparison of contrast-enhanced three-dimensional vibe with two-dimensional FISP sequences: preliminary experience. Invest. Radiol. 40(2), 89–96 (2005).
- Lomas DJ, Sood RR, Graves MJ, Miller R, Hall NR, Dixon AK: Colon carcinoma: MR imaging with CO2 enema – pilot study. Radiology 219(2), 558–562 (2001).
- Morrin MM, Hochman MG, Farrell RJ, Marquesuzaa H, Rosenberg S, Edelmann RR: MR colonography using colonic distention with air as the contrast material: work in progress. AJR Am. J. Roentgenol. 176(1), 144–146 (2001).
- Ajaj W, Lauenstein TC, Pelster G, Goehde SC, Debatin JF, Ruehm SG: MR colonography: how does air compare to water for colonic distention? J. Magn. Reson. Imaging 19(2), 216–221 (2004).
- Luboldt W, Bauerfeind P, Wildermuth S, Marincek B, Fried M, Debatin JF: Colonic masses: detection with MR colonography. Radiology 216(2), 383–388 (2000).
- Lam WW, Leung WK, Wu JK, So NM, Sung JJ: Screening of colonic tumors by air-inflated magnetic resonance (MR) colonography. J. Magn. Reson. Imaging 19(4), 447–452 (2004).
- Leung WK, Lam WW, Wu JC et al.: Magnetic resonance colonography in the detection of colonic neoplasm in high-risk and average-risk individuals. Am. J. Gastroenterol. 99(1), 102–108 (2004).
- Sumanac K, Zealley I, Fox BM et al.: Minimizing postcolonoscopy abdominal pain by using CO2 insufflation: a prospective, randomized, double blind, controlled trial evaluating a new commercially available CO2 delivery system. Gastrointest. Endosc. 56(2), 190–194 (2002).
- Saar B, Meining A, Beer A et al.: Prospective study on bright lumen magnetic resonance colonography in comparison with conventional colonoscopy. Br. J. Radiol. 80(952), 235–241 (2007).
- Pappalardo G, Polettini E, Frattaroli FM et al.: Magnetic resonance colonography versus conventional colonoscopy for the detection of colonic endoluminal lesions. Gastroenterology 119(2), 300–304 (2000).
- Morrin MM, Farrell RJ, Keogan MT, Kruskal JB, Yam CS, Raptopoulos V: CT colonography: colonic distention improved by dual positioning but not intravenous glucagon. Eur. Radiol. 12(3), 525–530 (2002).
- Chen SC, Lu DS, Hecht JR, Kadell BM: CT colonography: value of scanning in both the supine and prone positions. AJR Am. J. Roentgenol. 172(3), 595–599 (1999).
- Hartmann D, Bassler B, Schilling D et al.: Colorectal polyps: detection with dark-lumen MR colonography versus conventional colonoscopy. Radiology 238(1), 143–149 (2006).
- Kerker J, Albes G, Roer N, Montag M, Budde T, Schaefer A: MR-colonography in hospitalized patients: feasibility and sensitivity. Z. Gastroenterol. 46(4), 339–343 (2008).
- Saar B, Gschossmann JM, Bonel HM, Kickuth R, Vock P, Netzer P: Evaluation of magnetic resonance colonography at 3.0 Tesla regarding diagnostic accuracy and image quality. Invest. Radiol. 43(8), 580–586 (2008).
- Luboldt W, Steiner P, Bauerfeind P, Pelkonen P, Debatin JF: Detection of mass lesions with MR colonography: preliminary report. Radiology 207(1), 59–65 (1998).
- Ajaj WM, Ruehm SG, Ladd SC, Gerken G, Goyen M: Utility of dark-lumen MR colonography for the assessment of extracolonic organs. Eur. Radiol. 17, 1574–1583 (2007).
- Horsthuis K, Bipat S, Bennink RJ, Stoker J: Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 247(1), 64–79 (2008). & Systematic overview of studies on the diagnostic accuracy of MR colonography in inflammatory bowel disease.
- Desmond AN, O’Regan K, Curran C et al.: Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut 57(11), 1524–1529 (2008).
- Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F: Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 253(1), 241–248 (2009).
- Shoenut JP, Semelka RC, Magro CM, Silverman R, Yaffe CS, Micflikier AB: Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J. Clin. Gastroenterol. 19(1), 31–35 (1994).
- Florie J, Horsthuis K, Hommes DW et al.: Magnetic resonance imaging compared with ileocolonoscopy in evaluating disease severity in Crohn’s disease. Clin. Gastroenterol. Hepatol. 3(12), 1221–1228 (2005).
- Durno CA, Sherman P, Williams T, Shuckett B, Dupuis A, Griffiths AM: Magnetic resonance imaging to distinguish the type and severity of pediatric inflammatory bowel diseases. J. Pediatr. Gastroenterol. Nutr. 30(2), 170–174 (2000).
- Koh DM, Miao Y, Chinn RJ et al.: MR imaging evaluation of the activity of Crohn’s disease. AJR Am. J. Roentgenol. 177(6), 1325–1332 (2001).
- Wiarda BM, Horsthuis K, Dobben AC et al.: Magnetic resonance imaging of the small bowel with the true FISP sequence: intra- and interobserver agreement of enteroclysis and imaging without contrast material. Clin. Imaging 33(4), 267–273 (2009).
- Zijta F, Stoker J: Magnetic resonance imaging of the colon (colonography): results. In: Magnetic Resonance Imaging of the Gastrointestinal Tract. Stoker J (Ed.). Springer Verlag, Berlin, Germany, 185–204 (2010).
- Horsthuis K, Bipat S, Stokkers PC, Stoker J: Magnetic resonance imaging for evaluation of disease activity in Crohn’s disease: a systematic review. Eur. Radiol. 19(6), 1450–1460 (2009). & Systematic overview of studies on diagnostic accuracy of MR colonography in establishing disease activity in Crohn’s disease.
- Schreyer AG, Golder S, Scheibl K et al.: Dark lumen magnetic resonance enteroclysis in combination with MRI colonography for whole bowel assessment in patients with Crohn’s disease: first clinical experience. Inflamm. Bowel Dis. 11(4), 388–394 (2005).
- Schreyer AG, Rath HC, Kikinis R et al.: Comparison of magnetic resonance imaging colonography with conventional colonoscopy for the assessment of intestinal inflammation in patients with inflammatory bowel disease: a feasibility study. Gut 54(2), 250–256 (2005).
- Punwani S, Rodriguez-Justo M, Bainbridge A et al.: Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology 252(3), 712–720 (2009).
- Langhorst J, Kuhle CA, Ajaj W et al.: MR colonography without bowel purgation for the assessment of inflammatory bowel diseases: diagnostic accuracy and patient acceptance. Inflamm. Bowel Dis. 13(8), 1001–1008 (2007).
- Röttgen R, Herzog H, Lopez-Haninnen E, Felix R: Bowel wall enhancement in magnetic resonance colonography for assessing activity in Crohn’s disease. Clin. Imaging 30(1), 27–31 (2006).
- Ergen FB, Akata D, Hayran M et al.: Magnetic resonance colonography for the evaluation of colonic inflammatory bowel disease: correlation with conventional colonoscopy. J. Comput. Assist. Tomogr. 32(6), 848–854 (2008).
- Rimola J, Rodriguez S, Garcia-Bosch O et al.: Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 58(8), 1113–1120 (2009).
- Stoker J, van Randen A, Laméris W, Boermeester MA: Imaging patients with acute abdominal pain. Radiology 253(1), 31–46 (2009). & Overview of the state-of-the-art in imaging of patients with acute abdominal pain.
- Laméris W, van Randen A, van Es HW et al.: Imaging strategies for detection of urgent conditions in patients with acute abdominal pain: diagnostic accuracy study. BMJ 338, b2431 (2009).
- van Randen A, Bipat S, Zwinderman AH et al.: Acute appendicitis: meta-analysis of diagnostic performance of CT and graded compression US related to prevalence of disease. Radiology 249(1), 97–106 (2008).
- Laméris W, van Randen A, Bipat S et al.: Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur. Radiol. 18(11), 2498–2511 (2008).
- Birchard KR, Brown MA, Hyslop WB, Firat Z, Semelka RC: MRI of acute abdominal and pelvic pain in pregnant patients. AJR Am. J. Roentgenol. 184(2), 452–458 (2005).
- Cobben LP, Groot I, Haans L, Blickman JG, Puylaert J: MRI for clinically suspected appendicitis during pregnancy. AJR Am. J. Roentgenol. 183(3), 671–675 (2004).
- Israel GM, Malguria N, McCarthy S, Copel J, Weinreb J: MRI vs. ultrasound for suspected appendicitis during pregnancy. J. Magn. Reson. Imaging 28(2), 428–433 (2008).
- Oto A, Ernst RD, Shah R et al.: Right-lowerquadrant pain and suspected appendicitis in pregnant women: evaluation with MR imaging – initial experience. Radiology 234(2), 445–451 (2005).
- Pedrosa I, Lafornara M, Pandharipande PV, Goldsmith JD, Rofsky NM: Pregnant patients suspected of having acute appendicitis: effect of MR imaging on negative laparotomy rate and appendiceal perforation rate. Radiology 250(3), 749–757 (2009).
- Basaran A, Basaran M: Diagnosis of acute appendicitis during pregnancy: a systematic review. Obstet. Gynecol. Surv. 64(7), 481–488 (2009). & Provides a systematic overview of studies on the diagnostic accuracy of MRI in the detection of appendicitis during pregnancy.
- Oto A, Srinivasan PN, Ernst RD et al.: Revisiting MRI for appendix location during pregnancy. AJR Am. J. Roentgenol. 186(3), 883–887 (2006).
- Vu L, Ambrose D, Vos P, Tiwari P, Rosengarten M, Wiseman S: Evaluation of MRI for the diagnosis of appendicitis during pregnancy when ultrasound is inconclusive. J. Surg. Res. 156(1), 145–149 (2009).
- Yilmaz HG, Akgun Y, Bac B, Celik Y: Acute appendicitis in pregnancy – risk factors associated with principal outcomes: a case control study. Int. J. Surg. 5(3), 192–197 (2007).
- Cobben L, Groot I, Kingma L, Coerkamp E, Puylaert J, Blickman J: A simple MRI protocol in patients with clinically suspected appendicitis: results in 138 patients and effect on outcome of appendectomy. Eur. Radiol. 19(5), 1175–1183 (2009). & Prospective clinical study in a large population of patients suspected for acute appendicitis.
- Heverhagen JT, Sitter H, Zielke A, Klose KJ: Prospective evaluation of the value of magnetic resonance imaging in suspected acute sigmoid diverticulitis. Dis. Colon Rectum. 51(12), 1810–1815 (2008).
- Stoker J: Magnetic resonance imaging and the acute abdomen. Br. J. Surg. 95(10), 1193–1194 (2008).
- Barth MM, Smith MP, Pedrosa I, Lenkinski RE, Rofsky NM: Body MR imaging at 3.0 T: understanding the opportunities and challenges. Radiographics 27, 1445–1464 (2007).
- Rimola J, Rodriguez S, Garcia-Bosch O et al.: Role of 3.0-T MR colonography in the evaluation of inflammatory bowel disease. Radiographics 29(3), 701–719 (2009).
- Nederveen AJ, Gonçalves SI: Imaging of the gastrointestinal tract at high-field strength. In: Magnetic Resonance Imaging of the Gastrointestinal Tract. Stoker J (Ed.). Springer Verlag, Berlin, Germany, 21–32 (2010).
- Thornton E, Morrin MM, Yee J: Current status of MR colonography. Radiographics 30(1), 201–218 (2010).
- Rottgen R, Herzog H, Bogen P, Freund T, Felix R, Bruhn H: MR colonoscopy at 3.0 T: comparison with 1.5 T in vivo and a colon model. Clin. Imaging 30(4), 248–253 (2006).
- Wessling J, Fischbach R, Borchert A et al.: Detection of colorectal polyps: comparison of multi-detector row CT and MR colonography in a colon phantom. Radiology 241(1), 125–131 (2006).
- Koh DM, Collins DJ: Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am. J. Roentgenol. 188(6), 1622–1635 (2007).
- Padhani AR, Liu G, Koh DM et al.: Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11(2), 102–125 (2009).
- Goh V, Taylor NJ: Dynamic contrastenhanced and diffusion-weighted MRI of the gastrointestinal tract. In: Magnetic Resonance Imaging of the Gastrointestinal Tract. Stoker J (Ed.). Springer Verlag, Berlin, Germany, 51–64 (2010).
- Rao SX, Zeng MS, Chen CZ et al.: The value of diffusion-weighted imaging in combination with T2-weighted imaging for rectal cancer detection. Eur. J. Radiol. 65(2), 299–303 (2008).
- Hosonuma T, Tozaki M, Ichiba N et al.: Clinical usefulness of diffusion-weighted imaging using low and high b-values to detect rectal cancer. Magn. Reson. Med. Sci. 5(4), 173–177 (2006).
- Ichikawa T, Erturk SM, Motosugi U et al.: High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am. J. Roentgenol. 187(1), 181–184 (2006).
- Kiryu S, Dodanuki K, Takao H et al.: Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J. Magn. Reson. Imaging 29(4), 880–886 (2009).
- Weishaupt D, Patak MA, Froehlich J, Ruehm SG, Debatin JF: Faecal tagging to avoid colonic cleansing before MRI colonography. Lancet 354(9181), 835–836 (1999).