Research Article - Clinical Investigation (2017) Volume 7, Issue 2
Multi-Center Study of the 99th Percentile Value of High-Sensitivity Troponin I in A Normal Healthy Chinese Population
- Corresponding Author:
- Renqian Zhong
Department of Laboratory
Shanghai Changzheng Hospital, No. 415 Fengyang Road, Shanghai 200003, China
E-mail: rqzhong@hotmail.com
Submitted: 17 May 2017; Accepted: 05 June 2017; Published online: 12 June 2017
Abstract
Background: The aim of this study was to establish the 99th percentile value for high sensitivity troponin I in a normal healthy Chinese population and to analyze the differences between different age groups and sex and regions. Methods and Findings: The study included 1,537 cases of normal healthy subjects who were chosen according to a strict inclusion criterion. They were recruited from different regions of China and separated in different age groups. All patients were carefully scanned for any diseases or factors that might influence cardiac troponin levels. The measurement of high-sensitivity cardiac troponin was standardized and tests were repeated several times and all results recorded. We used ARCHITECT-i2000 (Abbott Laboratories, Lake Bluff, Illinois, USA) for measurement of all high sensitivity cardiac troponins. The overall value of the 99th percentile value was 26.42 pg/ml. For the male group 99th percentile value was 29.40 pg/ml and for the female group 99th percentile value was 19.46 pg/ml. The difference in cardiac troponin 99th percentile value for the male and female normal group was statistically significant (P<0.01). The age group >65 years old had a higher level of high sensitivity cardiac troponin and the difference was statistically significant when compared with other groups. The 99th percentile value corresponded to an imprecision of less than 10%. Conclusions: We established the 99th percentile value for cardiac troponin in the Chinese population and the normal range for males and females and we evaluated the analytical performance of ARCHITECT high sensitivity troponin I assays.
Keywords
High-Sensitivity Troponin; Acute coronary syndrome; Multi-center study
Introduction
Acute coronary syndrome (Acute Coronary Syndrome, ACS) has a high incidence, morbidity and mortality such that early diagnosis and treatment can significantly reduce mortality and improve long-term prognosis. Cardiac biomarker is one of the main basis for the diagnosis of ACS.
Compared to other available cardiac markers, cardiac troponin I had good sensitivity and tissue specificity and it was preferably used to detect myocardial injury markers [1,2]. In 2007, European Society of Cardiology introduced a redefinition of acute myocardial infarction (AMI) and the third universal definition of myocardial infarction 2012 both recommended that cardiac troponins (cTn) be used to support the diagnosis of AMI, making them the preferred laboratory diagnostic tool [3].
When myocardial ischemia leads to myocardial injury, the myocardial cell membrane integrity is compromised and free Cardiac Troponin I (cTnI) from myocardial cell cytoplasm is rapidly released into the blood circulation causing peripheral blood concentration to also rise rapidly. A very short time after the onset, high sensitivity testing methods can be used to measure this increase [4,5]. Studies have shown that patients with even a slight increase in highsensitivity cardiac troponin assays are at increased risk of AMI or death [6-9]. In the clinical setting, the use of an assay for diagnosis is dependent on how sensitive the assay is, on a level of imprecision which would be considered acceptable and also on clearly defining the normal range of values for a general population, according to sex and according to age. In the world, currently, the 99th percentile value of serum troponin of the normal healthy population is used as a basis for disease disagnosis. Obtaining the correct 99th percentile value for high-sensitivity (hs) cardiac troponin assays is very important for the appropriate diagnosis and classification of AMI at the emergency level and also for use in wards. The correct reference values can also be important for future clinical studies and can have important implications for patient prognosis. Furthermore, cardiac troponins have a potential role in the primary prevention of acute coronary syndrome. This study is the first multi-center study to evaluate cardiac troponin 99th percentile values in a normal Chinese population, to differentiate in terms of sex, age and region and to aim at providing reference values for cardiac troponins for use in a Chinese population. Similar small scale studies have been carried out before in both Yunnan and Kunming, but due to the relatively small sample size, geographic distribution, inadequate age groups distributions and other limitations, China is yet to have adequate troponin reference values from a large scale study [10].
Methods
Patients
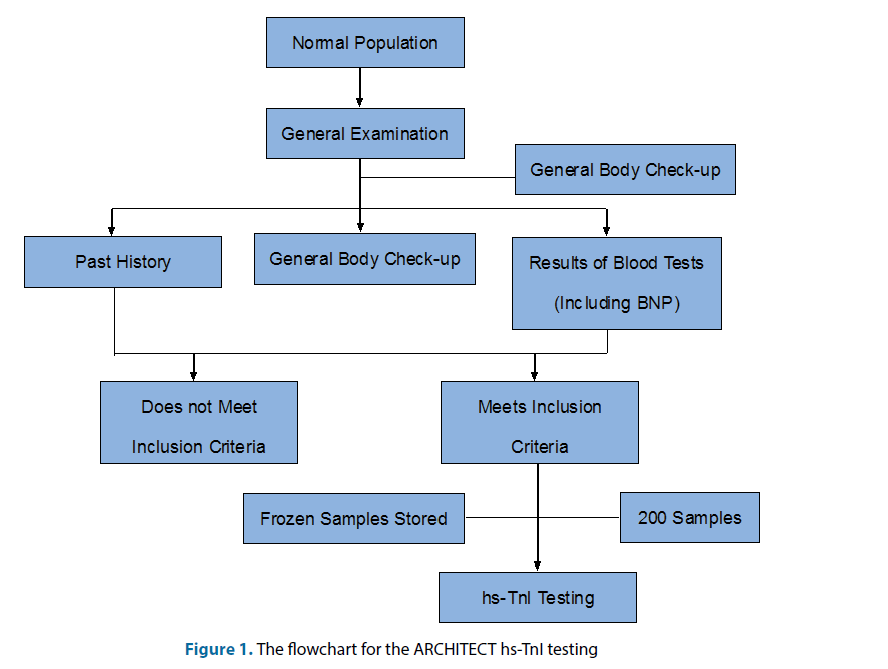
The distribution of subjects into groups was done by a stratified random sampling method. The subjects were divided into four groups according to age and the groups were as follows: 18-35 years old, 36-50 years old, 51-65 years old and >65 years of age. Each group was also divided according to sex. The sample included in this study came from eight different cities in mainland China which were Shanghai, Xi’an, Lanzhou, Anhui, Harbin, Beijing, Hangzhou, Fujian and Chongqing. All subjects signed an informed consent, filled in a past medical history questionnaire, underwent a thorough physical examination, and had their blood collected for laboratory examination. The process can be referred to Figure 1. The inclusion criteria included: healthy people aged between 18 years old and 85 years old; No history of heart disease and heart-related diseases; No high blood pressure, no diabetes and no history of high cholesterol; no abnormal liver function test results (ALT, AST, GGT, TBIL); no abnormal renal function test results (Scr); no abnormal blood count test results (RBC, WBC, Hb, PLT); glycated hemoglobin (HbA1c) <6.0% or normal fasting blood glucose; normal electrocardiogram (ECG) examination.
The exclusion criteria was as follows: Patients suspected of having coronary syndrome; patients with acute myocarditis; patients who previously had an episode of unstable angina; patients who were previously diagnosed with myocardial infarction; patients with a history of cerebrovascular disease; patients with known underlying diseases such as diabetes, acute and chronic kidney disease, high blood pressure (patients who even after use of medications had normal blood sugar and normal blood pressure were not enrolled); Males with BNP ≥100 pg/mL, females with BNP ≥150 pg/ mL; patients aged ≤40 years old with eGFR <90 mL/ min, patients aged >40 years old with eGFR <60 mL/ min (MDRD-Modification of Diet in Renal Disease formula of the NKF-National Kidney Foundation was used for calculations)
Testing method
After centrifugation of freshly collected EDTA- anticoagulated plasma, B-type natriuretic peptide (BNP) was tested according to steps in the operating manual of ARCHITECT-i2000 (Abbott Laboratories, Lake Bluff, Illinois, USA). The collected serum samples were simultaneously separated in two tubes with one tube being stored at -80°C as backup. Uniform batches of high-sensitivity troponin I (hs-TnI) reagents, calibrators and control materials were used for testing of the collected specimens. The quality control data and test results were printed and archived.
Limit of blank, limit of detection and limit of quantification
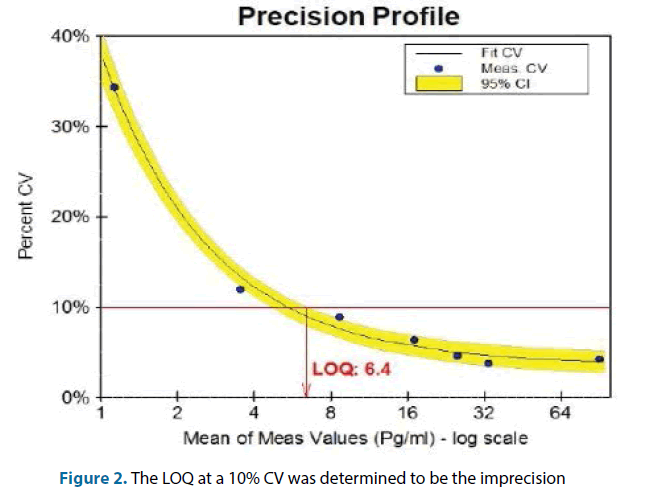
Before the specimen of the normal population was tested, the performance of ARCHITECT hs-TnI reagents was evaluated. We used the Clinical Laboratory Standards Institute (Clinical Laboratory Standard Institute, CLSI) EP17 protocols for the evaluation of the limit of blank (LoB), the Limit of Detection (LoD) and Limit of Quantitation (LoQ). Using a buffer and low-concentration samples, each sample was measured 20 times, verification was carried out at 2 times the standard deviation of LoB, the entire process was repeated twice. Using seven different concentrations of the test specimen, the test was repeated three times a day; it was measured for a total of 25 days. Using the EP Calculator®, we calculated the LoQ value at 10% Coefficient of Variation (CV) and 20% CV.
The measurement of imprecision
The EP5-A2 protocol was used to validate a method against user requirements. Using the Abbott ARCHITECT high-sensitivity troponin three levels control and Thermo Scientific laboratory solutions (CXL1407L) third-party quality control, the measurements were carried out twice daily, each time four levels of quality control material was tested, each quality control material were measured three times.
Calculation of the 99th percentile value
The 99th percentile value of the normal population was statistically analyzed according to sex, region and age. Age groups were as follows: 18-35 years old, 35- 50 years old, 50-65 years old and 65 years old and older. The 99th percentile value was also calculated and analyzed for regions and the eight different regions included in this study are Shanghai, Xi’an, Lanzhou,Anhui, Harbin, Beijing, Hangzhou, Fujian and Chongqing.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (IBM, New York, USA). Descriptive statistical methods were used to show the frequency distribution of cTnI. Non-parametric Kruskal-Wallis H test was used to compare difference between cTnI test values between different groups. We used the Mann-Whitney U non-parametric test to compare cTnI values between each group. P<0.05 was used to determine whether the difference was statistically significant.
Results
The study included 1,537 cases of normal healthy persons, of which 801 cases were males and 736 cases were female. As shown in Table 1, there were 446 cases in the 18-35 age group, 440 cases in the 35-50 year-old age group, 407 cases in the 50-65 year-old group, and 244 cases in the >65-year-old group.
| Age groups | Sex | Total | |
|---|---|---|---|
| Male | Female | ||
| 18-35 | 224 | 222 | 446 |
| 35-50 | 220 | 220 | 440 |
| 50-65 | 205 | 202 | 407 |
| >65 | 152 | 92 | 244 |
| Total | 801 | 736 | 1537 |
Table 1: The distribution by age and sex of the sample that was included in the study
According to the EP17 protocol, the assessment was carried out in duplicate. The value of LoB was 0.34 pg/ ml and 0.8 pg/ml, which was not in accordance with the manual where it is quoted as being consistently at 1.3 pg/ml; the lowest detection limit results was 1.0 pg/ml, whereas in the manualit is quoted as being below 1.9 pg/ml. The calculations were verified with the EP Evaluator. Cardiac troponin detection rate with ARCHITECT hsTnI in in the normal populations enrolled in this study was 97.5%. Detection rate among men was 99.2% and detection rate among women was 95.4%. Between the lowest LoD and the 99th percentile value, when an imprecision within 20% (95% of Confidence Interval was 11.4%-20.0%) was allowed, the lowest limit of quantification of the results was 3.29 pg/ml; when the imprecision allowed was at 10% (95% of Confidence Interval was 8.0% - 10.0%), the lowest limit of quantification of the results was 6.40 pg/ml (As shown in Figure 2).
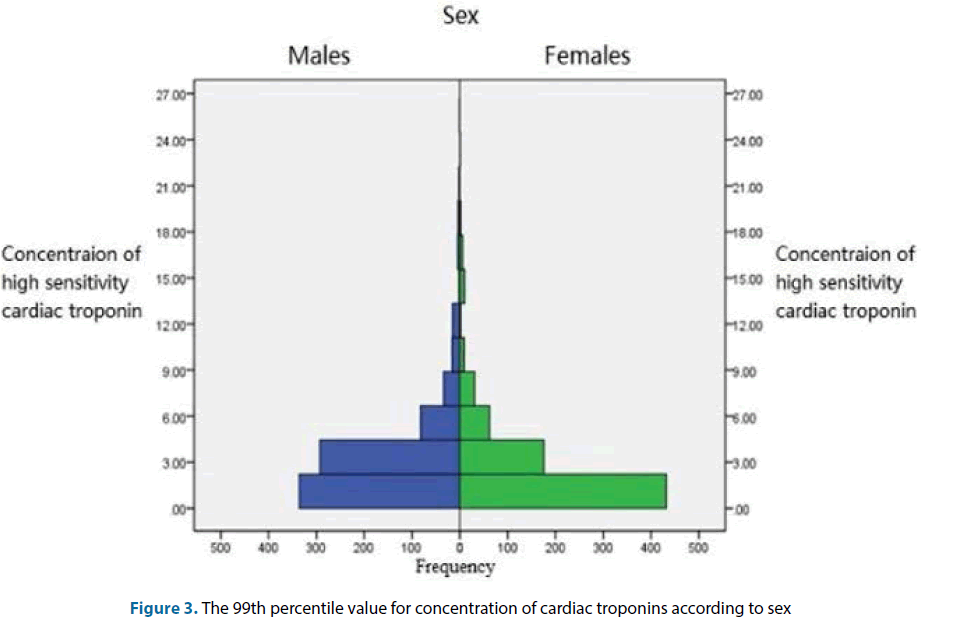
The overall cardiac troponin values of the healthy population showed a skewed distribution. The overall value of the 99th percentile value was at 26.42 (90% CI: 19.21-35.33) pg/ml corresponding CV was 4.90%. Male cardiac troponin 99th percentile value was 29.40 (90% CI: 21.34-41.79) pg/ml, corresponding CV was 4.77%, with a median of 2.60 and a mean of 3.82; the female 99th percentile value was 19.46 (90% CI: 13.45-26.53) pg/ml, corresponding CV was 5.37%, with a median of 2.00 and a mean of 3.05. The difference in cardiac troponin 99th percentile values for the male and female normal population was statistically significant (P<0.01) (Table 2). The concentration of high-sensitivity cardiac troponins for males and females was shown in Figure 3.
| Sex | High-sensitivity troponin concentration (pg/ml) | |||||
|---|---|---|---|---|---|---|
| Mean | Median | 99th percentile value | 90% CI | Corresponding CV (%) | P Value | |
| Male | 3.82 | 2.60 | 29.40 | 21.34-41.79 | 4.77 | P<0.01 |
| Female | 3.05 | 2.00 | 19.46 | 13.45-26.53 | 5.37 | |
| Both Sex | / | / | 26.42 | 19.21-35.33 | 4.90 | |
Table 2: Cardiac Troponin Values of the Healthy Population
When the distribution of the cardiac troponin 99th percentile value of the normal population was analyzed for different regions, we collected hs-TnI quality control specimens curve during the research centers, monitoring the average level of quality control and found that the difference in level of average quality control between all region was not statistically significant (P>0.05), showing that the differences in the test results were not due to the analysis system. The regional distribution of the 99th percentile value were as seen in Table 3, wherein for the Heilongjiang region, the total value, the value for males and the value for females were 29.8 pg/ml, 35.8 pg/ml and 27.5 pg/ml respectively, which was higher than the value of the corresponding groups in other regions. The total value and the value for males and the value for females in Beijing were 13.5 pg/ml, 23.9 pg/ml and 8.8 pg/ml respectively, which were lower than in other regions and the difference was statistically significant (P<0.05).
| Regions | No. | The 99th percentile (pg/ml) | ||
|---|---|---|---|---|
| Total | Male | Female | ||
| Beijing | 238 | 13.46 | 23.85 | 8.8 |
| Fujian | 208 | 26.62 | 28.40 | 13.60 |
| Helongjiang | 144 | 29.76 | 35.80 | 27.50 |
| Gansu | 206 | 22.15 | 29.60 | 19.20 |
| Shanghai | 191 | 19.84 | 22.00 | 14.40 |
| Zhejiang | 210 | 19.72 | 29.07 | 17.20 |
| Sichuang | 164 | 16.50 | 17.20 | 13.60 |
| Shanxi | 176 | 16.16 | 16.17 | 13.74 |
| Total | 1537 | 26.42 | 29.40 | 19.46 |
Table 3: The 99th percentile value of hs-TnI for the normal population of different regions
Discussion
This study was the first multi-center study to research the 99th percentile values of highly sensitive cardiac troponin in a healthy normal population in mainland China. Adequate selection of the cohort was of prime importance when doing such studies. The literature had several studies that evaluate the use of hs-cardiac troponins in AMI, but in several of these studies, what constitutes a normal population was poorly defined [11-17], while in other research works only health questionnaires and baseline clinical characteristics were evaluated [18-24]. Several studies had also made sure that a more thorough evaluation of the cohort was performed prior to enrollment in the study by making use of blood tests, electrocardiograms and physical examination [25-33]. The definition of normality was of uppermost importance in the evaluation of an assay and as such a strict inclusion criterion is warranted. Collinson et al. showed that with certain assays the results obtained will vary according to how a normal healthy person was defined [31]. Therefore, the inclusion and exclusion criteria were very strictly enforced and age and sex stratification was taken into account. In the course of this research, we not only considered the past medical history and current medical history of the normal population, but we also carried out laboratory liver and kidney function and blood sugar tests, electrocardiogram, and BNP tests so as to rule out the possibility of any disease. This study, to the maximum extent possible, defined a normal population. We found that according to the strict inclusion and exclusion criteria, it was more difficult to enroll women over the age of 65 years old in the normal population, mainly due to past history and current medical history. In our study, the group of people that were studied not only covers different regions of China and also includes central cities such as Beijing and Shanghai. The distribution of factors among the population was comprehensively evaluated. The results of this study aims at the establishment of the 99th percentile values for cardiac troponin among the Chinese population and also to provide a preliminary experimental data and repeatable processes and methods, with a high reference value, while also providing a cardiac troponin range of reference values for the clinical management of ACS which will contribute to the early diagnosis and evaluation of prognosis for ACS.
Although, at present there are several markers for myocardial ischemia and myocardial injury in use in the clinical setting, cardiac troponin is still the preferred marker for myocardial injury [2,3,5]. Acute coronary syndrome is a group of symptoms which presents with a main manifestation of acute myocardial ischemia and generally known as coronary artery disease, including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina (UA). For a long time the diagnosis has been made according to the definition of the World Health Organization and also based on clinical symptoms, ECG and comprehensive myocardial enzymes assay, with emphasis on the role of myocardial enzymes [34]. With the advent of testing of highly sensitive cardiac marker and development of imaging technology, we can now very early discover microscopic myocardial ischemia conditions [2]. European Society of Cardiology (ESC), American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) in 2000 and in 2007 introduced the first edition and the second edition of the universal definition of myocardial infarction and they placed a clear emphasis on the fact that cardiac troponin protein has to gradually replace cardiac enzymes, thus becoming the preferred diagnostic markers for ACS.
The 2007 United States laboratory medicine on ACS biomarkers practice guidelines [35] and the 2012 third edition of the global common definition of myocardial infarction [3], recommended that the 99th percentile value of cardiac troponin of a normal population be used as cut-off to determine the diagnosis of cardiac injury. The determination of the cardiac troponin 99th percentile values in a normal population can be used to more effectively judge any abnormal tracings on ECG of ACS patients (unstable angina and non-ST segment elevation myocardial infarction), determine the thrombolytic efficacy of acute myocardial infarction (AMI) patients, diagnose perioperative myocardial infarction and evaluate the prognosis. The clinical value is very significant [36,37].
According to 2014 update of “clinical application of high-sensitivity troponin Chinese expert consensus”, currently very few clinical laboratories have sufficient resources to carry out research on the Chinese population for the determination of the 99th percentile value. The prevailing practice is to use reference values provided in high quality benchmark reference papers or the use of values in the data product manuals provided by the manufacturer, whereby each laboratory to establish its own reference range [38]. The main purpose of this study is to select a representative research center and establish the high-sensitivity cardiac troponin 99th percentile value for the Chinese population based on the ARCHITECT platform.
The verification results of LoB and LoD are in line with the specifications of the equipment and reagents as per the manual of the manufacturer and verification was also done with EP Evaluator. Imprecision was good. On the overall, for males and females, the 99th percentile value corresponded to an imprecision of less than 10%, achieving a standard which would be acceptable for use as guideline and reference. The consensus is that when testing high-sensitivity cardiac troponins in a healthy population, the maximum imprecision should be 10% coefficient of variation, which is a requirement that is only met by a few assays [39,40]. The chemiluminescent detection method - ARCHITECT high-sensitivity cardiac troponin testing method has a detection rate of more than 95% in the normal population, which corresponds to the “highest level” of the third generation of high sensitivity troponin [38,41-43].
This study found that the value of the 99th percentile was higher in males as compared to females which were also noted in many other studies conducted abroad. Research study by Tar-Choon Aw et al. using the ARCHITECT platform found that in the normal male population the 99th percentile value was 34.3 pg/ ml and in women it was 15.6 pg/ml [44]; Apple et al. found the value in men to be 36.0 ng/ml and was 15.0 pg/ml in women [41]. In previous clinical applications, the cut-off value used for cardiac troponin did not differentiate on the basis of sex with one cut-off value being used for both sexes. Due to the fact that the cutoff value for women is lower than the overall cut-off value for both sexes, cases of NSTEMI in females could be misdiagnosed if the level of cardiac troponin is below that of the overall cut-off value. A systematic review on mortality in patients with ACS in both men and women published by El-Menyar showed that the proportion of female patients with myocardial infarction, in an ACS center, was significantly less than in men, 45% of female ACS patients were diagnosed with unstable angina with a good prognosis, but the mortality rate of female patients with ACS is far higher than that of men [45], which also suggest that there may be the possibility of female NSTEMI patients being misdiagnosed. The results of this study may provide a preliminary basis for the differentiation of cut-off values for cardiac troponin according to sexes in the Chinese population. As was also noted by Sandoval et al., sex-specific 99th percentile value need to be determined for hs-cardiac troponin assays to be used in the clinical setting [46].
The difference in cardiac troponin concentrations between different age groups was statistically significant. This study found that in the group with patients older than 65 years, cardiac troponin increased significantly. Eggers et al. also showed, in a study conducted on Swedish community residents aged 70-75 years, cardiac troponin concentrations in the elderly increases with increase in age [47]. These findings in a normal population of 60 years or older have been confirmed by Tar-Choon et al. in Singapore where it was found that troponin concentrations were significantly increased in that age bracket [44]. When using cardiac troponins in the clinical settings in the older population for the clinical diagnosis of cardiac ACS, doctors need to exercise a degree of caution because in the older population the baseline value is likely to be higher. It is therefore important to allow for a dynamic observation of the changes in the value of cardiac troponin concentrations over time, as according to clinical guidelines [3,48].
Study Limitations
There was relatively less biological variability in the normal reference values in the current study. At present, China is basically in a state of lack of data. Although this was a preliminary study on the 99th percentile value of cardiac troponin, but as far as clinical applications are concerned, particularly in the diagnosis of suspected cases of NSTEMI within 0-3 hours, further research and discussion is warranted for the cardiac troponin concentration value because of the biological variability.
When the specimens were tested in each research centers, we collected an average quality control level to determine the error between systems; ideally specimen should be collected together and sent to a central laboratory for testing in order to avoid errors.
In this study, we used frozen serum specimens, because we wanted to ensure that we made use of the same batch of reagents, calibration and quality control for testing purposes, thus excluding bias caused by reagents and instruments. There may be some difference in results between fresh and frozen specimens.
In terms of regional differences, Lanzhou and Harbin are at a higher altitude than other regions, but there were no further follow-ups and more in depth studies are required.
Conclusion
This multi-center study established the 99th percentile value for high sensitivity cardiac troponin in the Chinese population. We determined the normal range for males and females and we evaluated the analytical performance of ARCHITECT high sensitivity troponin I assays. This will help in the early diagnosis and clinical decision-making for patients presenting with suspected cases of ACS.
Competing and Conflicting Interests
The authors stated that they have no conflict of interest regarding the publication of this article.
References
- Cheng X, Xia L, Cen X, Yan S. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: The clinical features of acute coronary syndrome and biomarkers [Article in Chinese]. Chinese J Clin Laboratory Sci. 05: (2009).
- Hochholzer W, Morrow DA, Giugliano RP. Novel biomarkers in cardiovascular disease: update 2010. Am Heart J. 160: 583-594 (2010).
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Eur Heart J. 33: 2551-2567 (2012).
- Chen X, Yao G. Early complications of acute myocardial infarction and the changes in serum Cardiac troponin I. Chinese J Cardiovasc Rev. 11: (2005).
- Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 361: 868-877 (2009).
- Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 113: 1071-1078 (2006).
- Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long-term mortality. Circulation.116: 1907-1914 (2007).
- Kavsak PA, Newman AM, Lustig V, MacRae AR, Palomaki GE, et al. Long-term health outcomes associated with detectable troponin I concentrations. Clin Chem. 53: 220-227 (2007).
- James S, Flodin M, Johnston N, Lindahl B, Venge P. The antibody configurations of cardiac troponin I assays may determine their clinical performance. Clin Chem. 52: 832-837 (2006).
- Liu Z, Zhang Z, Zhao G, Gao H, Cao S, Sun X. 99th percentile value of high-sensitivity cardiac troponin I for normal healthy Chinese population from different regions of China. Chinese J Laboratory Sci. 35 (2012).
- Apple FS, Murakami MM. Serum and plasma cardiac troponin I 99th percentile reference values for 3 2nd-generation assays. Clin Chem. 53: 1558-1560 (2007).
- Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 56: 254-261 (2010).
- Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 53: 1990-1995 (2007).
- Venge P, Lagerqvist B, Diderholm E, Lindahl B, Wallentin L. Clinical performance of three cardiac troponin assays in patients with unstable coronary artery disease (a FRISC II substudy). Am J Cardiol. 89: 1035-1041 (2002).
- Lam Q, Black M, Youdell O, Spilsbury H, Schneider HG. Performance evaluation and subsequent clinical experience with the Abbott Automated Architect STAT Troponin-I assay. Clin Chem. 52: 298-300 (2006).
- Pagani F, Stefini F, Chapelle JP, Lefevre G, Graine H, Luthe H, et al. Multicenter evaluation of analytical performance of the Liaison troponin I assay. Clin Biochem. 37: 750-757 (2004).
- Christenson RH, Cervelli DR, Bauer RS, Gordon M. Stratus CS cardiac troponin I method: performance characteristics including imprecision at low concentrations. Clin Biochem. 37: 679-683 (2004).
- Sthaneshwar P, Jamaluddin FA, Fan YS. Reference value for cardiac troponin I in a multi-ethnic group. Pathology. 42: 454-456 (2010).
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem. 49: 1331-1336 (2003).
- Kavsak PA, MacRae AR, Yerna MJ, Jaffe AS. Analytic and clinical utility of a next-generation, highly sensitive cardiac troponin I assay for early detection of myocardial injury. Clin Chem. 55: 573-577 (2009).
- Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 412: 748-754 (2011).
- Uettwiller-Geiger D, Wu AH, Apple FS, Jevans AW, Venge P, Olson MD, et al. Multicenter evaluation of an automated assay for troponin I. Clin Chem. 48: 869-876 (2002).
- Apple FS, Murakami MM. Serum 99th percentile reference cutoffs for seven cardiac troponin assays. Clin Chem. 50: 1477-1479 (2004).
- Tate JR, Ferguson W, Bais R, Kostner K, Marwick T, Carter A. The determination of the 99th centile level for troponin assays in an Australian reference population. Ann Clin Biochem. 45: 275-288 (2008).
- Venge P, Johnston N, Lindahl B, James S. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol. 54: 1165-1172 (2009).
- Apple FS, Simpson PA, Murakami MM. Defining the serum 99th percentile in a normal reference population measured by a high-sensitivity cardiac troponin I assay. Clin Biochem. 43: 1034-1036 (2010).
- Koerbin G, Tate JR, Hickman PE. Analytical characteristics of the Roche highly sensitive troponin T assay and its application to a cardio-healthy population. Ann Clin Biochem. 47: 524-528 (2010).
- Collinson PO, Clifford-Mobley O, Gaze D, Boa F, Senior R. Assay imprecision and 99th-percentile reference value of a high-sensitivity cardiac troponin I assay. Clin Chem. 55: 1433-1434 (2009).
- Venge P, Johnston N, Lagerqvist B, Wallentin L, Lindahl B; FRISC-II Study Group. Clinical and analytical performance of the Liaison cardiac troponin I assay in unstable coronary artery disease, and the impact of age on the definition of reference limits. A FRISC-II substudy. Clin Chem. 49: 880-886 (2003).
- Eggers KM, Jaffe AS, Lind L, Venge P, Lindahl B. Value of cardiac troponin I cutoff concentrations below the 99th percentile for clinical decision-making. Clin Chem. 55: 85-92 (2009).
- Collinson PO, Heung YM, Gaze D, Boa F, Senior R, Christenson R, et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. ClinChem. 58: 219-25 (2012).
- Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 116: 1242-1249 (2007).
- McKie PM, Heublein DM, Scott CG, Gantzer ML, Mehta RA, Rodeheffer RJ, et al. Defining high-sensitivity cardiac troponin concentrations in the community. Clin Chem. 59: 1099-1107 (2013).
- No authors listed. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 59(3): 607-609 (1979).
- Zhang Yun, Huo Yong, Tang Hong. High -sensitivity troponin consensus - 2nd draft. [Book in Chinese]. 2014.
- Pracon R, Kruk M, Jakubczak B, Demkow M, Bilinska ZT. Superior early diagnostic performance of a sensitive cardiac troponin assay ascompared to a standard troponin test in the diagnosis of acute myocardialinfarction. Kardiol Pol. 70: 131-138 (2012).
- Mills NL, Churchhouse AM, Lee KK, Anand A, Gamble D, Shah AS, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA. 305: 1210-1216 (2011).
- 2014 Finalized Version " Clinical application of high-sensitivity troponin Chinese expert consensus ". Chinese Journal of Cardiovascular Disease [Article in Chinese]. 54: 899-904 (2015).
- Apple FS, Jesse RL, Newby LK, Panteghini M, Christenson RH, Cannon CP, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Clin Chem. 115: 222-226 (2007).
- NACB Writing Group, Wu AH, Jaffe AS, Apple FS, Jesse RL, Francis GL, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 53: 2086-2096 (2007).
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 58: 1574-1581 (2012).
- Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 53: 552-574 (2007).
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 58: 54-61 (2012).
- Aw TC, Phua SK, Tan SP. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin Chim Acta. 422: 26-28 (2013).
- El-Menyar A, Zubaid M, Rashed W, Almahmeed W, Al-Lawati J, Sulaiman K, et al. Comparison of men and women with acute coronary syndrome in six Middle Eastern countries. Am J Cardiol. 104: 1018-1022 (2009)
- Sandoval Y, Apple FS. The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem. 60: 455-462 (2014).
- Eggers KM, Lind L, Venge P, Lindahl B. Factors Influencing the 99th Percentile of Cardiac Troponin I Evaluated in Community-Dwelling Individuals at 70 and 75 Years of Age. Clin Chem. 59: 1068-1073 (2013).
- Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, et al. Serial changes in highly sensitive troponin I assay and early diagnosis ofmyocardial infarction. JAMA. 306: 2684-2693 (2011).