Review Article - Interventional Cardiology (2013) Volume 5, Issue 3
Multimodality imaging in the cardiac catheterization laboratory: a new era in sight
- Corresponding Author:
- Amar Krishnaswamy
Department of Cardiovascular Medicine
Cleveland Clinic, Cleveland, OH, USA
E-mail: krishna2@ccf.org
Abstract
Keywords
3D echocardiography, catheterization laboratory, fluoroscopic overlay, intracardiac echocardiography, multimodality imaging multidetector computed tomography, paravalvular leak closure, percutaneous mitral balloon valvuloplasty, transcatheter aortic valve replacement
Multimodality imaging in the cardiac catheterization laboratory continues to develop with rapid advancements in the use of intracardiac echocardiography (ICE), 3D echocardiography (3DE) and multidetector computed tomography (MDCT), with fluoroscopic overlay to produce 3D images of intracardiac structures. The improvement in these imaging tools have coincided with innovation and procedural advancement in the field of interventional cardiology, especially with regard to structural and valvular cardiac interventions. As advances in transcatheter interventions continue and the availability of these procedures become more common; an understanding of the various imaging modalities used in the interventional catheterization laboratory is important for practicing clinicians.
2D fluoroscopic and echocardiographic imaging in the catheterization laboratory have been, and will likely continue to be, routine methods for real-time imaging of the heart during transcatheter interventions, given their widespread availability and clinicians’ familiarity with their use. Inherent to fluoroscopic imaging, however, are significant inadequacies in defining accurate dimensions, distances and depth of intracardiac anatomy, especially the nonradiopaque structures found in the heart. These limitations in 2D planar imaging have led to the increasing availability and use of 3DE and MDCT with fluoroscopic overlay to generate 3D, real-time, detailed and accurate images for procedural guidance in the cardiac catheterization laboratory. Although ICE also produces 2D planar views, the images it provides are of greater detail and anatomic accuracy than fluoroscopy and transesophageal echocardiography (TEE) alone. As such, these imaging modalities may reduce procedural time, radiation exposure, TEE probe dwell time and procedural complications, while improving procedural success for many transcatheter interventions.
In this article, the authors will demonstrate applications of the commonly used multimodality imaging resources in the contemporary cardiac catheterization laboratory, including 3DE, ICE and computed tomography (CT):fluoroscopy overlay.
3D echocardiography
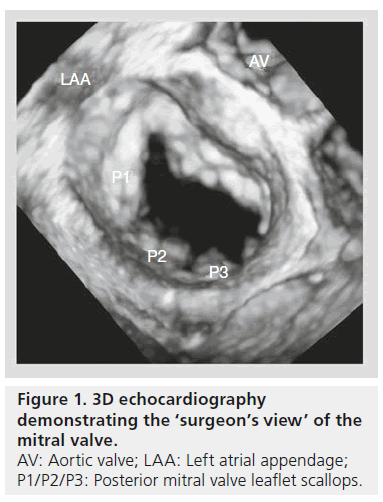
Fluoroscopy and cineangiography in the catheterization laboratory provide 2D images without any clear structural imaging of intracardiac anatomy. While standard 2D echocardiography (2DE) provides the advantage over fluoroscopy of giving spatial and temporal resolution, 2DE is limited in visualizing intracardiac catheters and devices in relation to the surrounding anatomic environment, given only two spatial dimensions are depicted. Real-time 3DE has become a useful modality with which to assess cardiac anatomy and function, leading the European Association of Cardiovascular Imaging (formerly European Association of Echocardiography) and the American Society of Echocardiography to provide guidelines for image acquisition and display using this technology [1,2]. 3DE provides a better context in which to assess anatomical features [3]. For instance, the ‘en face’ view of the left atrium (LA), also known as the ‘surgeon’s view’, provides images of the mitral valve (MV) from the perspective of the LA (Figure 1).
These types of images provide a more accurate depiction of the valve than standard 2DE alone. Investigators have demonstrated that 3D-TEE correlates more closely with operative surgical findings than standard 2D-TEE, especially with respect to MV pathology [4–6]. With continued advances in echocardiography transducers, 3DE has become an important imaging tool to use in the catheterization laboratory for diagnosis, as well as for procedural guidance in prosthetic paravalvular leak (PVL) repair, percutaneous mitral balloon valvuloplasty (PMBV), transcatheter edge-to-edge repair of mitral regurgitation (MR), atrial septal defect (ASD) closure and transcatheter aortic valve replacement (TAVR).
▪ Prosthetic paravalvular leak closure
PVL is common, and while many patients remain asymptomatic, up to 5% of patients who have undergone valve replacement have significant clinical manifestations [7]. Redo surgery is associated with greater risk than first-time surgery, and results of reoperation for PVL are unpredictable. Therefore, transcatheter closure is a promising treatment modality and requires accurate assessment of valvular anatomy and identification of regurgitant flows.
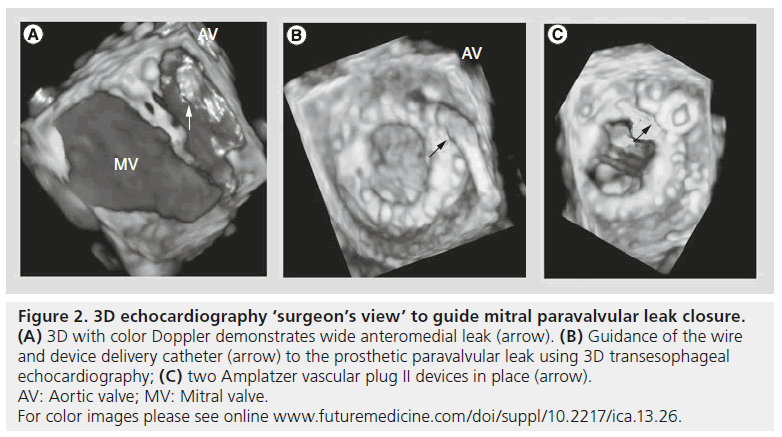
Using 3DE in conjunction with standard 2D-TEE allows for accurate identification of paravalvularregurgitant flow (Figure 2A). 2D-TEE alone in this circumstance is sometimes limited by prosthetic valve shadowing that may obscure regurgitant flow areas that otherwise could have been visualized. In addition, identifying the proportion of the sewing ring circumference with regurgitant flow is important for classifying the severity of the paravalvular leak. For example, paravalvular jet width for the MV from 1 to 2, 3 to 6 and >6 mm determines whether the leak is mild, moderate or large [8]. 3DE with color Doppler is an important adjuvant imaging modality to appropriately classify the severity of the paravalvular leak, and also to aid in the identification of leak origin. This allows for better preprocedural planning and assessment of leak-closure feasibility or necessity.
Figure 2: 3D echocardiography ‘surgeon’s view’ to guide mitral paravalvular leak closure. (A) 3D with color Doppler demonstrates wide anteromedial leak (arrow). (B) Guidance of the wire and device delivery catheter (arrow) to the prosthetic paravalvular leak using 3D transesophageal echocardiography; (C) two Amplatzer vascular plug II devices in place (arrow). AV: Aortic valve; MV: Mitral valve. For color images please see online www.futuremedicine.com/doi/suppl/10.2217/ica.13.26.
Once the regurgitant flow and defect have been identified and the decision has been made to close the leak, 3DE allows for real-time guidance for safely advancing wires and catheters across the regurgitant paravalvular space in the catheterization laboratory (Figures 2B & 2C) [9]. Subsequently, after crossing the defect and directing equipment to the valve, device deployment can be undertaken using real-time 3DE for accurate and precise placement of the device. Similarly to 2DE, 3DE has the ability to immediately evaluate for procedural complications, including pericardial effusion, ASD formation, catheter thrombus formation and/or prosthetic valve leaflet entrapment [7].
Despite the more detailed images that 3DE provides, it does have the disadvantage of creating ‘stitch artifacts’ created by motion of the heart that 2DE is unlikely to reproduce [1,7]. It is also important to note that shadow artifacts created by valve prostheses can result in ‘dropout’, which may be mistaken in some locations for a paravalvular defect. The use of color Doppler to demonstrate flow (or lack thereof) may provide confirmation in these situations. In some cases, the anterior cardiac structures including the aortic and tricuspid valves are not visualized well using 3DE, and traditional echocardiographic assessment of those valves may be required. Regardless, 3DE has proven to be advantageous in visualizing paravalvular leaks and guiding the proceduralist for percutaneous repair in the catheterization laboratory.
▪ Percutaneous mitral balloon valvuloplasty
PMBV is performed for symptomatic mitral stenosis as an alternative to open heart surgery. The well known ‘splitability score’ uses the transthoracic 2DE findings of severity and the extent of leaflet calcifications, leaflet thickening, leaflet mobility and involvement of the subvalvular apparatus to provide a numerical score that has an inverse relationship with PMBV success [10].
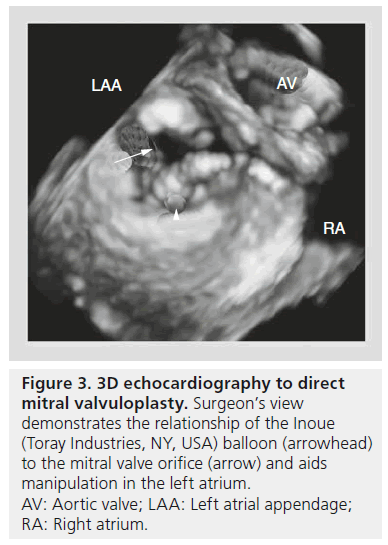
While TTE and 2DE are mainstays in the evaluation of mitral stenosis and associated MR that may affect the performance of PMBV, 3DE may also provide incremental benefit in both procedural planning and execution. 3DE allows operators a unique view of the MV leaflets and commissures. This may provide a better understanding of asymmetric commissural fusion than 2DE alone, with implications regarding the feasibility of performing PMBV. In addition, manipulation of the Inoue (Toray Industries, NY, USA) balloon in the LA and direction of the catheter through the MV into the left ventricle (LV) can be facilitated by the surgeon’s view of the LA on 3DE (Figure 3). Post-PMBV, 3DE provides more accurate measurement of the MV area, commissure opening, and transvalvular gradients than standard 2D-TEE [11,12] and has been shown to be better at revealing leaflet tear severity than TTE, 2D-TEE or ICE [13].
Figure 3: 3D echocardiography to direct mitral valvuloplasty. Surgeon’s view demonstrates the relationship of the Inoue (Toray Industries, NY, USA) balloon (arrowhead) to the mitral valve orifice (arrow) and aids manipulation in the left atrium. AV: Aortic valve; LAA: Left atrial appendage; RA: Right atrium.
▪ Transcatheter edge-to-edge repair of MR
Transcatheter intervention to repair severe MR has become a well-known option for patients suffering from MR [14]. The MitraClip (Abbott Laboratories, IL, USA) replicates the double MV orifice created by Alfieri’s surgical intervention [15]. Appropriate anatomic considerations for use of the clip include a coaptation length of >2 mm and a coaptation depth of <11 mm, as well as a flail gap of <10 mm and a flail width of <15 mm [7]. Furthermore, use of the clip is often most successful in patients whose MR jet originates at the A2/P2 segment of the valve. While 2DE (TTE and TEE) can provide this information, 3DE may more clearly identify the areas of regurgitation and allow the operator to understand the pathology of MR.
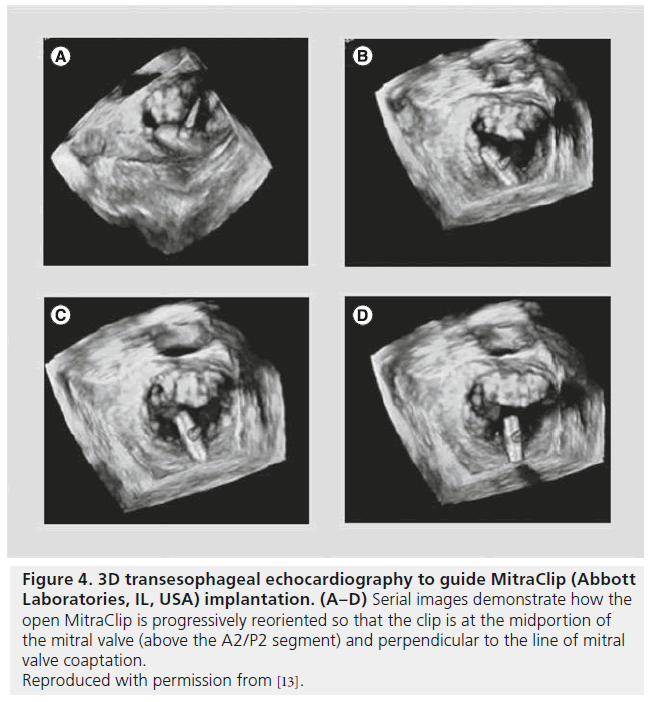
Intraprocedurally, 3DE, 2D-TEE and ICE all allow visualization of septal crossing with catheter guidance to the middle of the MV orifice. Real-time 3DE via the surgeon’s view allows the operator to direct the clip to the valve leaflets in the appropriate perpendicular orientation (Figure 4) [16]. The same guidance may be achieved using transgastric 2D-TEE in the short-axis view of the MV, but adequate views are difficult to achieve in nearly one-third of patients [7]. 3DE can also be used to visualize real-time grasping of both MV leaflets, although may be inferior to 2DE in this situation [17]. In cases of persistent regurgitation after one clip is placed, 3DE with color can help identify whether an additional clip should be placed more medially or laterally.
Figure 4: 3D transesophageal echocardiography to guide MitraClip (Abbott Laboratories, IL, USA) implantation. (A–D) Serial images demonstrate how the open MitraClip is progressively reoriented so that the clip is at the midportion of the mitral valve (above the A2/P2 segment) and perpendicular to the line of mitral valve coaptation. Reproduced with permission from [13].
As with many of the other imaging modalities discussed in this review, very limited studies exist that compare clinical end points, comparative procedural duration and other procedural factors between 3DE and 2D-TEE for transcatheter edge-to-edge. The data that do exist are largely comprised of small studies that have evaluated subjective ratings of procedural difficulty based on the perception of the interventionalist involved in using 3DE. Altiok and colleagues demonstrated an advantage in using 3DE for MitraClip in 28 patients with respect to the interventionalists’ subjective perception of improved procedural guidance by reducing time between steps during the procedure, as well as providing a more detailed and accurate definition of the trans-septal puncture site and catheter/ balloon direction to the MV orifice [17].
▪ Transcatheter aortic valve replacement
TAVR is a quickly growing and readily available interventional therapy for severe, symptomatic aortic valve stenosis in patients who are either deemed inoperable or have a high degree of surgical risk. 3DE has been successfully used to provide a detailed assessment of the aortic annular size in preprocedural planning, visualization of the valve deployment catheter in the aortic position to assure appropriate positioning, and for immediate postprocedural assessment of possible complications [18–21]. Multiple studies have demonstrated that 3DE has good reproducibility and accuracy in determination of aortic annular size, which is of vital importance for preprocedural planning for sizing of the aortic valve prosthesis [20,22–24].
The importance of 3D imaging in sizing the annulus is the result of the elliptical nature of this structure; by definition, single-plane imaging is, therefore, inaccurate [25–29]. Contrast-enhanced MDCT has been demonstrated to provide the most accurate assessment of the aortic annulus for TAVR prosthesis sizing [30,31]. It also provides the opportunity to plan the appropriate C-arm angulation to optimal valve deployment, obviating the need for multiple intraprocedural aortic root angiograms [32]. Unfortunately, some patients with chronic kidney disease are unable to undergo contrasted MDCT. Therefore, 3DE still holds an important place in the evaluation of patients for TAVR.
Given the advantages in anatomical structure characterization, direct visualization of trans-septal puncture and device delivery system advancement to the area of interest, as well as the ability to provide detailed, real-time visualization of intracardiac structures, 3DE will undoubtedly continue to grow and evolve. It is likely that the temporal and spatial resolution of the 3DE transducers and computer technology that processes the images will also continue to improve and allow even better characterization of intracardiac anatomy. The xMATRIX 3DE transducers and SONOS 7500 (Koninklijke Philips Electronics, NV, USA) are commonly used systems for live 3DE, and multiple other systems are in development. Multiple reports, institutional anecdotal experiences, descriptions of echocardiographic measurement differences and small patient studies using subjective ratings for assessment of intraprocedural 3DE exist and have shown an overall favorable profile for 3DE over standard 2D-TEE for a variety of percutaneous procedures [1–6,18,20,22–25]. While these findings are thoughtprovoking, trials are necessary to adequately assess the clinical and procedural benefits of using 3DE.
MDCT overlay
Generating 3D CT images can be very helpful in accurately defining cardiovascular anatomy for procedures in the catheterization laboratory. Preprocedural CT images can be used to overlay images onto real-time fluoroscopic imaging during transcatheter interventions using the syngo DynaCT Cardiac C-arm CT system (Siemens Healthcare, PA, USA). This imaging modality has proven helpful to procedural guidance in paravalvular leak closure and pulmonary vein stenting in the authors’ own center, although its use has also been described in patients undergoing septal ablation of hypertrophic cardiomyopathy [33], electroanatomic mapping of the LA and pulmonary veins [34], in assessing aortic anatomy prior to endovascular aneurysm repairs [35], in catheter navigation through previously placed carotid stents [36], MV repair [37], TAVR [36,38] and left atrial appendage occlusion [39], among others.
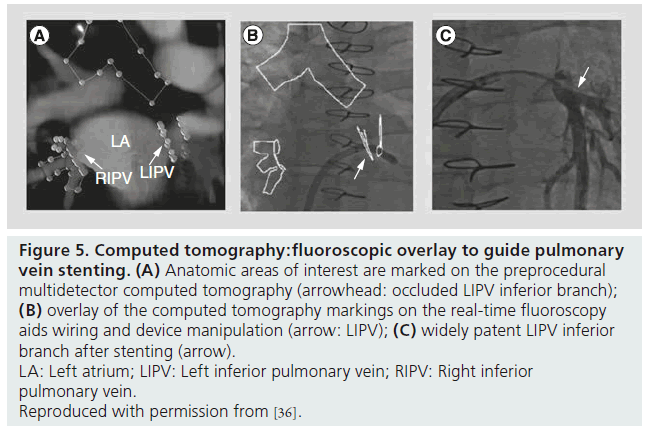
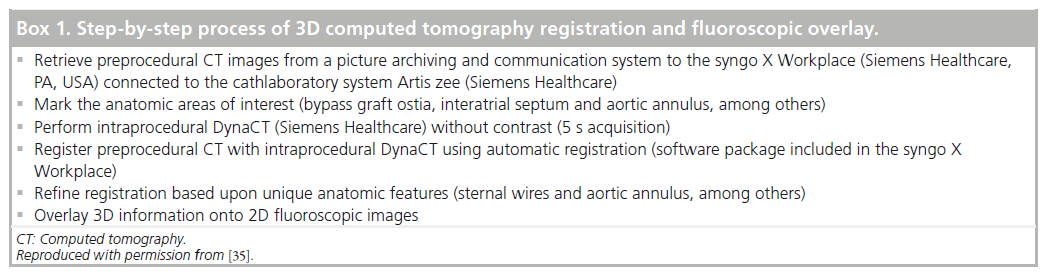
The process of creating 3D CT images with fluoroscopic overlay has been previously described and is summarized in box 1 [40]. In brief, preprocedural CT images are used to mark areas of anatomic interest (Figure 5A) [41]. Subsequently, with the patient in the catheterization laboratory, the syngo DynaCT is acquired without contrast administration. Images are produced by high-speed C-arm rotation around the patient with subsequent 3D volume reconstruction. Both scans are necessary as the preprocedural MDCT provides a greater degree of anatomic clarity than the noncontrasted DynaCT alone, and the DynaCT establishes the position of the patient on the catheterization laboratory table. Once the two data sets are ‘registered’ to one another, the previously marked anatomic areas are then overlaid on real-time fluoroscopy images to provide procedural guidance (Figure 5B). The overlay markings are displayed on the typical fluoroscopic screen, do not require a second screen and can be turned off as necessary.
Figure 5: Computed tomography:fluoroscopic overlay to guide pulmonary vein stenting. (A) Anatomic areas of interest are marked on the preprocedural multidetector computed tomography (arrowhead: occluded LIPV inferior branch); (B) overlay of the computed tomography markings on the real-time fluoroscopy aids wiring and device manipulation (arrow: LIPV); (C) widely patent LIPV inferior branch after stenting (arrow). LA: Left atrium; LIPV: Left inferior pulmonary vein; RIPV: Right inferior pulmonary vein. Reproduced with permission from [36].
Box 1. Step-by-step process of 3D computed tomography registration and fluoroscopic overlay..
In the authors’ experience, CT-overlay can be particularly useful in patients presenting for paravalvular leak closure or pulmonary vein stenting. With accurate identification of intracardiac structures, the need for ‘test’ or repeated contrast injections for angiography and/or prolonged fluoroscopy duration and prolonged TEE probe dwell time are all potentially improved. As such, 3D CT with fluoroscopic overlay provides accurate and safe guidance for catheter manipulation, trans-septal punctures and various device deployments other than fluoroscopy or 2D TEE alone. While multiple promising reports exist in the literature, studies comparing clinical outcomes, contrast dye exposure, procedure duration, TEE probe dwell time and radiation exposure need to be performed.
While the noncontrast-enhanced DynaCT does not provide the ability to appropriately analyze cardiac structures (due to significant cardiac motion), image quality of the great cardiac vessels is substantially enhanced using contrast injection, and contrast-enhanced DynaCT in the peripheral vessels is similarly useful [42,43]. However, as many patients presenting for structural cardiac intervention have already had MDCT preprocedurally (with or without contrast), and visualization of intracardiac structures even with contrasted DynaCT is poor, we prefer fusion of the previously obtained CT with the real-time fluoroscopy. Furthermore, while it is possible to fuse the preprocedural CT with the fluoroscopic image using a ‘phantom’ (i.e., without a C-arm acquisition), we find this method cumbersome and less accurate. Given the relative low x-ray dose of the DynaCT acquisition, we do believe this is a necessary technique in most cases [44].
Some studies have used cardiac MRI (CMR) to assess aortic valve morphology and function in conjunction with TAVR [45–48]. CMR provides the advantage of not requiring contrast dye and it does not use ionizing radiation. Some reports have demonstrated using fluoroscopic overlay of previously performed CMR studies to help with procedural guidance in the catheterization laboratory [49,50], and very limited case-series exist for which real-time CMR has been used in animal studies for TAVR [51]. While this imaging modality may prove beneficial in the future, limited studies exist that describe and compare the use of CMR with CT for preprocedural guidance in the catheterization laboratory. Further investigations into the feasibility and potential advantages that CMR provides need to be performed.
▪ Intracardiac echocardiography
ICE was first utilized in the 1980s as a tool for visualization of the coronary arteries and for guidance with radiofrequency ablation. The ICE catheter, usually introduced via the femoral vein, provides more detailed visualization of trans-septal puncture with greater patient comfort than TEE. As opposed to fluoroscopic guidance alone, the use of ICE (or TEE) provides operators the ability to more precisely puncture the septum (in the anterior/posterior and superior/ inferior plane) on the basis of the procedure being performed [52].
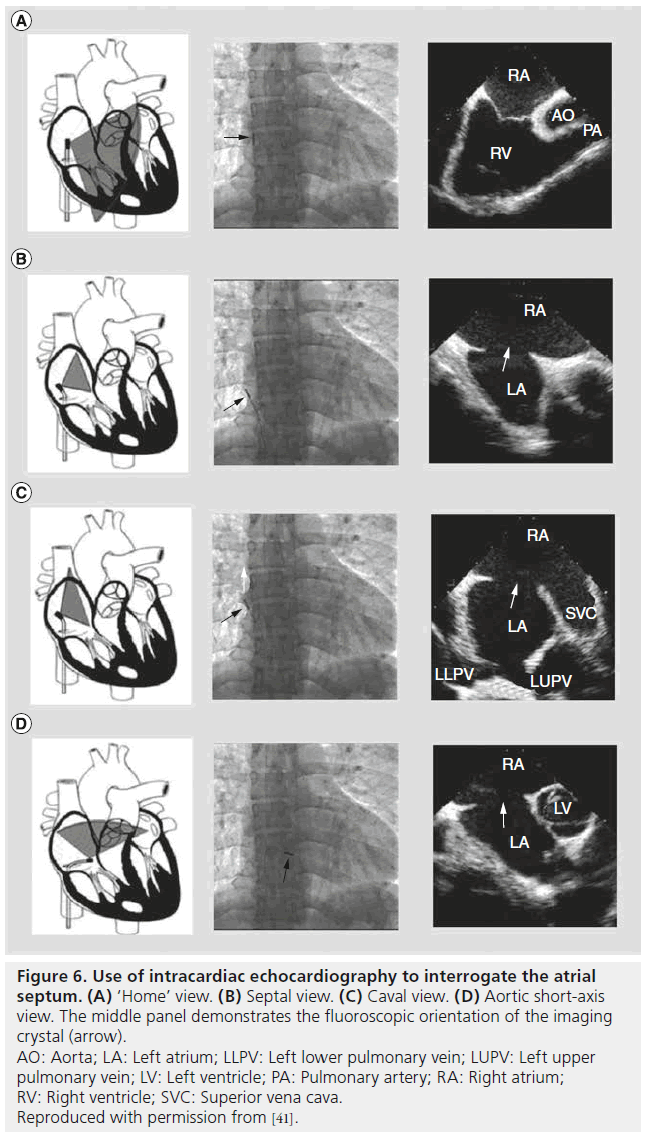
Pandian used ICE catheters for the first time in humans in 1990 for the evaluation of iliofemoral arterial disease and later that same year for coronary angioplasty guidance [53]. Valdes-Cruz and colleagues used ICE in the successful percutaneous closure of ASDs in piglets in 1991 [54]. In 2001, Gonzalez and colleagues first described the use of ICE for guidance in performing device closures for ASDs and patent foramen ovales in humans [55]. For ASD closures in particular, ICE is an important imaging modality to properly assess the ‘rims’ of the defect and assure the safety of percutaneous device closure. In addition to fluoroscopy, it is also routinely used for sizing the defect, where it can demonstrate residual shunt during balloon occlusion sizing of the defect. Furthermore, ICE demonstrates device efficacy and stability prior to final release of the occluder device. Figure 6 demonstrates the common views obtained by ICE and the fluoroscopic position of the imaging crystal in the right atrium.
Figure 6: Use of intracardiac echocardiography to interrogate the atrial septum. (A) ‘Home’ view. (B) Septal view. (C) Caval view. (D) Aortic short-axis view. The middle panel demonstrates the fluoroscopic orientation of the imaging crystal (arrow). AO: Aorta; LA: Left atrium; LLPV: Left lower pulmonary vein; LUPV: Left upper pulmonary vein; LV: Left ventricle; PA: Pulmonary artery; RA: Right atrium; RV: Right ventricle; SVC: Superior vena cava. Reproduced with permission from [41].
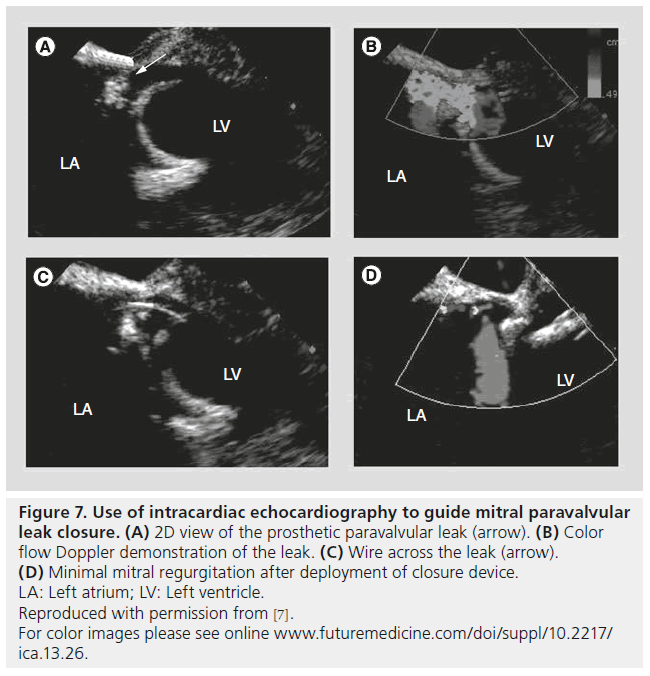
ICE has also become a versatile tool for use in other transcatheter interventions including PVL closure and left atrial appendage closure (Figure 7) [9]. ICE is helpful not only in guiding deployment catheters, balloons and devices safely but also for assessing for any procedural complications such as pericardial effusion.
Figure 7: Use of intracardiac echocardiography to guide mitral paravalvular leak closure. (A) 2D view of the prosthetic paravalvular leak (arrow). (B) Color flow Doppler demonstration of the leak. (C) Wire across the leak (arrow). (D) Minimal mitral regurgitation after deployment of closure device. LA: Left atrium; LV: Left ventricle. Reproduced with permission from [7].
Currently, five different ICE transducers are available for commercial use (adapted from [56]):
▪ The ultraICE™ mechanical single-element system (Boston Scientific, MA, USA);
▪ The AcuNavsystem™ from Siemens (Siemens Healthcare);
▪ The Clear ICE system (St Jude Medical, MN, USA);
▪ The SoundStar™ Catheter system (Stryker Medical, MI, USA);
▪ The ViewMate Z Intracardiac Ultrasound System (St Jude Medical) and ViewFlex Plus ICE Catheter (St Jude Medical).
These devices vary significantly in their steerability, Doppler ability, image depth and clarity. The Clear ICE device has the advantage of being able to be integrated with 3D localization and 3D-ICE transducers are likely to become more commonplace in the near future.
Complications from ICE are typically quite rare and are usually related to access site issues also common for right heart catheterization (access site bleeding, transient arrhythmias from probe contact with the right ventricular wall/ septum, and thrombus formation on the probe with prolonged insertion). Despite the advantages that ICE can offer, the images that ICE provides can vary in their quality and technical issues during catheterization procedures can arise in determining the proper image planes. Commonly used ICE catheters currently provide only 2D images that are inherently limited in their ability to provide the most accurate and detailed visualization of intracardiac structures. 3D-ICE is in development and will likely become more common in the near future.
Limitations to currently available catheterization laboratory imaging
Undoubtedly, each of the imaging modalities discussed in this review have their own limitations. For each of these (3DE, ICE, CT:fluoroscopy fusion), one of the greatest barriers to use is the operator’s experience and understanding of a given modality. When working together in the catheterization laboratory with a specialist in cardiovascular imaging, it is also important that all those involved in a given procedure use a standard nomenclature to define the location of defects and devices, and that there is a ‘realtime’ updating of the imaging specialist by the interventional operator.
Typical limitations of echocardiographic imaging (TTE, TEE and ICE) include difficulty in obtaining necessary views due to distance from the transducer (i.e., LV apex for TEE and leftsided structures for ICE, among others), shadowing artifacts from valve prostheses [1]. Typical limitations of MDCT include the need for contrast enhancement, as well as the radiation dose provided to patients, though contemporary imaging algorithms have substantially reduced both contrast and radiation doses.
Future perspective
The primary goal of developing new imaging techniques in the catheterization laboratory is to provide procedural guidance that will help make various percutaneous interventions more feasible, safer and more effective for patients. As these technologies continue to improve and evolve, the role for interventional cardiologists in structural heart disease will continue to expand and an understanding of the various imaging modalities used in the interventional catheterization laboratory is important for structural interventionalists. It should also be noted that the variety of imaging modalities discussed in this review are not mutually exclusive of one another. Rather, these various modalities should be considered complementary in providing the most accurate, detailed and descriptive images of intracardiac structures.
While 3DE, MDCT with fluoroscopic overlay and ICE have shown significant advantages over more traditional imaging modalities (2DE, TEE, fluoroscopy and cineangiography) in the catheterization laboratory, future studies that specifically evaluate and define the clinical and procedural benefits of using these advanced imaging modalities are necessary and will most likely continue to be performed. In the coming years, these imaging modalities, including CMR with fluoroscopic overlay and real-time CMR, will become more common and interventionalists will need to become more familiar with their use. Future studies are likely to show procedural and clinical benefit in employing these various imaging modalities.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Multimodality imaging in the catheterization laboratory
▪ Percutaneous interventions for a variety of cardiovascular conditions continue to become more commonplace and an understanding of the various imaging modalities that can be utilized in the catheterization laboratory is vital for any practicing interventional cardiologist.
▪ Imaging modalities continue to rapidly improve and new technologies are in development to provide procedural guidance.
3D echocardiography
▪ Has been successfully used in the catheterization laboratory for prosthetic paravalvular leak closure, percutaneous mitral balloon valvuloplasty, transcatheter edge-to-edge repair of mitral regurgitation and transcatheter aortic valve replacement.
▪ There are some imaging advantages over 2D echocardiography for the same procedures, but there are limited data to show actual clinical and procedural benefit for using 3D echocardiography and future studies should be undertaken to evaluate these potential advantages.
Multidetector computed tomography overlay
▪ Provides both preprocedural and intraprocedural guidance with accurate anatomical definition using computed tomography with real-time fluoroscopic overlay.
▪ This imaging modality has a number of advantages over other imaging modalities in the catheterization laboratory and can be safely used for procedural guidance.
▪ Future studies should focus on a comparative assessment of clinical and procedural benefits using this modality.
▪ Periprocedural cardiac MRI with real-time imaging has evolved out of multidetector computed tomography with overlay to provide another imaging modality for use in the catheterization laboratory. Its use is very early in development for routine use in the catheterization laboratory.
Intracardiac echocardiography
▪ Like the other imaging modalities that are discussed, intracardiac echocardiography can further and more accurately define cardiac anatomy than 2D echocardiography or fluoroscopy alone for procedural guidance in the catheterization laboratory.
▪ 3D intracardiac echocardiography transducers are in development and in the future may provide an even greater advantage for use in the catheterization laboratory.
References
Papers of special note have been highlighted as:
▪ of interest
- Lang R, Badano L, Tsang W et al. EAE/ASE Recommendations for image acquisition and display using three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 25(1), 3–46 (2012).
- Lang R, Badano L, Tsang W et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 13(1), 1–46 (2012).
- Agricola E, Badano L, Mele D et al. Real-time three dimensional transesophageal echocardiography; technical aspects and clinical applications. Heart Int. 5(1), e6 (2010).
- Pepi M, Tamborini G, Maltagliati A et al. Head-to head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J. Am. Coll. Cardiol. 48(12), 2524–2530 (2006).
- Garcia-Orta R, Moreno E, Vidal M et al. Three dimensional versus two-dimensional transesophageal echocardiography in mitral valve repair. J. Am. Soc. Echocardiogr. 20(1), 4–12 (2007).
- Sugeng L, Shernan SK, Weinert L et al. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J. Am. Soc. Echocardiogr. 21(12), 1347–1354(2008).
- Cavalcante J, Rodriquez LL, Kapadia S, Tuzcu EM, Stewart WJ. Role of echocardiography in percutaneous mitral valve interventions. JACC Cardiovasc. Imaging 5(7), 733–746 (2012).
- Vitarelli A, Conde Y, Cimino E et al. Assessment of severity of mechanical prosthetic mitral regurgitation by transesophageal echocardiography. Heart 90(5), 539–544 (2004).
- Krishnaswamy A, Tuzcu EM, Kapadia SR. Paravalvular leak closure. In: Interventional Procedures for Structural Heart Disease. RogersJ, Lasala J (Eds). Elsevier, PA, USA (2014) (In Press).
- Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br. Heart J. 60(4), 299–308 (1988).
- Naqvi TZ. Echocardiography in percutaneous valve therapy. JACC Cardiovasc. Imaging 2(10), 1226–1237 (2009).
- Zamorano J, Perez de Isla L, Sugeng L et al. Non-invasive assessment of mitral valve area during percutaneous balloon mitral valvuloplasty: role of real-time 3D echocardiography. Eur. Heart J. 25(23), 2086–2091 (2004).
- Applebaum RM, Kasliwal RR, Kanojia A et al. Utility of three dimensionalechocardiography during balloon mitral valvuloplasty. J. Am. Coll. Cardiol. 32(5), 1405–1409 (1998).
- Vassiliades TA Jr, Block PC, Cohn LH et al. The clinical development of percutaneous heart valve technology: a position statement of the Society of Thoracic Surgeons (STS), the American Association for Thoracic Surgery (AATS), and the Society for Cardiovascular Angiography and Interventions (SCAI). Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA). J. Am. Coll. Cardiol. 3(45), 1554–1560 (2005).
- Alfieri O, Maisano F, De Bonis M et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J. Thorac. Cardiovasc. Surg. 122, 674–681(2001).
- Siegel RJ, Luo H, Biner S. Transcatheter valve repair/implantation. Int. J. Cardiovasc. Imaging 27(8), 1165–1177 (2011).
- Altiok E, Becker M, Hamada S, Reith S, Marx N, Hoffmann R. Optimized guidance of percutaneous edge-to edge repair of the mitral valve using real-time 3-D transesophageal echocardiography. Clin. Res. Cardiol. 100(8), 675–681 (2011).
- Lee AP, Lam YY, Yip GW, Lang RM, Zhang Q, Yu CM. Role of real time three-dimensional transesophageal echocardiography in guidance of interventional procedures in cardiology. Heart 96(18), 1485–1493 (2010).
- Scohy TV, Soliman OI, Lecomte PV et al. Intraoperative real time three-dimensional transesophageal echocardiographic measurement of hemodynamic, anatomic and functional changes after aortic valve replacement. Echocardiography 26(1), 96–99 (2009).
- Ng AC, Delgado V, van der Kley F et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ. Cardiovasc. Imaging 3(1), 94–102 (2010).
- Filgueiras-Rama D, Lopez T, Moreno-Gomez R et al. 3D transesophageal echocardiographic guidance and monitoring of percutaneous aortic valve replacement. Echocardiography 27(1), 84–86 (2010).
- Shahgaldi K, da Silva C, Back M, Ruck A, Manouras A, Sahlen A. Transesophageal echocardiography measurements of aortic annulus diameter using biplane mode in patients undergoing transcatheter aortic valve implantation. Cardiovasc. Ultrasound 11, 5 (2013).
- Messika-Zeitoun D, Serfaty JM, Brochet E et al. Multimodal assessment of theaorticannulus diameter: implications for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 55(3), 186–194 (2010).
- Jánosi RA, Kahlert P, Plicht B et al. Measurement of the aortic annulus size by real-time three-dimensional transesophageal echocardiography. Minim. Invasive Ther. Allied Technol. 20(2), 85–94 (2011).
- Altiok E, Koos R, Schröder J et al. Comparison of two-dimensional and three-dimensional imaging techniques for measurement of aortic annulus diameters before transcatheter aortic valve implantation. Heart 97(19), 1578–1584 (2011).
- Wood DA, Tops LF, Mayo JR et al. Role of multislice computed tomography in transcatheter aortic valve replacement. Am. J. Cardiol. 103(9), 1295–1301 (2009).
- Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ. Cardiovasc. Intervent. 1(1), 74–81(2008).
- Schoenhagen P, Numburi U, Halliburton SS et al. Three-dimensional imaging in thecontext of minimally invasive and transcatheter cardiovascular interventions using multi-detector computed tomography: from pre-operative planning to intra-operative guidance. Eur. Heart J. 31(22), 2727–2740 (2010).
- Zamorano JL, Badano LP, Bruce C et al.; Document Reviewers: European Association of Echocardiography (EAE): American Society of Echocardiography (ASE): The ASE Guidelines and Standards Committee and the ASE Board of Directors. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur. Heart J. 12, 557–584 (2011).
- Hayashida K, Bouvier E, Lefevre T et al. Impact of CT-guided valve sizing on post-procedural aortic regurgitation in transcatheter aortic valve implantation. EuroIntervention 8(5), 546–555 (2012).
- Jilaihawi H, Kashif M, Fontana G et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J. Am. Coll. Cardiol. 59(14), 1275–1286 (2012).
- Kurra V, Kapadia SR, Tuzcu EM et al. Pre-procedural imaging of aortic root orientation and dimensions: comparison between x-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc. Interv. 3(1), 105–113 (2010).
- Krishnaswamy A, Tuzcu EM, Kapadia SR. Use of intraprocedural CT imaging to guide alcohol septal ablation of hypertrophic cardiomyopathy in the cardiac catheterization laboratory. Catheter Cardiovasc. Interv. 80(6), 991–994 (2012).
- Nolker G, Asbach S, Gutleben KJ et al. Image-integration of intraprocedural rotational angiography-based 3D reconstructions of left atrium and pulmonary veins into electroanatomical mapping: accuracy of a novel modality in atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 21(3), 278–283 (2010).
- Nordon IM, Hinchliffe RJ, Malkawi AH et al. Validation of DynaCT in the morphological assessment of abdominal aortic aneurysm for endovascular repair. J. Endovasc. Ther. 17(2), 183–189 (2010).
- Mordasini P, Al-Senani F, Gralla J et al. The use of flat panel angioCT (DynaCT) for navigation through a deformed and fracturedcarotid stent. Neuroradiology 52(7), 629–632 (2010).
- Choure AJ, Garcia MJ, Hesse B et al. In vivo analysis of the anatomical relationship of coronary sinus to mitralannulus and left circumflex coronary artery using cardiac multidetector computed tomography: implications for percutaneous coronary sinus mitral annuloplasty. J. Am. Coll. Cardiol. 48(10), 1938–1945 (2006).
- Schoenhagen P, Tuzcu EM, Kapadia SR, Desai MY, SvenssonLG. Three-dimensional imaging of the aortic valve and aortic root with computed tomography: new standards in an era of transcatheter valve repair/ implantation. Eur. Heart J. 30(17), 2079–2086 (2009).
- Krishnaswamy A, Patel NS, Ozkan A et al. Planning left atrial appendage occlusion using cardiac multidetector computed tomography. Int. J. Cardiol. 158(2), 313–317 (2012).
- Krishnaswamy A, Tuzcu EM, Kapadia SR. Three-dimensional computed tomography in the cardiac catheterization laboratory. Catheter Cardiovasc. Interv. 77(6), 860–865 (2011).
- Duggal B, Krishnaswamy A, Kapadia SR. Relentless pulmonary vein stenosis: a contemporary approach to a recurring problem. Catheter Cardiovasc. Interv. doi:10.1002/ccd.24882 (2013) (Epub ahead of print).
- Glockler M, Halbfab J, Koch A et al. Multimodality 3D-roadmap for cardiovascular interventions in congenital heart disease – a single-center, retrospective analysis of 78 cases. Catheter Cardiovasc. Interv. doi:10.1002/ccd.24646 (2012) (Epubahead of print).
- Biasi L, Ali T, Thompson M. Intra-operative DynaCT in visceral-hybrid repair of an extensive thoracoabdominal aortic aneurysm. Eur. J. Cardiothorac. Surg. 34(6), 1251–1252(2008).
- Bai M, Liu B, Liu X, Jiang Y. The comparison of radiation dose between C-arm flat-detector CT (DynaCT) and multi-slice CT (MSCT): a phantom study. Eur. J. Radiol. 81(11), 3577–3580 (2012).
- Quail M, Nordmeyer J, Schievano S et al. Use of cardiovascular magnetic resonance imaging for TAVR assessment in patients with bioprosthetic aortic valves: comparison with computed tomography. Eur. J. Radiol. 81(12), 3912–3917 (2012).
- Jabbour A, Ismail T, Moat N et al. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. JACC 58(21), 2165–2173 (2011).
- Paelinck B, Van Herck P, Rodrigus I et al. Comparison of magnetic resonance imaging of aortic valve stenosis and aortic root to multimodality imaging for selection of transcatheter aortic valve implantation candidates. Am. J. Cardiol. 108(1), 92–98 (2011).
- La Manna A, Sanfilippo A, Capodanno et al. Cardiovascular magnetic resonance for the assessment of patients undergoing transcatheter aortic valve implantation: a pilot study. J. Cardiovasc. Magn. Reson. 13, 82 (2011).
- Dori Y, Sarmiento M, Glatz A et al. X-ray magnetic resonance fusion to internal markers and utility in congenital heart disease catheterization. Circ. Cardiovasc. Imaging 4(4), 415–424 (2011).
- Saikus C, Lederman R. Interventional cardiovascular magnetic resonance imaging: a new opportunity for image-guided interventions. JACC Cardiovasc. Imaging 2(11), 1321–1331 (2009).
- Kahlert P, Parohl N, Albert J et al. Towards real-time cardiovascular magnetic resonance guided transarterial CoreValve implantation: in vivo evaluation in swine. J. Cardiovasc.Magn. Reson. 14, 21 (2012).
- Yuksel UC, Tuzcu EM, Kapadia SR. Percutaneous closure of a postero–medial mitral paravalvular leak: the triple telescopic system. Catheter Cardiovasc. Interv. 77(2), 281–285 (2011).
- Pandian NG. Intravascular and intracardiac ultrasound imaging. An old concept, now on the road to reality. Circulation 80(4), 1091–1094 (1989).
- Valdes-Cruz LM, Sideris E, Sahn DJ et al. Transvascular intracardiac applications of aminiaturized phased-array ultrasonic endoscope. Initial experience with intracardiac imaging in piglets. Circulation 83(3), 1023–1027 (1991).
- Banchs JE, Patel P, Naccarelli GV, Gonzalez MD. Intracardiac echocardiography in complex cardiac catheter ablation procedures. J. Interv. Card. Electrophysiol. 28(3), 167–184(2010).
- Gonzalez I, Cao QL, Hijazi Z. Role of intracardiac echocardiography (ICE) in transcatheter occlusion of atrial septal defects. In: Atrial Septal Defect. Rao PS (Ed.). InTech, NY, USA, 99–118 (2012).
▪ Provides an excellent overview of the advantages of 3D echocardiography and recommendations for its use in various scenarios.
▪ Provides technical details of 3D echocardiography.
▪ Provides a closer look at the use of cardiac MRI in conjunction with transcatheter aortic valve replacement.
▪ Further reading on cardiac MRI with fluoroscopic overlay.