Short Communication - Clinical Investigation (2019) Volume 9, Issue 1
Mutation analysis of circadian clock gene BMAL1 in 21 Pakistani congenital cataract families
Udita Bagchi1,2, Shazia Micheal1, Sorath Noorani Siddiqui3, Muhammad Imran Khan4, Jacoline B Ten Brink1, Marie- Paule Felder-Schmittbuhl2, Arthur A Bergen1,5*
1Department of Clinical Genetics, Academic Medical Centre, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
2Centre National de la Recherche Scientifique, University of Strasbourg, Institute of Cellular and Integrative Neuroscience (UPR3212), Strasbourg, France.
3Department of Pediatric Ophthalmology and Strabismus, Al-Shifa Eye Trust Hospital, Jhelum Road, Rawalpindi, Pakistan.
4Department of Human Genetics, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands.
5Netherlands Institute for Neuroscience (NN-KNAW), Meibergdreef, Amsterdam, The Netherlands.
- *Corresponding Author:
- Arthur A Bergen
Department of Clinical Genetics,
Academic Medical Centre,
Netherlands Institute for Neuroscience (NN-KNAW)
Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
E-mail: aabergen@amc.uva.nl
Abstract
Background: Given the cataract phenotype in BMAL1-/- mice, we aimed to identify potential disease-causing variants in the human circadian clock candidate gene BMAL1 in 21 probands of consanguineous Pakistani congenital cataract families.
Methods: Ophthalmic examinations were performed for the probands and available family members. Genomic DNA was isolated from a volume of 5 ml of peripheral blood. The entire coding region of the candidate gene BMAL1 was analyzed in the probands of 21 families with targeted Sanger sequencing.
Results: A heterozygous missense variant c.41A>T; p.(Asp14Val) was detected in 1 of the 21 patients, which has a rare allele frequency of 0.000065 (2/30576 individuals) exclusively in the South-Asian population. The variant did not co-segregate with the disease phenotype in the family. A non-synonymous variant (rs2290037) in the heterozygous state was also identified in 5 out of 21 probands with a higher allele frequency of 0.1190 as compared to the global population (0.06626; 15570/234984 individuals).
Conclusion: Our study is the first to investigate the core circadian clock gene BMAL1 in humans for their association with congenital cataract. Unfortunately, no clear association between human genetic BMAL1 variants and cataract was found. Compared with targeted NGS technologies, traditional Sanger sequencing remains an indispensable cost-effective tool especially to report mutation profiles in small study cohorts. Our study may act to guide further studies in the molecular clockwork pathway from other (disease) populations.
Abstract
Background: Given the cataract phenotype in Bmal1-/- mice, we aimed to identify potential disease-causing variants in the human circadian clock candidate gene BMAL1 in 21 probands of consanguineous Pakistani congenital cataract families.
Methods: Ophthalmic examinations were performed for the probands and available family members. Genomic DNA was isolated from a volume of 5 ml of peripheral blood. The entire coding region of the candidate gene BMAL1 was analyzed in the probands of 21 families with targeted Sanger sequencing.
Results: A heterozygous missense variant c.41A>T; p.(Asp14Val) was detected in 1 of the 21 patients, which has a rare allele frequency of 0.000065 (2/30576 individuals) exclusively in the South-Asian population. The variant did not co-segregate with the disease phenotype in the family. A non-synonymous variant (rs2290037) in the heterozygous state was also identified in 5 out of 21 probands with a higher allele frequency of 0.1190 as compared to the global population (0.06626; 15570/234984 individuals).
Conclusion: Our study is the first to investigate the core circadian clock gene BMAL1 in humans for their association with congenital cataract. Unfortunately, no clear association between human genetic BMAL1 variants and cataract was found. Compared with targeted NGS technologies, traditional Sanger sequencing remains an indispensable cost-effective tool especially to report mutation profiles in small study cohorts. Our study may act to guide further studies in the molecular clockwork pathway from other (disease) populations.
Keywords
Congenital Cataract, Consanguineous, Circadian, Clockwork, Infantile, Mutation
Background
Congenital or infantile cataract is defined by the opacity of the crystalline lens resulting in the (predisposition for) partial to complete pediatric visual disability. The World Health Organization (WHO) describes cataract as the primary cause of blindness throughout the world affecting 16 million people worldwide [1]. The incidence of congenital cataract (CC) is estimated to be 1-6 cases per 10,000 live births in developed countries, and 5-15 cases per 10,000 in the underdeveloped countries [2]. Approximately 200,000 children every year are affected by lifelong vision impairment due to congenital cataract [3]. Inherited cataracts represent a significant contribution to CC [4-6]. Currently, over 48 genes have been delineated in the Cat-Map database [7] underlying the pathogenesis of congenital cataract. Nearly 50% of the disease is accounted for by mutations in the crystalline genes [8-10]. Connexin genes comprise approximately a share of 25% [11- 13] along with the causative gene mutations described in other structural proteins, namely beaded filament structural protein 2 (BFSP2) [14], lens intrinsic membrane protein (LIM2) [15,16], aquaporin0 (MIP) [17,18], enzymes like glucosaminyl (N-acetyl) transferase 2 (GCNT2) [16] and in transcription factors such as paired-like homeodomain 3 (PITX3) [19], avian musculoaponeurotic fibrosarcoma (MAF) [20], heat shock transcription factor 4 gene (HSF4) [21].
Identification of new genetic mutations in cataract patients will improve our understanding of cataractogenesis during childhood and could provide further insights into lens biology. There is mounting experimental evidence that suggests a direct or indirect involvement of the circadian clock in cataract [22-27]. Circadian rhythms are 24-hour temporal programmes, widely distributed in mammalian tissues and synchronized by a master-hypothalamic clock [28]. In mammals, the core clock genes, including Bmal1, Clock, Cry, and Per, are rhythmically expressed in the Suprachiasmatic Nuclei (SCN)-the master clock in the hypothalamus and also in almost all peripheral cells/ tissues, including the lens and retina of the eyes [28]. The clock transcription factors control the expression of numerous target genes in a circadian manner, influencing many physiological and biochemical processes [29], including those in the eye [30]. Clock factors act upstream of or in cooperation with tissuespecific transcription factors to temporally modulate RNA polymerase II loading, histone modification or three-dimensional conformation of the chromatin [31]. Thus, cycling transcriptomes are rather tissuespecific [32]. In addition, the clock orchestrates the temporal expression of genes during the development of the eye per se [33-36]. Notably, circadian clock genes Bmal1 and Clock have been observed to be involved in the pathophysiology of cataract in mice [37-39]. It has been reported that genes implicated in cataract development in humans, may also be the key players in animal models like rodents and vice versa [40,41]. Daily variations in levels of crystalline mRNAs and proteins in the retinal photoreceptor cells of rats highlight the role of circadian processes in retinal crystalline synthesis [42]. In humans, decreased potential for circadian photo-entrainment is known to be associated with cataract development [43]. Moreover, visual acuity and circadian photoreception via the photosensitive retinal ganglion cells is profoundly impaired in cataract patients [44-46]. Finally, progressive loss of vision leading to blindness fails to render the required input signals to the biological clock [47,48]. These data prompted us to investigate the possible association between cataract development and the clock.
The primary transcriptional regulator of the circadian clock, the brain, and muscle aryl hydrocarbon receptor nuclear translocator-like protein BMAL1 is implicated in the regulation of early to premature ocular aging [37,38,49]. More than 50% of Bmal1 deficient mice developed cataract before the 40th week of life [37]. Also, deletion of Bmal1 disrupts clock-dependent oscillatory gene expression and behavioral rhythmicity coincident with eye pathologies, reduced body weight, impaired hair growth, abnormal bone calcification, neurodegeneration, and a shortened lifespan [49- 52]. Recently, the conditional deletion of Bmal1 in endothelial and hematopoietic cells of the murine retina [53] demonstrated pathologic hallmarks of diabetic retinopathy, thereby expanding on the ocular pathology as a consequence of molecular Bmal1 defects. It has been identified that BMAL1-mediated activation of the DNA repair system can render remedial mechanisms for treating photodamage, including photoaging [54].
Due to the heterogeneous nature of congenital cataracts, the involvement of additional genetic and environmental factors cannot be dismissed. In this study, we aimed to identify the disease-causing variants in the BMAL1 gene associated with the CC phenotype in the consanguineous Pakistani families to explore any existing links between the circadian clock and ocular abnormalities.
Materials and Methods
Subjects
The patients were recruited at the pediatric ophthalmology department of Al-Shifa Eye Trust Hospital, Rawalpindi, Pakistan. The study was approved by the Institutional Review Board of the Al- Shifa Eye Trust Hospital (Rawalpindi, Pakistan), and adhered to the tenets of the Declaration of Helsinki with the approval code PK2014:102. Written informed consent was obtained for study participation from the participants and/or their parents, as appropriate. Comprehensive, ocular, medical, and family histories were obtained from the parents/available family member. A detailed ophthalmic examination was performed for both affected and unaffected individuals of families. Blood samples were collected from affected and unaffected siblings, and from the parents. Genomic DNA was extracted using QIAGEN DNA Blood Midi Kit (QIAGEN, Germantown, Maryland, USA).
PCR and sanger sequencing
PCR amplification of the 16 coding exons of the BMAL1 gene was performed in the (n=21) probands of the consanguineous cataract families using a PE 9700 thermocycler (Applied Biosystems, Foster City, CA). Primers for the BMAL1 gene (NM_001351814.1) were designed using Primer 3 [55] to cover exon/intron boundaries up to 100 base pairs into introns and are presented in Table 1. All amplicons were subjected to the following cycling conditions: initial denaturation at 94°C for 5 min followed by 35 cycles of 94°C for 30s, 64°C for 30s, and 72°C for 30s. PCR products were analyzed on 2% agarose gels followed by Sanger sequencing using ABI BigDye chemistry (Applied Biosystems Inc., Foster City, CA, USA), and were processed through an automated ABI 3730 Sequencer (Applied Biosystems, Inc.) using standard protocols.
| Primer Name | Sequence 5’-3’ | Product size |
|---|---|---|
| ARNTL_E5_Forward | GCTCTTTCCATTCTATCACATGC | 476 |
| ARNTL_E5_Reverse | TGTCGCCACCTAGAGTTGG | |
| ARNTL_E6-7_Forward | TGGCTGTTCGAACTTTATGA | 567 |
| ARNTL_E6-7_Reverse | ACAGGACCAAACATGCAGAG | |
| ARNTL_E8_Forward | TGCCTTGTCAGATGAACATTGA | 568 |
| ARNTL_E8_Reverse | GGCATACTACTGAAGGCTACAT | |
| ARNTL_E9_Forward | AGAGACTAGGCCACTTACAGA | 345 |
| ARNTL_E9_Reverse | AGAAATGTGAAGCCTGTCCA | |
| ARNTL_E10_Forward | TCCTGTGCTTTGGATGCTT | 442 |
| ARNTL_E10_Reverse | TGCAGCAATAGAAGAAAGCCA | |
| ARNTL_E11_Forward | AACCTCCAGATGCCTCCTTC | 456 |
| ARNTL_E11_Reverse | GCCAAAGATAGCTCTGGTGC | |
| ARNTL_E12_Forward | AGTGAGGCAGGCAAGAAAAG | 390 |
| ARNTL_E12_Reverse | AGCCAGAAACCATGGAACC | |
| ARNTL_E13_Forward | TCCCTACCTACATCCCATCC | 487 |
| ARNTL_E13_Reverse | TTCTTAGAAAAGCCAGCTGATG | |
| ARNTL_E14_Forward | GCAGCTTTGACCTTGCTCTC | 347 |
| ARNTL_E14_Reverse | GGCTGGCTGACTCTACATCC | |
| ARNTL_E15_Forward | CTAAAGAGCGATGTCGTTGG | 415 |
| ARNTL_E15_Reverse | AGCTTCTGCCAGTCCTGAG | |
| ARNTL_E16_Forward | ACCTCTGCTGAACTGTGTCC | 470 |
| ARNTL_E16_Reverse | GAAATCCGCACATCATCC | |
| ARNTL_E17_Forward | ACTGCAAATGGATCATGGGA | 383 |
| ARNTL_E17_Reverse | TGTTTAACAAGCAGCATCCCT | |
| ARNTL_E18_Forward | GCTTGCCAAACCCTAATCTAGAT | 349 |
| ARNTL_E18_Reverse | CCTCACACAGATGCATTTACTTC | |
| ARNTL_E19_Forward | AGAAAACTGAAGCCATTTGAAGC | 399 |
| ARNTL_E19_Reverse | CTCCACCAAAACTCAAATACTGG | |
| ARNTL_E20_Forward | AAGCAGCATCTCACCCTACC | 423 |
| ARNTL_E20_Reverse | TCAATGGCTCTGAGATGGCT |
Table 1: Primer sequences of BMAL1 (ARNTL).
Data processing
The obtained sequences were aligned with the reference sequence (NM_001351814.1) using CodonCode Aligner (version 6.1) (CodonCode Co., Centerville, MA, USA). Intra-familial segregation analysis was also performed upon the identification of variant in the exon 5 of the BMAL1 gene in the respective family.
Potential pathogenicity of the identified DNA variants was evaluated by publicly available tools including PhyloP, Grantham and polymorphism phenotyping v-2 (PolyPhen-2) (version 2.1.0 r367) [56] MutationTaster [57], and Sorting Intolerant From Tolerant (SIFT, [58]) to predict the functional impact of the sequence variants on the encoded protein. To determine the amino acid conservation among different species, protein sequences were obtained from UniProt [59] database. Kalign (2.0) was used for the multiple nucleotides and amino acid sequence alignment.
Results
Mutation detection
All coding sequences of BMAL1 of 21 cataract probands were screened. In one proband, we identified a c.41A>T; p.(Asp14Val) variant; in five probands, we detected a non-synonymous variant (rs2290037). The remainder of the sequences were wild-type.
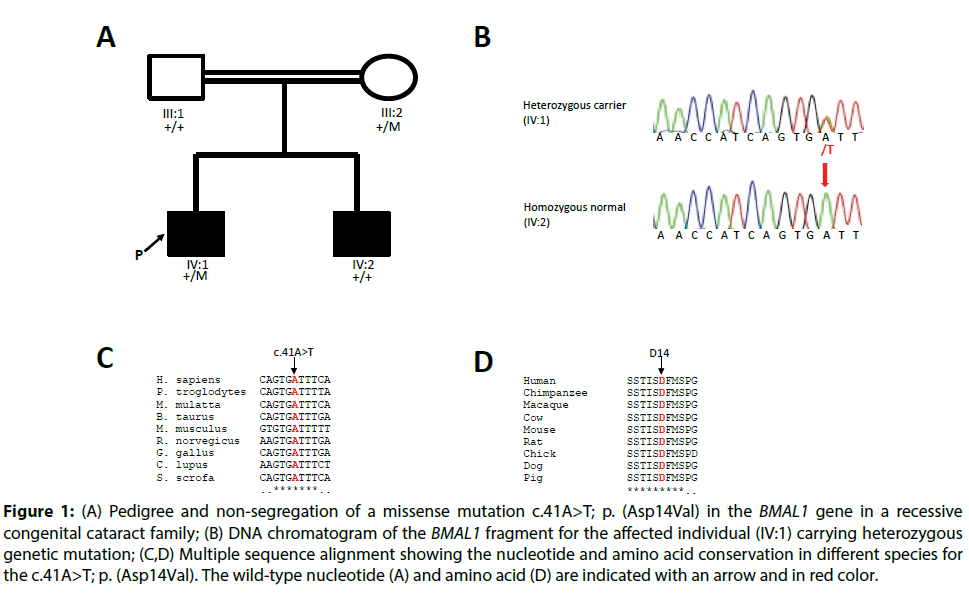
In one proband, a BMAL1 c.41A>T; p.(Asp14Val) a missense variant in exon 5 was present in a heterozygous form (Figure 1B). This particular variant c.41A>T; p.(Asp14Val) was bioinformatically predicted to be deleterious by SIFT, damaging by PolyPhen-2 and disease-causing by Mutation Taster. The wildtype nucleotide and amino acid residues were highly conserved with a PhyloP score of 4.73, and a grantham score 152 respectively. The nucleotide and amino acid residues were found to be highly conserved among different orthologous species (Figure 1C and 1D). The p.Asp14Val variant was present with a rare allele frequency of 0.000065 (2/30576 individuals) exclusively in the South-Asian population [60]. Segregation analysis of the variant was performed, but the c.41A>T; p.(Asp14Val) variant did not co-segregate with the disease phenotype in the family (Figure 1A).
Figure 1: (A) Pedigree and non-segregation of a missense mutation c.41A>T; p. (Asp14Val) in the BMAL1 gene in a recessive congenital cataract family; (B) DNA chromatogram of the BMAL1 fragment for the affected individual (IV:1) carrying heterozygous genetic mutation; (C,D) Multiple sequence alignment showing the nucleotide and amino acid conservation in different species for the c.41A>T; p. (Asp14Val). The wild-type nucleotide (A) and amino acid (D) are indicated with an arrow and in red color.
In addition, we detected a non-synonymous variant (rs2290037) in BMAL1 intron 7 in 5 out of 21 probands. In our patient set, the variant occurred with a much higher allele frequency of 0.1190 than the general population.
Discussion and Conclusion
Circadian clock genes influence disease susceptibility due to their pleiotropic activities on gene expression by involvement in multiple pathways or via direct involvement with circadian clock function [61]. Earlier studies have reported BMAL1 gene variants in humans contributing to fertility and seasonality [62] as well as in hypertension and type-2 diabetes [63]. Given the occurrence of cataract in Bmal1-/- and Clock-/- mice, we tested the hypothesis that human BMAL1 mutations could be involved in human cataract.
In this study, we showed that genetic variations in BMAL1 in 21 patients with CC disease did not account for the disease phenotype. Although the identified missense variant c.41A>T; p.(Asp14Val) altered the wild-type amino acid sequence, occurred in a highly evolutionary conserved residue, and was also determined to be “probably pathogenic” by bioinformatics, it happened in the heterozygous state in a single patient and did not segregate in the family. Thus, we could not correlate the Asp14Val variant with the occurrence of congenital cataract. We did not exclude potential additional pathogenic mutations in our CC probands occurring outside of the coding exons and the flanking intron splice sites. A non-synonymous heterozygous variant (rs2290037) with a higher allele frequency was detected in the intronic region. It has been previously estimated that 5% of the rare non-synonymous heterozygous variants carry at least ~22 pathogenic derived alleles, which if turns out to be homozygous due to consanguineous marriages, can lead to recessive diseases [64].
To the best of our knowledge, our study is the first attempt to evaluate the presence of genetic variants in the BMAL1 gene for congenital cataract. The samples were identified from a well-characterized epidemiological cohort, which has a high degree of genetic heterogeneity [65]. Based on results in the mice, it will be apt to state that the human circadian clock genes may not be such an attractive target for mutation analysis in cataract families. Yet, we cannot entirely exclude the involvement of BMAL1 in human cataract. BMAL1 gene is an intricate member of the clockwork web, and since BMAL1 is not a sole member, BMAL1-CLOCK complex drives the clockwork machinery. Therefore, some more specific and additional screening of congenital cataract patients with (other) circadian clock genes (sequences) may be justified in subsequent studies.
Acknowledgment
The authors thank the family members for their cooperation during the course of the study. Udita Bagchi was supported by “Neurotime” Erasmus Mundus program.
Conflict of Interest
The authors declare no competing conflict of interests.
Funding
The study was supported by the Algemene Nederlandse Vereniging ter Voorkoming van Blindheid.
Availability of Data and Materials
All relevant datasets are used in the manuscript. The analyzed data is available from the corresponding author upon request.
Authors’ Contributions
U.B and A.A.B.B. conceived and designed the experiments; U.B. performed the experiments; S.M and S.N.S. recruited patients and collected samples; J.B, M.P.F.S., and A.A.B.B. contributed reagents/materials/ analysis tools; and U.B. wrote the manuscript. All authors have read and approved the final manuscript.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Review Board of the Al-Shifa Eye Trust Hospital (Rawalpindi, Pakistan), adhering to the tenets of the Declaration of Helsinki with the approval code PK2014:102.
Consent for Publication
The family members of the probands signed a written informed consent form for publication of all data.
Executive summary
Background: Given the cataract phenotype in Bmal1-/- mice, we aimed to identify potential disease-causing variants in the human circadian clock candidate gene BMAL1 in 21 probands of consanguineous Pakistani congenital cataract families.
Methods: Ophthalmic examinations were performed for the probands and available family members. Genomic DNA was isolated from a volume of 5 ml of peripheral blood. The entire coding region of the candidate gene BMAL1 was analyzed in the probands of 21 families with targeted Sanger sequencing.
Results: A heterozygous missense variant c.41A>T; p.(Asp14Val) was detected in 1 of the 21 patients, which has a rare allele frequency of 0.000065 (2/30576 individuals) exclusively in the South-Asian population. The variant did not co-segregate with the disease phenotype in the family. A non-synonymous variant (rs2290037) in the heterozygous state was also identified in 5 out of 21 probands with a higher allele frequency of 0.1190 as compared to the global population (0.06626; 15570/234984 individuals).
Conclusion: Our study is the first to investigate the core circadian clock gene BMAL1 in humans for their association with congenital cataract. Unfortunately, no clear association between human genetic BMAL1 variants and cataract was found. Compared with targeted NGS technologies, traditional Sanger sequencing remains an indispensable cost-effective tool especially to report mutation profiles in small study cohorts. Our study may act to guide further studies in the molecular clockwork pathway from other (disease) populations.
References
- Thylefors B, AD Negrel, Ramachandra Pararajasegaram, KY Dadzie. Global data on blindness. Bull World Health Organ 73: 115-121 (1995).
- Apple DJ, Ram J, Foster A, Peng Q. Elimination of cataract blindness: A global perspective entering the new millenium. Surv Ophthalmol 45: 196 (2000).
- Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: A global perspective. J Cataract Refract Surg 23: 601-604 (1997).
- Stoll C, Alembik Y, Dott B, Roth MP. Epidemiology of congenital eye malformations in 131,760 consecutive births. Ophthalmic Paediatr Genet 13:179-186 (1992).
- Gregg NM. Congenital cataract following German measles in the mother 1941. Epidemiol Infect 170: 3-14 (1991).
- Blohme J, Tornqvist K. Visual impairment in Swedish children: III. Diagnoses. Acta Ophthalmol Scand 75: 681-687 (1997).
- https://cat-map.wustl.edu/
- Sun Z, Zhou Q, Li H, Yang L, Wu S, Sui R. Mutations in crystallin genes result in congenital cataract associated with other ocular abnormalities. Mol Vis 23: 977-986 (2017).
- Bateman JB, von-Bischhoffshaunsen FR, Richter L, Flodman P, Burch D, Spence MA. Gene conversion mutation in crystallin, beta-B2 (CRYBB2) in a Chilean family with autosomal dominant cataract. Ophthalmol 114: 425-432 (2007).
- AlFadhli S, Abdelmoaty S, Al-Hajeri A, Behbehani A, Alkuraya F. Novel crystallin gamma B mutations in a Kuwaiti family with autosomal dominant congenital cataracts reveal genetic and clinical heterogeneity. Mol Vis 8: 2931-2936 (2012).
- Shen C, Wang J, Wu X, et al. Next-generation sequencing for D47N mutation in Cx50 analysis associated with autosomal dominant congenital cataract in a six-generation Chinese family. BMC Ophthalmol 17: 73 (2017).
- Mohebi M, Chenari S, Akbari A, et al. Mutation analysis of connexin 50 gene among Iranian families with autosomal dominant cataracts. Iran J Basic Med Sci 20: 288-93 (2017).
- Berthoud VM, Ngezahayo A. Focus on lens connexins. BMC Cell Biol 18: 6 (2017).
- Jakobs PM, Hess JF, FitzGerald PG, Kramer P, Weleber RG, Litt M. Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am J Hum Genet 66: 1432-1436 (2000).
- Irum B, Khan SY, Ali M, et al. Mutation in LIM2 is responsible for autosomal recessive congenital cataracts. PloS One 11: e0162620 (2016).
- Pras E, Raz J, Yahalom V, et al. A nonsense mutation in the glucosaminyl (N-acetyl) transferase 2 gene (GCNT2): Association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci 45: 1940-1945 (2004).
- Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant 'polymorphic' and lamellar cataracts linked to 12q. Nat Genet 25: 15-17 (2000).
- Qin L, Guo L, Wang H, et al. A novel MIP mutation in familial congenital nuclear cataracts. Eur J Med Genet 59: 488-491 (2016).
- Semina EV, Ferrell RE, Mintz-Hittner HA, et al. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet 19: 167 (1998).
- Vanita V, Singh D, Robinson PN, Sperling K, Singh JR. A novel mutation in the DNA-binding domain of MAF at 16q23. 1 associated with autosomal dominant “cerulean cataract” in an Indian family. Am J Med Genet A 140: 558-566 (2006).
- Behnam M, Imagawa E, Chaleshtori ARS, et al. A novel homozygous mutation in HSF4 causing autosomal recessive congenital cataract. J Hum Genet 61: 177 (2015).
- Khorsand M, Akmali M, Sharzad S, Beheshtitabar M. Melatonin reduces cataract formation and aldose reductase activity in lenses of streptozotocin-induced diabetic rat. Iran J Med Sci 41: 305-313 (2016).
- Yan SS, Wang W. The effect of lens aging and cataract surgery on circadian rhythm. Int J Ophthalmol 9: 1066-1074 (2016).
- Nishi T, Saeki K, Obayashi K, et al. The effect of blue-blocking intraocular lenses on circadian biological rhythm: protocol for a randomised controlled trial (CLOCK-IOL colour study). BMJ Open 5: e007930 (2015).
- Saeki K, Obayashi K, Nishi T, et al. Short-term influence of cataract surgery on circadian biological rhythm and related health outcomes (CLOCK-IOL trial): study protocol for a randomized controlled trial. Trials 15: 514 (2014).
- Bai J, Dong L, Song Z, et al. The role of melatonin as an antioxidant in human lens epithelial cells. Free Radic Res 47: 635-642 (2013).
- Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom (2018).
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15: R271-R277 (2006).
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517-549 (2010).
- Felder-Schmittbuhl MP, Buhr ED, Dkhissi-Benyahya O, et al. Ocular clocks: Adapting mechanisms for eye functions and health. Invest Ophthalmol Vis Sci 59: 4856-4870 (2018).
- Vakili H, Jin Y, Cattini PA. Evidence for a circadian effect on the reduction of human growth hormone gene expression in response to excess caloric intake. J Biol Chem 291: 13823-13833 (2016).
- Lech K, Ackermann K, Revell VL, Lao O, Skene DJ, Kayser M. Dissecting daily and circadian expression rhythms of clock-controlled genes in human blood. J Biol Rhythms 31: 68-81 (2016).
- Curran KL, LaRue S, Bronson B, et al. Circadian genes are expressed during early development in Xenopus laevis. PLoS One 3: e2749 (2008).
- Kobayashi Y, Ye Z, Hensch TK. Clock genes control cortical critical period timing. Neuron 86: 264-275 (2015).
- Sawant OB, Horton AM, Zucaro OF, et al. the circadian clock gene bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep 21: 692-706 (2017).
- Vallone D, Lahiri K, Dickmeis T, Foulkes NS. Start the clock! Circadian rhythms and development. Dev Dyn 236: 142-155 (2007).
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20: 1868-1873 (2006).
- Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany NY) 1: 979-987 (2009).
- Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2: 936-944 (2010).
- Graw J. Mouse models of cataract. J Genet 88: 469-486 (2009).
- Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B Biol Sci 366: 1234-1249 (2011).
- Organisciak D, Darrow R, Barsalou L, Rapp C, McDonald B, Wong P. Light induced and circadian effects on retinal photoreceptor cell crystallins. Photochem Photobiol 87: 151-159 (2011).
- Kessel L, Siganos G, Jorgensen T, Larsen M. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep 34: 1215-1219 (2011).
- Brondsted AE, Sander B, Haargaard B, et al. The effect of cataract surgery on circadian photoentrainment: a randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmol 122: 2115-2124 (2015).
- Gimenez M, Beersma D, Daan S, et al. Melatonin and sleep-wake rhythms before and after ocular lens replacement in elderly humans. Biology (Basel) 5 (2016).
- Turner PL, Mainster MA. Circadian photoreception: ageing and the eye's important role in systemic health. Br J Ophthalmol 92: 1439-1444 (2008).
- Skene DJ, Lockley SW, Arendt J. Melatonin in circadian sleep disorders in the blind. Biol Signals Recept 8: 90-95 (1999).
- Skene DJ, Lockley SW, Thapan K, Arendt J. Effects of light on human circadian rhythms. Reprod Nutr Dev 39: 295-304 (1999).
- Yang G, Chen L, Grant GR, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med 8: 324ra16 (2016).
- Geyfman M, Andersen B. Clock genes, hair growth and aging. Aging (Albany NY) 2: 122-128 (2010).
- McDearmon EL, Patel KN, Ko CH, et al. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314: 1304-1308 (2006).
- Samsa WE, Vasanji A, Midura RJ, Kondratov RV. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 84: 194-203 (2016).
- Bhatwadekar AD, Beli E, Diao Y, et al. Conditional deletion of Bmal1 accentuates microvascular and macrovascular injury. Am J Pathol 187: 1426-1435 (2017).
- Joo JH, Hong IK, Kim NK, Choi E. Trichosanthes kirilowii extract enhances repair of UVB radiationinduced DNA damage by regulating BMAL1 and miR1423p in human keratinocytes. Mol Med Rep 17: 877-883 (2018).
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 3: 479-493 (2011).
- http://bioinfo.ut.ee/primer3-0.4.0/
- http://genetics.bwh.harvard.edu/pph2/
- http://www.mutationtaster.org/
- http://sift.bii.a-star.edu.sg/
- https://www.uniprot.org/uniprot/O00327
- http://gnomad.broadinstitute.org
- Kovanen L, Saarikoski ST, Aromaa A, Lonnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One 5: e10007 (2010).
- Woon PY, Kaisaki PJ, Braganca J, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci 104: 14412-14417 (2007).
- Li MX, Kwan JS, Bao SY, et al. Predicting mendelian disease-causing non-synonymous single nucleotide variants in exome sequencing studies. PLoS Genet 9: e1003143 (2013).
- Chen J, Wang Q, Cabrera PE, et al. Molecular genetic analysis of Pakistani families with autosomal recessive congenital cataracts by homozygosity screening. Invest Ophthalmol Vis Sci 58: 2207-2217 (2017).