Mini Review - Interventional Cardiology (2024)
Myval transcatheter aortic valve series: An innovation in transcatheter aortic valve implantation technology
- Corresponding Author:
- Teoman Kilic
Structural Heart Interventions Unit, Department of Cardiology, Kocaeli University School of Medicine, Kocaeli, Turkey,
E-mail: kilicteoman@yahoo.com
Received date: 02-Nov-2024, Manuscript No. FMIC-24-151636; Editor assigned: 04-Nov-2024, PreQC No. FMIC-24-151636 (PQ); Reviewed date: 19-Nov-2024, QC No. FMIC-24-151636; Revised date: 26-Nov-2024, Manuscript No. FMIC-24-151636 (R); Published date: 03-Dec-2024, DOI: 10.37532/1755- 5310.2024.16(S25).632
Abstract
The Myval Transcatheter Heart Valve (THV) represents a significant innovation in Transcatheter Aortic Valve Implantation (TAVI) technology, particularly for patients with severe Aortic Stenosis (AS) and complex anatomies. This balloon-expandable, tri-leaflet THV utilizes a nickel-cobalt alloy frame, offering enhanced conformability within the aortic annulus. Structural advancement in the Myval THV series reduces paravalvular leaks and minimizes the need for Permanent Pacemaker Implantation (PPI). Approved for high and intermediate-risk AS patients, Myval has demonstrated consistent safety and efficacy, marked by low rates of procedural complications and mortality in clinical studies. Comparative analyses reveal Myval’s superior hemodynamic performance and reduced vascular complications, attributed to its unique design and range of sizes, including intermediate and extra-large options for anatomies up to 840 mm2 in annulus area. Next-generation developments of Myval also aim to further optimize patient outcomes and procedural safety, promising a valuable expansion of TAVI treatment options. This brief review highlights the design innovations, clinical effectiveness and potential future impact of the Myval THV within the evolving TAVI landscape.
Keywords
Valve • Annulus area • Design innovations • Patient outcomes
Introduction
The European and American Cardiovascular Societies, both, recommend TAVI as an established, minimally invasive technique for treating severe AS, predominantly in older (aged ≥ 75 years) and high-risk patients unsuitable for surgery. Recently, TAVI indications have expanded to include select low-risk and younger patients [1]. The advancement of THVs has developed since the introduction of the first Balloon-Expandable (BE) Edwards-Sapien [2]. However, until 2018, all commercially available alternatives to Edwards technology were Self-Expanding (SE) devices with indications limited to aortic positions. The first SE valve was CoreValve introduced in 2014 featuring leaflets positioned above the aortic annulus (Supra-annular design). Later, SE valve designs advanced with the introduction of Evolut R and Evolut PRO demonstrating improved clinical outcomes [3]. SE THVs typically offered a larger Effective Orifice Area (EOA) and lower gradients, especially in supra-annular designs, however were often associated with higher risks of Permanent PPI and Paravalvular Leaks (PVLs) [4].
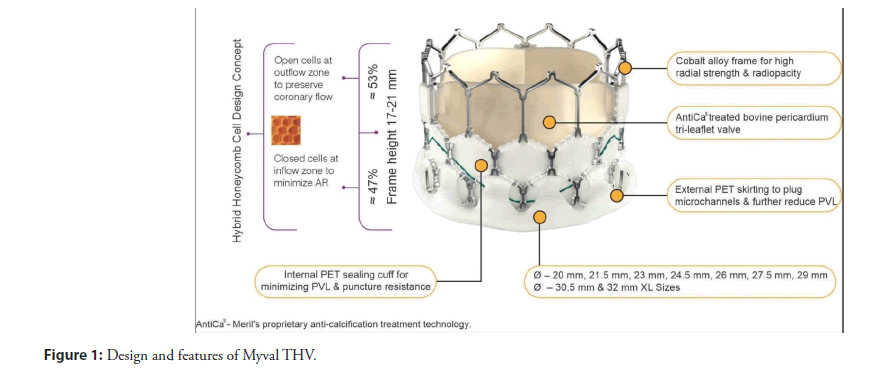
A new-generation Myval THV is a BE THV developed by Meril Life Sciences, Vapi, India (Conformité Européenne mark in 2018). It is notable for its innovative unique design, made from bovine pericardial tissue mounted in a nickel-cobalt frame embedded with an anti-calcification coating to reduce valve degeneration and an external Polyethylene Terephthalate (PET) buffing to avoid PVL [5]. The frame is composed of hexagons arranged in a hybrid honeycomb structure including closed cells (47%) at the ventricular end to provide higher annular radial force and open cells (53%) at the aortic end to ensure optimal coronary artery perfusion. Under fluoroscopy, this design creates an alternative dark/light band-like pattern on crimping, which serves as reference markers for precise positioning and deployment of THV across the native annulus [6,7]. The device description of the Myval THV is shown in (Figure 1). It has been engineered to reduce procedural complications such as vascular injuries, conduction disturbances and Prosthetic Valve Regurgitation (PVR) thereby enhancing clinical outcomes. Additionally, it has diverse sizing options, (Conventional: 20 mm, 23 mm, 26 mm and 29 mm; Intermediate: 21.5 mm, 24.5 mm and 27.5 mm; Extra-large: 30.5 mm and 32 mm) with 1.5 mm increments (e.g., 20.0 mm, 21.5 mm and 23.0 mm) to match a broad range of aortic annulus dimensions (270 mm2 to 840 mm2). The availability of intermediate sizes is one of the unique features of the Myval THV series which is not present in other contemporary devices. The intermediate sizes of Myval THV series provide a more calibrated THV choice to the operator addressing the unmet need of the TAVI operators for accurate sizing. This is particularly helpful in reducing the hazardous selection of undersized or oversized valves that may lead to complications such as PVR, new PPI and annular ruptures. When compared to established THVs, Myval THV has been demonstrating its versatility and success in treating native AS, as well as complex anatomies such as bicuspid AS and non-calcified Aortic Regurgitation (AR) [8].
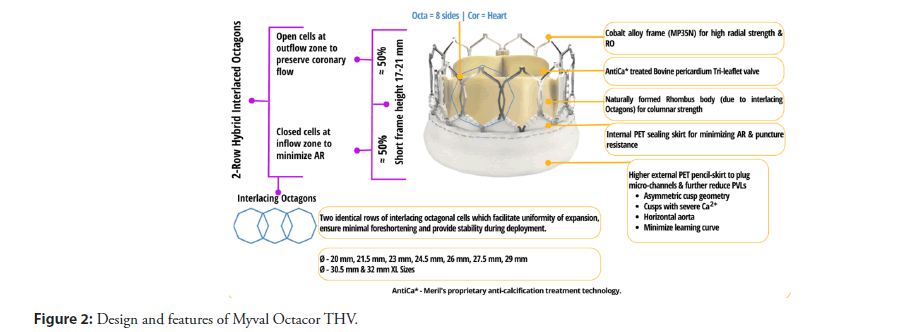
Myval Octacor and Myval Octapro THVs represent advanced versions of the Myval THV, retaining most of the features same as its predecessor but featuring two rows of geometrically identical, interlacing octagonal cells, instead of three rows of hexagonal cells of first-generation Myval THV. This modified design aims to minimize the foreshortening during expansion (19%-20% of Octacor vs. 21%-24% of Myval), thereby enhancing deployment accuracy [9,10]. The distinct features of Myval Octacor THV have been designed to facilitate precise positioning and deployment. A landing zone marker towards the ventricular end of the Navigator Inception THV delivery system facilitates precise positioning of Myval Octacor THV at the annulus. The BE Myval Octacor THV is designed to be implanted with a novel OctaAlign technique, which aims for accurate commissural alignment. Myval Octacor THV is recommended to be crimped directly onto the Navigator Inception THV balloon delivery system (Meril Life Sciences Pvt. Ltd., India) prior to insertion through the expandable 14Fr Python introducer sheath (Meril Life Sciences Pvt. Ltd., India). The device description of the Myval Octacor THV is shown in (Figure 2).
Literature Review
Several studies demonstrated Myval’s safety and efficacy in different ethnic populations and risk categories across the globe. The Myval THV has shown to be effective in high, intermediate and low surgical risk severe symptomatic AS and bicuspid anatomies [11,12]. In another experience with bicuspid anatomy at the procedural and 30-day observations, the Myval THV system was found to be safe and the hemodynamic parameters did not differ between tricuspid and bicuspid patients [13]. In the SAPPHIRE prospective registry, Myval THV proved to have promising safety and efficacy at 2 years [14].
A cohort study investigation on clinical outcomes for patients who underwent TAVI for severe AS patients revealed that Myval was safe and effective with 99% technical success, 91% device success and 79% clinical efficacy [15]. Similarly, the MATCHBALL study found favorable early outcomes of Myval over Sapien in terms of safety, with a low PPI incidence and with favorable residual gradients and PVL. The EVAL registry which compared Myval vs. Evolut R, showed non-significant differences in all-cause mortality and stroke rates, while PPI rates were lower at 30 days and 6 months’ follow-up and both THVs offered similar 2-year clinical outcomes, whereas Myval THV revealed advantages with higher clinical efficacy and reduced PVL rates [16,17]. The Myval THV also has advantages with higher clinical efficacy and lower PVL incidence. A study comparing BE Myval with SE Evolut in severe AS found both valves have similar safety and efficacy outcomes, with lower rates of complications and mortality. However, Myval had a significantly lower PPI rates compared to the Evolut group (4% vs. 15%, p=0.01) [18]. An independent Academic European registry found similar procedural and in-hospital outcomes, similar early new PPI amongst Myval (7.4%), Sapien-3 (13.4%) and Acurate (9.1%), but Evolut, Portico and Allegra presented significantly higher rates (18.5%, p=0.003; 29.5%, p<0.001 and 22%, p=0.001, respectively) [19,20].
Discussion
Further, studies demonstrated that intermediate sizes could reduce annular overexpansion, thus minimizing the rates of conduction system abnormalities and the need for new PPI. The recent LANDMARK trial compared the Myval THV series with contemporary (Evolut THV series and Sapien THV series) THV series and established the non-inferiority of Myval THV series for primary combined safety and effectiveness endpoint at 30 days in treating symptomatic severe native AS. A recent systematic review and meta-analysis demonstrated the safety and efficacy of Myval, with a 30-day mortality rate of 1.3%, good hemodynamic performance, lower PPI incidences (8.8%) and ≥ moderate PVL (1.3%). In the same systematic review, Myval was safe and effective in bicuspid AS, noncalcified AR, aortic valve-in-valve procedures and mitral valve-in-valve/in-ring procedures. In a real-world study, a newly approved 32 mm Myval THV in AS patients with extremely large aortic annuli showed promising initial results [21].
The early outcomes of the next-generation Myval Octacor THV proved its safety and effectiveness in treating severe AS in the OCTACOR India Study where overall mortality was 1.6% with no major vascular complications and significant improvements in the mean pressure gradient and EOA [22]. A recent review elucidated Myval and Myval OCTACOR key findings from the existing studies with complex scenarios such as large aortic annuli and bicuspid aortic valves. Experts demonstrated comparable early safety and efficacy profiles to contemporary THVs characterized by low rates of PVL and PPI. Further, the broad range of sizes in the Myval family reduced the risks of under or over-sizing that potentially led to reduce PVL and PPI rates.
Based on the above scientific evidence, the Myval THV has emerged as a significant innovation in TAVI technology, offering clinical benefits, safety and efficacy compared to standard contemporary THVs. Clinical evidence highlights its adaptability to a wide range of patient anatomies, including bicuspid AS, noncalcified AR, complex cardiac conditions, as well as valve-in-valve procedures. Its promising clinical outcomes particularly lower PPI rates and reduced conduction disturbances, can be attributed to its innovative design features. Multiple comparative studies confirmed Myval’s performance extremely well in terms of lower mortality, stroke, PPI and advantageous in terms of residual gradients and reduced PVL incidences. The recent innovations in Myval devices represent a leap in advancement in THV technologies, with several key modernizations enhancing its clinical performance with greater precision during implantation, reducing the risk of complications. Moreover, different valve sizes with its improved delivery system, ensure that Myval can cater to a wider demography across all risk categories, including anatomically challenging aortic roots. New iterations of the Myval THV by Meril Life Sciences continue to explore future advancements in TAVI technology incorporating next-generation materials and enhanced delivery systems aimed at further improving patient outcomes and procedural safety.
Conclusion
The Myval THV has set an innovative standard in TAVI technology, offering significant benefits over established valves. Its innovative design, excellent hemodynamic performance and reduced complications and mortality rates have positioned it as a leading treatment option for AS including extra-large sizes and complex anatomies as well. As clinical data continues to accumulate, Myval’s role in the TAVI landscape is expected to expand further, offering a highly effective and safe solution. Further randomized controlled trials and real-world registries with Myval THV are expected to support the long-term safety and efficacy, ensuring Myval’s durability
References
- Baumbach A, Royen N, Amat-Santos IJ, et al. LANDMARK comparison of early outcomes of newer-generation Myval transcatheter heart valve series with contemporary valves (Sapien and Evolut) in real-world individuals with severe symptomatic native aortic stenosis: A randomised non-inferiority trial. Lancet. 403(10445):2695-2708 (2024).
[CrossRef] [Google Scholar] [PubMed]
- Andersen HR, Knudsen LL, Hasenkam JMm, et al. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 13(5):704-708 (1992).
[CrossRef] [Google Scholar] [PubMed]
- Forrest JK, Kaple RK, Tang GH, et al. Three generations of self-expanding transcatheter aortic valves: A report from the STS/ACC TVT registry. JACC Cardiovasc Interv. 13(2):170-179 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA. 311(15):1503-1514 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Delgado-Arana JR, Gordillo-Monge MX, Halim J, et al. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart. 108(9):725-732 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Seth A, Kumar V, Singh VP, et al. Myval: A novel transcatheter heart valve for the treatment of severe aortic stenosis. Interv Cardiol. (2023).
[CrossRef] [Google Scholar] [PubMed]
- Sengottuvelu G, Kumar V, Seth A, et al. The Myval transcatheter heart valve system for the treatment of severe aortic stenosis-current evidence and future directions. Heart Int. 14(2):86 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Kawashima H, Serruys PW, Mylotte D, et al. Operator preference and determinants of size selection when additional intermediate-size aortic transcatheter heart valves are made available. Int J Cardiol. 338:168-173 (2021).
[CrossRef] [Google Scholar] [PubMed]
- García-Gómez M, Fernández-Cordón C, González-Gutiérrez JC, et al. The novel balloon-expandable myval transcatheter heart valve: Systematic review of aortic, mitral, tricuspid and pulmonary indications. Rev Esp Cardiol (Engl Ed). (2024).
[CrossRef] [Google Scholar] [PubMed]
- Montonati C, Pellegrini D, d’Atri DO, et al. A novel balloon-expandable transcatheter aortic valve bioprosthesis: Myval and myval Octacor. Expert Rev Cardiovasc Ther. 22(7):325-337 (2024).
[CrossRef] [Google Scholar] [PubMed]
- Elkoumy A, Jose J, Terkelsen CJ, et al. Safety and efficacy of myval implantation in patients with severe bicuspid aortic valve stenosis-a multicenter real-world experience. J Clin Med. 11(2):443 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Amat-Santos IJ, García-Gómez M, de Marco F, et al. Latest-iteration balloon-and self-expandable transcatheter valves for severe bicuspid aortic stenosis: The TRITON study. Rev Esp Cardiol (Engl Ed). 76(11):872-880 (2023).
[CrossRef] [Google Scholar] [PubMed]
- Magyari B, Kittka B, Goják I, et al. Single-center experience with the balloon-expandable myval transcatheter aortic valve system in patients with bicuspid anatomy: Procedural and 30-day follow-up. J Clin Med. 13(2):513 (2024).
[CrossRef] [Google Scholar] [PubMed]
- Testa L, Criscione E, Rubbio AP, et al. Safety and performance parameters of the myval transcatheter aortic valve bioprosthesis: The SAPPHIRE prospective registry. Cardiovasc Revasc Med.55:22-27 (2023).
[CrossRef] [Google Scholar] [PubMed]
- Kilic T, Ielasi A, Ninios V, et al. Clinical outcomes of the myval transcatheter heart valve system in patients with severe aortic valve stenosis: A two-year follow-up observational study. Arch Med Sci. 20(2):410 (2024).
[CrossRef] [Google Scholar] [PubMed]
- Barki M, Ielasi A, Buono A, et al. Clinical comparison of a novel balloon-expandable versus a self-expanding transcatheter heart valve for the treatment of patients with severe aortic valve stenosis: The EVAL registry. J Clin Med. 11(4):959 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Moscarella E, Ielasi A, Montonati C, et al. Comparing two-year outcomes of balloon-expandable myval and self-expanding evolut R in severe aortic valve stenosis. Int J Cardiol. 400:131701 (2024).
[CrossRef] [Google Scholar] [PubMed]
- Halim J, Rooijakkers M, den Heijer P, et al. Assessing the novel Myval balloon-expandable valve with the Evolut valve: A propensity-matched study. J Clin Med.12(13):4213 (2023).
[CrossRef] [Google Scholar] [PubMed]
- Santos-Martinez S, Halim J, Castro-Mejía A, et al. Myval versus alternative balloon-and self-expandable transcatheter heart valves: A central core lab analysis of conduction disturbances. Int J Cardiol. 351:25-31 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Halim J, den Heijer P, Vos J, et al. Balloon‐expandable TAVR bioprostheses: Area or perimeter sizing? A prospective pilot study. J Interv Cardiol. 2022(1):3139476 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Holzamer A, Bedogni F, van Wyk P, et al. Performance of the 32 mm Myval transcatheter heart valve for treatment of aortic stenosis in patients with extremely large aortic annuli in real‐world scenario: First global, multicenter experience. Catheter Cardiovasc Interv. 102(7):1364-1375 (2023).
[CrossRef] [Google Scholar] [PubMed]
- Jose J, Mandalay A, Cholenahally MN, et al. Safety and effectiveness of the novel myval octacor transcatheter heart valve in severe, symptomatic aortic valve stenosis-A real-world Indian experience (The OCTACOR India Study). Cardiovasc Revasc Med. 63:1-7 (2024).
[CrossRef] [Google Scholar] [PubMed]